Abstract
Background
The Dog Erythrocyte Antigen (DEA) 1 blood group system remains poorly defined.
Objectives
The purpose of the study was to determine the DEA 1 mode of inheritance and to characterize the DEA 1 antigen and alloantibodies.
Animals
Canine research colony families, clinic canine patients, and DEA 1.2+ blood bank dogs were studied.
Methods
Canine blood was typed by flow cytometry and immunochromatographic strips using anti-DEA 1 monoclonal antibodies. Gel column experiments with polyclonal and immunoblotting with monoclonal anti-DEA 1 antibodies were performed to analyze select samples. Cross-reactivity of human typing reagents against canine RBCs and one monoclonal anti-DEA 1 antibody against human RBC panels was assessed.
Results
Typing of 12 families comprising 144 dogs indicated an autosomal dominant inheritance with ≥4 alleles: DEA 1− (0) and DEA 1+ weak (1+), intermediate (2+) and strong (3+ and 4+). Samples from 6 dogs previously typed as DEA 1.2+ were typed as DEA 1+ or DEA 1− using monoclonal antibodies. Human typing reagents produced varied reactions in tube agglutination experiments against DEA 1+ and DEA 1− RBCs. Polypeptide bands were not detected on immunoblots using a monoclonal anti-DEA 1 antibody, therefore the anti-DEA 1 antibody may be specific for conformational epitopes lost during denaturation.
Conclusions
The autosomal dominant inheritance of DEA 1 with ≥4 alleles indicates a complex blood group system; the antigenicity of each DEA 1+ type will need to be determined. The biochemical nature of the DEA 1 antigen(s) appears different from human blood group systems tested.
Keywords: Blood groups, Blood typing, Canine, Polymorphism, Transfusion
Introduction
Dog Erythrocyte Antigens (DEA) refer to canine blood group systems that were originally defined by an international committee for canine immunogenetics, and were based on studies utilizing induced alloantibodies.1, 2 Since then, a few additional blood groups and high frequency RBC antigens, such as Dal3, have been proposed, but the biochemical and molecular characteristics of the DEA system remain undefined. Dogs are either positive or negative for each DEA blood group, e.g. DEA 4+ or DEA 4−.4 The DEA 1 blood group system represents an exception, as it was originally reported to contain 2–3 types: DEA 1.1 (A1), 1.2 (A2), and possibly 1.3 (A3) based upon experiments using polyclonal alloantibodies. The DEA 1.1+ type appeared to be dominant over DEA 1.2 and DEA 1.2+ seemed dominant over DEA 1.3; thus dogs had to be DEA 1.1− in order to be DEA 1.2+ and only DEA 1.2− dogs could be DEA 1.3+.5, 6
However, our recent flow cytometric and immunochromatographic studies utilizing a murine monoclonal anti-DEA 1 antibody (Alvedia, Lyon, France) found that antigenic binding was quantitatively different among dogs, indicating that DEA 1 is a complex blood group system with varied surface expression levels. Consequently, a dog could be DEA 1− or weakly to strongly DEA 1+.7 Experimentally, as well as clinically, DEA 1 incompatibilities have been associated with serious blood transfusion reactions. As approximately half of all dogs are DEA 1+8, 9, it is important to understand how the weak to strong DEA 1+ phenotypes are inherited and how they relate to DEA 1+ antigenicity in order to provide sound clinical transfusion guidelines.
Here we determined the mode of inheritance of DEA 1 in several canine families, attempted to characterize the DEA 1 antigen using immunoblotting procedures, and screened canine RBCs with typing reagents against human blood groups to check for cross-reactivity. We also screened one monoclonal anti-DEA 1 antibody against human RBC panels expressing different blood group antigens used in human blood banking and transfusion medicine to determine if potential orthologs exist. Identification of a conserved RBC membrane protein in dogs could improve characterization of the DEA 1 antigen. It can also contribute to the development of more specific blood-typing reagents.
