Highlights
-
•
Portal vein thrombosis after liver transplantation is one of serious complications.
-
•
Indocyanine green (ICG)-fluorescence imaging can visualize the impaired perfusion or congestive area on the liver graft surface clearly.
-
•
ICG-fluorescence imaging can visualize regions with impaired hepatic perfusion during liver translantation in addition to visualization of hepatic flows of reconstructed vessels and evaluation of regions with venous occlusion.
Abbreviations: D-IOUS, Doppler intraoperative ultrasonography; FI, fluorescence intensity; ICG, indocyanine green; LT, liver transplantation; PVT, portal vein thrombosis
Keywords: Portal vein thrombosis, Living-donor liver transplantation, Indocyanine green, Fluorescence imaging technique
Abstract
Introduction
Portal vein thrombosis (PVT) after liver transplantation (LT) is one of serious complications and reportedly ranges from 2% to 13%. PVT impairs the blood perfusion to the grafts and causes the graft dysfunction.
Presentation of case
A 60-year-old female underwent living-donor LT with the left liver graft for end-stage liver disease related to chronic hepatitis C. After reperfusion, Indocyanine green (ICG)-fluorescence imaging was performed to confirm the graft perfusion, which pointed out an insufficient perfusion on the surface of segment 4. Following intraoperative ultrasonography revealed thrombus in the portal vein of segment 4, which was successfully removed by heparinized saline flush.
Discussion
The most of patients with PVT developed graft failure and resulted in retransplantation. This enhances the importance of the surveillance for PVT in the postoperative period as well as the intraoperative period. However, the modality to identify PVT during surgery is limited mainly to intraoperative ultrasound. ICG-fluorescence imaging can visualize regions with impaired hepatic perfusion due to PVT in real time during LT in addition to visualization of hepatic flows of reconstructed vessels and evaluation of regions with venous occlusion.
Conclusion
ICG-fluorescence imaging can be simply performed with single ICG injection and is expected to have potential roles to enhance the safety of LT.
Portal vein thrombosis (PVT) after liver transplantation (LT) is one of serious complications and reportedly ranges from 2% to 13% [1,2]. PVT impairs the blood perfusion to the grafts and causes the graft dysfunction. The most of patients with PVT developed graft failure and resulted in retransplantation [1,2]. This enhances the importance of the surveillance for PVT in the postoperative period as well as the intraoperative period. However, the modality to identify PVT during surgery is limited mainly to intraoperative ultrasound. Herein, we describe an alternative method to identify PVT during liver transplantation using indocyanine green (ICG)-fluorescence imaging.
A 60-year-old female underwent living-donor LT for end-stage liver disease related to chronic hepatitis C. The left liver graft with caudate lobe (372 mL, 36.7% of the recipient standard liver volume) was procured, and venoplasty with a circular cuff vein patch at the stump of the middle and left hepatic veins using cryopreserved veins was performed to avoid outflow obstruction [3] (Fig. 1A). After reconstruction of all hepatic vessels, ICG (0.93 mg; 2.5 μg per 1 mL of graft LV) was administered intravenously to evaluate anastomotic sites of reconstructed hepatic artery and portal vein and venous congestion in the graft related to venous reconstruction [4]. ICG-fluorescence imaging visualized continuous arterial and portal flow passing anastomotic sites. Fluorescence intensity (FI) on the left liver graft surface increased gradually while FI on the surface of segment 4 was lower than that on left lateral sector (Fig. 1B and C, and Supplementary video 1). Doppler intraoperative ultrasonography (D-IOUS) showed no evidence of impaired venous flow in the tributary of the segment 4, but revealed thrombus in the portal vein flowing into the segment 4 (Fig. 1D). The heparinized saline was injected and flushed from the portal venous anastomosis, which successfully melted away the thrombus. The postoperative course was uneventful except she suffered from herpes zoster, and the patient was discharged on postoperative days 48 without the development of PVT any more.
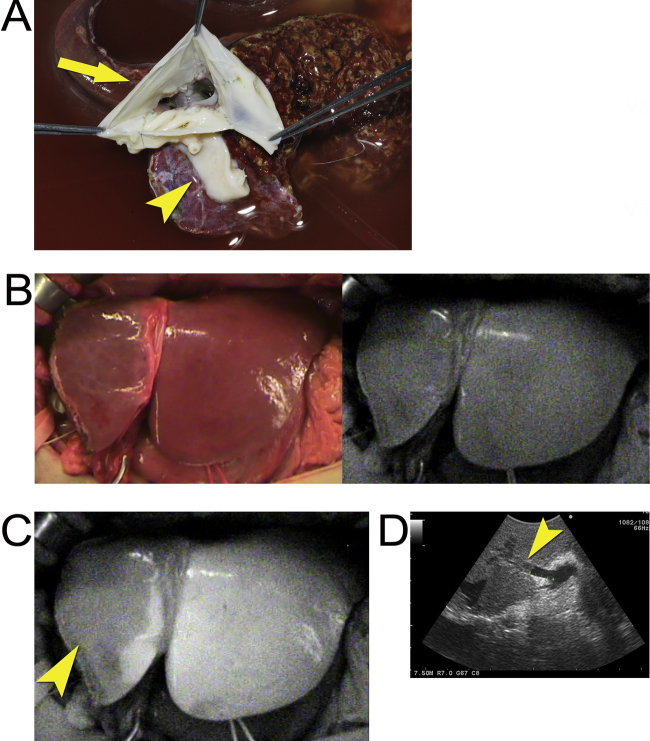
Fig. 1.
Identification of portal vein thrombus using ICG-fluorescence imaging.
(A) Venoplasty with a circular cuff vein patch (arrow) at the stump of the middle and left hepatic veins which was combined with the stump of the short hepatic vein (arrowhead).
(B) Intraoperative gross appearance of the left liver graft (left) and corresponding fluorescence images prior to intravenous injection of ICG (right).
(C) Fluorescence imaging following intravenous injection of ICG provided demarcation of FI between the segment 4 (arrowhead) and left lateral sector.
(D) IOUS reveals a thrombus in the portal vein flowing into the segment 4 (arrow head).
Supplementry material related to this article found, in the online version, at doi:10.1016/j.ijscr.2015.07.031.
Video S1. Evaluation of hepatic blood flows and identification of thrombus in the liver graft using ICG-fluorescence imaging. Following the reconstruction of the hepatic vessels in the left liver graft with caudate lobe, ICG is intravenously injected to evaluate hepatic blood flows and regions with venous occlusion. Fluorescent imaging visualizes continuous arterial blood and portal venous flows at the anastomotic sites and hepatic perfusions in the liver graft. Despite reconstruction of the all hepatic vessels, demarcation of FI on the liver surface between the segment 4 and the left lateral sector was provided. Subsequently, intraoperative ultrasound was performed and revealed a thrombus in the portal vein flowing into the segment 4.
Fluorescence imaging visualized regions in which hepatic perfusion was impaired due to PVT, and FI was lower in the regions with PVT than those without PVT. Hepatic perfusion levels in liver grafts can be evaluated in real-time by comparing FI values on the liver surface. The liver graft may develop hypoperfusion due to thrombosis, stricture, or occlusion in the portal vein when ICG-fluorescence imaging reveals FI heterogeneity on the liver surface. Previously, our groups demonstrated that FI was lower in veno-occlusive regions than in non-veno-occlusive regions [4,5]. These results imply that ICG-fluorescence imaging, during LT, can be utilized to visualize flows of reconstructed hepatic vessels, regions with venous occlusion, and regions with impaired hepatic perfusion caused by PVT as in this case. ICG-fluorescence imaging was applied to visualize anastomotic sites of arteries and lymphatic vessels in plastic surgery as well. In addition to this direct visualization of the reconstructed vessels, the present case might indicate the potential role of ICG-fluorescence imaging in visualizing the hypoperfusion area of the liver graft intraoperatively, which may help the real-time surveillance of hepatic circulation during LT. This technique was also applied for other type of LT; first, for the auxiliary partial orthotopic LT, in which perfusion of native liver found to be impaired while the satisfactory perfusion of the graft was confirmed (Fig. 2A), and second, for the whole liver graft from deceased-donor, in which the homogenous perfusion of the graft was confirmed (Fig. 2B). We believe that ICG-fluorescence imaging complement D-IOUS and contribute to demonstrate the problems of the graft circulation in real time during LT. These examinations are simply performed with the placement of fluorescence camera above the hepatic hilum or the graft surface after single ICG injection. Hepatic arterial and portal flows were visualized approximately 5–10 s after ICG injection and FI on the liver surface gradually increases spending 300 s and reaches a plateau. One of the drawbacks of this technique is that it cannot be applied repeatedly to evaluate hepatic perfusion of the graft in principle because the fluorescence on the liver surface lasts more than hours. By contrast, hepatic flows of the reconstructed vessels can be repeatedly evaluated using this technique. D-IOUS is the simplest and most reliable modality for the intraoperative evaluation of hepatic circulation. ICG-fluorescence imaging may have additive roles in demonstrating not only the vessels themselves but also hypoperfusion or venous congestion in liver parenchyma, which may further improve the safety and certainty of LT procedures.