Materials and Methods
Animals and Samples
Blood samples, anticoagulated with Ethylenediaminetetraacetic acid (EDTA) and kept at 4°C, were obtained from research colony dogs or received as left-over samples from canine patients at the Clinical Pathology Laboratory at the School of Veterinary Medicine of the University of Pennsylvania (Penn Vet). For confirmatory DEA 1 typing, blood samples previously tested as DEA 1.2+ using conventional polyclonal antibody typing methods were received from Animal Blood Resources International (ABRI, Dixon, CA) and HemoSolutions (Colorado Springs, CO). Samples from several canine families were provided by commercial research dog colonies (Covance, Cumberland, VA and Marshall, North Rose, NY) and the Penn Vet research dog colony for DEA 1 typing. These studies were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
DEA 1 Blood Typing by Immunochromatographic Strip and Flow Cytometry
Following the preparation of 20% RBC suspensions from each blood sample, blood typing was performed using immunochromatographic strip kits (Alvedia, Lyon, France). The band strength was read on a scale from 0 to 4+ by one author (KP) prior to densitometric analysis of the strip using a protocol previously described by our laboratory.7 For flow cytometry, diluted monoclonal murine anti-DEA 1 antibody identical to the antibody impregnated on the immunochromatographic strip and an additional murine monoclonal anti-DEA 1 antibody (DMS Laboratories Inc., Tempe, AZ) were separately incubated with a 10% washed RBC suspension followed by labeling with a fluorescein isothiocyanate (FITC)-conjugated polyclonal goat anti-mouse antibody (Dako North America Inc., Carpinteria, CA). Flow analysis was performed on a Becton Dickinson FACSCalibur instrument (Franklin Lakes, New Jersey, operated at the Flow Cytometry and Cell Sorting Resource Laboratory, University of Pennsylvania School of Medicine), mean fluorescent intensities (MFI) were measured, and the data were analyzed with CellQuest Pro software (Penn Vet, Philadelphia, PA) as previously described.7
We used related dogs from the Penn Vet research dog colony and families of Beagle and mongrel dogs from commercial research dog colonies (Covance and Marshall) to assess the inheritance pattern of DEA 1. Blood samples from these related dogs were typed using strip and flow methods, and the pedigrees were analyzed thereafter. Pedigrees were selected for completely DEA 1− families, mixed DEA 1− and DEA 1+ families, and DEA 1+ families with different strengths of antigen expression, and the patterns were analyzed for simple Mendelian inheritance.
Gel Column Typing of DEA 1.2+ Blood using Polyclonal Reagents
Using the gel column typing technique (DiaMed, Cressier, Switzerland; no longer available) with both anti-canine immunoglobulin-impregnated gel columns (DiaMed-Vet ID-Card, Anti-canine globulin, DiaMed-Vet) and plain saline gel columns (DiaMed-Vet ID-Card NaCl, enzyme test and cold agglutinins), typing with two batches of polyclonal anti-DEA 1.1 and anti-DEA 1.X antisera (Animal Blood Resources International, Dixon, CA) was performed on samples previously determined to be DEA 1.2, as well as DEA 1+ and DEA 1− control samples typed with a monoclonal antibody for comparison. All RBCs were diluted to a 0.8% suspension by adding 10 μL of the RBC pellet in 1 mL of phosphate buffered saline (PBS, 150 mM NaCl, 7.5 mM NaPO4, pH 7.4), and 50 μL of this suspension was combined with 25 μL of undiluted antiserum.9 The gel column cards were incubated at 37°C for 15 min and then centrifuged. Each gel column was assessed for agglutination and contained a saline control to rule out auto-agglutination.