Fig. 2.
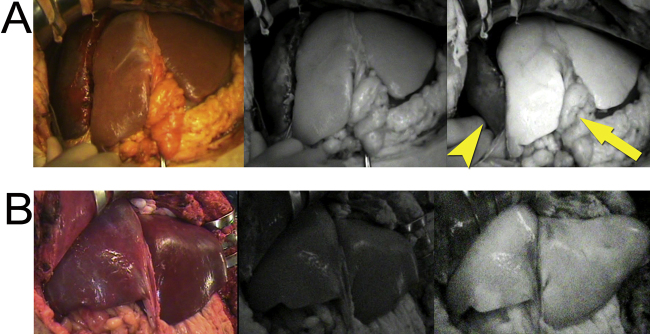
Application of ICG-fluorescence imaging for auxiliary partial orthotopic and deceased-donor LT.
(A) Fluorescence imaging visualized that the hepatic perfusion in the native liver (arrowhead) was impaired compared to the left liver graft (arrow) in auxiliary partial orthotopic LT (left; gross appearance, middle; fluorescence images before ICG injection, right; fluorescence images 240 s after the injection).
(B) Fluorescence images visualized the homogenous perfusion of the whole liver graft in the deceased-donor LT (left; gross appearance, middle; fluorescence images before ICG injection, right; fluorescence images 240 s after the injection).
In conclusion, ICG-fluorescence imaging visualized regions with impaired hepatic perfusion due to PVT in real time during LT in addition to visualization of hepatic flows of reconstructed vessels and evaluation of regions with venous occlusion. These evaluations are simply performed with single ICG injection and are expected to be potential roles of ICG-fluorescence imaging to enhance the safety of LT.
Conflict of interest
The authors disclose no conflicts.
Financial support
This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and Grants-in-aid for Research on HIV/AIDS, and Research on Measures for Intractable Diseases from the Ministry of Health, Labor, and Welfare of Japan.
Contributor Information
Nobuhisa Akamatsu, Email: nakamats-tky@umin.ac.jp.
Norihiro Kokudo, Email: KOKUDO-2SU@h.u-tokyo.ac.jp.
References
- 1.Llado L., Fabregat J., Castellote J., Ramos E., Torras J., Jorba R. Management of portal vein thrombosis in liver transplantation: influence on morbidity and mortality. Clin. Transplant. 2007;21:716–721. doi: 10.1111/j.1399-0012.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 2.Duffy J.P., Hong J.C., Farmer D.G., Ghobrial R.M., Yersiz H., Hiatt J.R. Vascular complications of orthotopic liver transplantation: experience in more than 4200 patients. J. Am. Coll. Surg. 2009;208:896–903. doi: 10.1016/j.jamcollsurg.2008.12.032. discussion 03-5. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto T., Sugawara Y., Tamura S., Kaneko J., Motomura N., Takamoto S. One orifice vein reconstruction in left liver plus caudate lobe grafts. Transplantation. 2007;83:225–227. doi: 10.1097/01.tp.0000244729.39485.7c. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi Y., Sugawara Y., Ishizawa T., Satou S., Kaneko J., Tamura S. Identification of veno-occlusive regions in a right liver graft after reconstruction of vein segments 5 and 8: application of indocyanine green fluorescence imaging. Liver Transplant. 2013;19:778–779. doi: 10.1002/lt.23657. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y., Ishizawa T., Miyata Y., Yamashita S., Masuda K., Satou S. Portal uptake function in veno-occlusive regions evaluated by real–time fluorescent imaging using indocyanine green. J. Hepatol. 2013;58:247–253. doi: 10.1016/j.jhep.2012.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Evaluation of hepatic blood flows and identification of thrombus in the liver graft using ICG-fluorescence imaging. Following the reconstruction of the hepatic vessels in the left liver graft with caudate lobe, ICG is intravenously injected to evaluate hepatic blood flows and regions with venous occlusion. Fluorescent imaging visualizes continuous arterial blood and portal venous flows at the anastomotic sites and hepatic perfusions in the liver graft. Despite reconstruction of the all hepatic vessels, demarcation of FI on the liver surface between the segment 4 and the left lateral sector was provided. Subsequently, intraoperative ultrasound was performed and revealed a thrombus in the portal vein flowing into the segment 4.