Ghost Membrane Preparation
Erythrocyte ghost lysates were produced from DEA 1+ and DEA 1− blood as previously described with slight modifications.10 Briefly, 2 mL of EDTA blood were centrifuged (Sero-Fuge 2002 Centrifuge 2 speed with 12-place head, Becton Dickinson) at 1000g for 20 min. After removing the plasma and buffy coat layer, the pellet was resuspended in and washed 3–4x with PBS (Gibco, Life Technologies, Grand Islands, NY; centrifugation for 2 min at 1,000g). The RBC pellet was washed 2x with isotonic PBS buffer and resuspended in 2 mL of the same buffer after the final wash. The RBC suspension was aliquoted into conical centrifuge tubes (Falcon, Fisher Scientific, Pittsburgh, PA), and the RBC were lysed by adding 40 volumes hypotonic buffer (75 mM NaCl, 5mM NaPO4, pH 7.5) to one volume RBC suspension. This suspension was stored on ice for 90 min to allow for complete cell lysis. The RBC membranes were then pelleted by centrifugation (IEC Multi RF, Thermo, Waltham, MA) at 20,000g for 25 min and washed 3x with the hypotonic buffer or until the supernatant was clear and the ghost pellet was white. After each spin the loose ghost pellet was separated from the small red underlying pellet of unlysed cells and transferred to a new tube for the subsequent wash. After the final wash, all aliquoted pellets from the same animal were combined and resuspended in 200 μL hypotonic buffer. Ghost lysates were aliquoted into microcentrifuge tubes (Seal-Rite 1.5 ml microcentrifuge tubes, USA Scientific, Ocala, FL), stored at −80°C and thawed only once for analysis. Protein concentration of lysates was determined with a Bradford assay (Bio-Rad Laboratories, Hercules, CA).
Electrophoresis of RBC Membrane Proteins
Prior to loading samples, ghost lysate aliquots were prepared using sample buffer (NuPAGE LDS, 4X, Bis-Tris gel, Invitrogen, Carlsbad, CA) and heated at 70°C for 10 min to denature the proteins. To reduce disulfide bonds in the protein samples, 500 mM dithiothreitol (DTT, NuPAGE Sample Reducing Agent, Thermo Fisher Scientific, Waltham, MA) were added fresh right before loading the gel as recommended by the manufacturer.
Erythrocyte membrane proteins were separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by loading gel wells (NuPAGE 4–12%) with 18 μg of membrane protein lysate or 10 μL of a standard protein ladder (MagicMark Standard, Invitrogen). Electrophoresis was run at 200 V with a SDS running buffer for 35 min (NuPAGE MES [2-{N-morpholino} ethane sulfonic acid] in the XCell SureLock Mini-Cell apparatus, Invitrogen).
Immunoblotting of Denatured RBC Membrane Proteins
Gels were processed immediately after electrophoresis was completed and blotting membranes (0.45 μm pore size polyvinylidene difluoride [PVDF], Immobilon-FL, Fisher Scientific), previously soaked in methanol and treated for fluorescence applications, were used for the protein transfer (XCell II Blot Module apparatus, Invitrogen). A regular transfer protocol (XCell II Blot Module technical manual, Invitrogen) was used. The PVDF blotting membrane was blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) diluted 1:1 with PBS. The PVDF membranes were incubated with a range of dilutions (1:50–1:3,000) of anti-DEA 1 monoclonal primary antibody (DMS, Flemington NJ) diluted in either Tris-buffered saline with 0.1% Tween-20 (TBS-T, Abcam, Cambridge, MA) or blocking buffer. The polyclonal rabbit anti-beta actin primary antibody (AbCam) loading control was used at the 1:1,000 dilution as recommended. The PVDF membranes were washed 3x for 5 min under agitation again with TBS-T and then incubated for 45 min with fluorescent monoclonal anti-mouse IRDye 800 nm and polyclonal goat anti-rabbit IRDye 680 nm secondary antibodies (LI-COR Biosciences, Lincoln, NE) at the recommended 1:10,000 dilution in either TBS-T or blocking buffer under agitation and protected from light. Once again the PVDF membranes were washed 3x for 5 min under agitation in the dark with TBS-T, and then rinsed with PBS to remove residual Tween-20. The PVDF membranes were scanned and visualized in 2 different fluorescence channels (700 and 800 nm; LI-COR Odyssey Infrared Imaging System).
Immunoblotting of Native (non-denatured) RBC Membrane Proteins
Erythrocyte membrane proteins were separated by loading 3–8% Tris-acetate gels (NuPAGE Novex 3–8% Tris-Acetate Protein Gels, Invitrogen) wells with 10–30 μg of ghost membrane protein lysate and using 5 μL of Standard ladder. Loading samples (in Novex Tris-Glycine native sample buffer, 2X, Invitrogen) were directly loaded into the gel wells. Electrophoresis was run at 125 V (Tris-Glycine Native running buffer for 1 hr in the XCell SureLock Mini-Cell apparatus, Invitrogen).
Immunoblotting techniques for the native gel protein transfer were the same as for the gel described for the denatured membrane protein experiments, except the native proteins were fixed to the PVDF blotting membrane by air-drying the PVDF membrane before the blocking step, which enables better binding to the PVDF membrane and prevents diffusion of the more soluble native proteins during the incubation steps.
Red Blood Cell and Antibody Panel Screening
Screening of the anti-DEA 1 monoclonal IgM antibody from DMS Laboratories against panels of human reagent RBCs (Human Red Blood Cell Panels I, II, Immucor, Norcross, GA and Human Red Blood Cell Panel C, Ortho Clinical Diagnostics, Raritan, NJ) for reactivity was done using saline gel column cards (Ortho Clinical Diagnostics). Briefly, 50 μL of washed 0.8% RBC suspension made with Micro Typing System (MTS, Fisher Scientific) Diluent 2 was mixed with 25 μL of serum/antibody in tubes and the total 75 μL was transferred to the gel columns. The gel column card was incubated at 37°C for 15 min and then centrifuged for 10 min at 85g using an Ortho-Clinical Diagnostics MTS Centrifuge. Results were interpreted as described in the product instructions.
Additionally, canine RBCs were screened with human polyclonal or monoclonal antigen typing reagents (Immucor) against human Rh antigens (C, c, E, e, D) as well as antigens of other human blood group systems (Kell, Duffy, ABO). The tube agglutination method was applied using manufacturer instructions for the particular blood group (Immucor). Canine RBCs were also treated with papain to check for enhancement or elimination of agglutination reactions with human antibodies. In human RBCs containing the Duffy (Fy) blood group treatment with the enzyme papain weakens or eliminates the agglutination reaction with anti-FyA or FyB antibodies because of Duffy antigen degradation. On the contrary, incubation of human RBCs containing the Rh antigen with papain enhances the agglutination reaction with anti-Rh antibodies because the enzyme removes proteins containing sialic acid, which usually confers a large negative charge to the RBCs.
RBCs were washed with 0.9% saline a minimum of 3x or until the supernatant wash fluid was clear, which was removed after the last wash. For each 1 mL packed RBCs, 2 drops of papain (Immucor) were added and mixed thoroughly and then incubated at room temperature (18–24°C) for 10 min. The enzyme-treated RBCs were washed 5x with 0.9% saline.
Results
DEA 1 Inheritance Studies
We constructed pedigrees for genetic analysis by typing 12 different dog families comprising a total of 144 dogs. Representative pedigrees and individual DEA 1 typing results are shown in Figure 1. The information on 32 matings between known DEA 1 type dogs producing 27 DEA 1− and 53 DEA 1+ offspring was included for analysis (Table 1). Other pedigrees were missing one parent, but their results were entirely consistent with the assessment below. Because both male and female dogs showed a similar frequency of DEA 1− and DEA 1+ dogs, sex-related effects were excluded (data not shown).
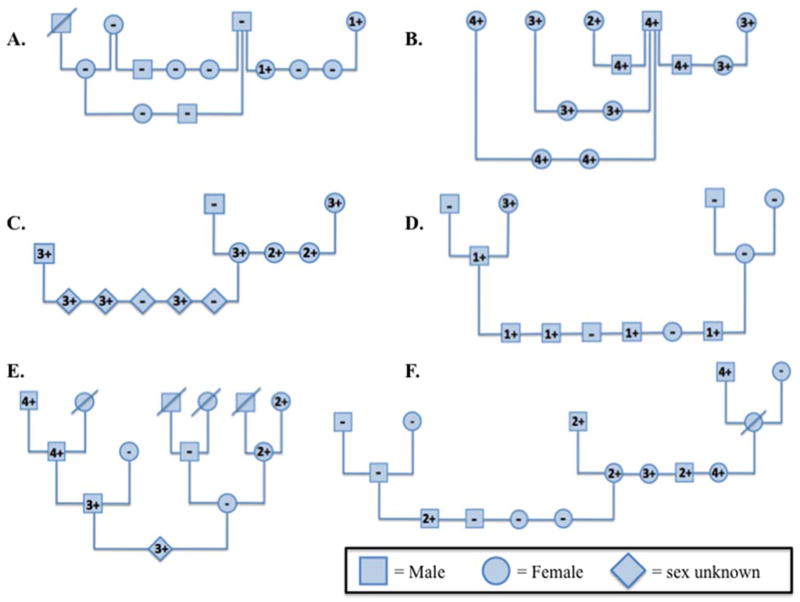
Figure 1.
Pedigrees of dogs with different combinations of Dog Erythrocyte Antigen (DEA) 1+ and DEA 1− blood types. A) Predominantly DEA 1−, B) DEA 1+ and C) DEA 1 mixed family of mongrel dogs at PennVet. D) Mixed DEA 1+ and DEA 1− pairs in a family of Beagles and E) and F) mongrel dog family.
Table 1.
Dog Erythrocyte Antigen (DEA) 1 of canine breeding pairs and offspring from parents with DEA 1 pedigrees typed by strip chromatography and densitometry. Pedigrees composed of a total of 32 matings from 12 different families (a total of 144 dogs) were analyzed.
| Breeding pair based upon DEA 1 type | # of breedings | DEA 1 typing results in # offspring | ||||
|---|---|---|---|---|---|---|
| DEA 1− | DEA 1+ strength | |||||
| 0 | 1+ | 2+ | 3+ | 4+ | ||
| 0 × 0 | 5 | 8 | 0 | 0 | 0 | 0 |
| 0 × 1+ | 3 | 7 | 7 | 0 | 0 | 0 |
| 0 × 2+ | 3 | 6 | 4 | 1 | 0 | 0 |
| 0 × 3+ | 4 | 2 | 1 | 2 | 5 | 1 |
| 0 × 4+ | 2 | 0 | 0 | 0 | 4 | 0 |
| 1+ × 1+ | 1 | 0 | 0 | 1 | 0 | 0 |
| 1+ × 3+ | 1 | 0 | 2 | 1 | 0 | 0 |
| 2+ × 3+ | 1 | 0 | 0 | 1 | 1 | 0 |
| 2+ × 4+ | 1 | 0 | 0 | 0 | 0 | 1 |
| 3+ × 3+ | 6 | 4 | 0 | 0 | 12 | 1 |
| 3+ × 4+ | 4 | 0 | 0 | 0 | 3 | 3 |
| 4+ × 4+ | 1 | 0 | 0 | 0 | 0 | 2 |
| Total | 32 | 27 | 14 | 6 | 25 | 8 |
Breeding pairs in which both parents were DEA 1− produced only DEA 1− offspring (Figure 1 and Table 1). Weakly DEA 1+ dogs (strength 1+) bred to DEA 1− dogs only produced DEA 1− or weakly DEA 1+ offspring. In contrast, strongly DEA 1+ dogs (strength 3+ or 4+) bred to DEA 1− or DEA 1+ dogs produced DEA 1− and weakly to strongly DEA 1+ dogs. One breeding between 2 strongly DEA 1+ dogs produced both DEA 1+ and DEA 1− puppies.
These patterns are consistent with an autosomal dominant inheritance with 4–5 alleles: DEA 1− (0), DEA 1+ weak (1+), intermediate (2+) and strong (3+ and 4+). The DEA 1+ blood samples that were subjectively assigned 3+ and 4+ strengths during the strip typing technique produced rather comparable MFI values during the quantitative flow cytometry typing technique. The mean MFI of the 4+ (mean MFI = 504) DEA 1 strength is only 1.4 times greater than that of strength 3+ (mean MFI = 348), whereas the mean MFI of strength 2+ (mean MFI = 97) is about 2.8 times greater than that of strength 1+ (mean MFI = 35) and therefore more distinct.7 It remains unclear whether the 3+ and 4+ strengths of DEA 1+ actually differ, as suggested by Table 1, or if there is just one strong DEA 1+ phenotype, as suggested by the flow cytometric MFI measurements.7
Re-typing previously typed DEA 1.1−, 1.1+, and 1.2+ blood samples
Samples previously typed as DEA 1.1+, 1.1− and 1.2+ by the ABRI laboratory using polyclonal antibodies were retyped in parallel with polyclonal and monoclonal antibodies to determine potential correlations. While the DEA 1.1+ sample reacted strongly with all reagents that detect DEA 1, both DEA 1.1− controls tested negative by monoclonal anti-DEA 1 (using either flow or strip method) but were weakly to moderately positive in agglutination reactions with the polyclonal anti-DEA 1.1 and anti-DEA 1.X antisera (Table 2).
Table 2.
Typing results of previously typed Dog Erythrocyte Antigen (DEA) 1.1−, 1.1+, and 1.2+ canine blood samples using polyclonal and monoclonal reagents. Flow cytometry and chromatographic strip techniques with monoclonal anti-DEA 1 antibody produced similar results. A saline or antiglobulin gel column system was used instead of a tube assay to type samples with the polyclonal 1.1 and 1.X antibodies. Auto-controls were negative for autoagglutination.
| Dog sample | Blood type according to ABRI | DEA 1 monoclonal antibody | DEA 1.1 and 1.X polyclonal antibodies | |||||
|---|---|---|---|---|---|---|---|---|
| DEA 1 Flow (MFI) | DEA 1 Strip (Subjective) | DEA 1 Type | Gel Column | |||||
| Anti-canine globulin | Saline | |||||||
| 1.1 | 1.X | 1.1 | 1.X | |||||
| A1 | DEA 1.1+ | 4+ (428) | 4+ | DEA 1+ | 4+ | 4+ | 4+ | 4+ |
| B1 | DEA 1.1− | 0 (4) | 0 | DEA 1− | 1+ | 2+ | 2+ | 1+ |
| B2 | DEA 1.1− | 0 (4) | 0 | DEA 1− | 1+ | 2+ | 2+ | 1+ |
| C1 | DEA 1.2+ | 1+ (24) | 2+ | DEA 1+ | 2+ | 3+ | 2+ | 1+ |
| C2 | DEA 1.2+ | 1+ (34) | 2+ | DEA 1+ | 2+ | 3+ | 2+ | 1+ |
| C3 | DEA 1.2+ | 1+ (23) | 2+ | DEA 1+ | 2+ | 3+ | 2+ | 1+ |
| C4 | DEA 1.2+ | 2+ (69) | 3+ | DEA 1+ | 3+ | 4+ | 3+ | 4+ |
| C5 | DEA 1.2+ | 2+ (63) | 3+ | DEA 1+ | 3+ | 4+ | 3+ | 4+ |
| C6 | DEA 1.2+ | 0 (5) | 0 | DEA 1− | 2+ | 2+ | 2+ | 0 |
ABRI indicates Animal Blood Resources International
Surprisingly, the 6 samples previously typed as DEA 1.2+ produced discordant and variably moderate reactions with both monoclonal and polyclonal reagents. Using the anti-DEA 1.1 reagent, DEA 1.2+ dogs tested weaker than the control DEA 1.1+ dog, but stronger than the DEA 1.1− control dog. Moreover, the agglutination reaction with the 1.X reagent inconsistently ranged from weak to strong among the 6 DEA 1.2+ samples (Table 2). Thus, none of these samples could be confirmed to have blood type DEA 1.2+ using the polyclonal antibodies and all typed as moderately strong DEA 1+ with monoclonal antibodies except one dog typed as DEA 1−.
Red Blood Cell and Antibody Panel Screening
The DMS anti-DEA 1 monoclonal antibody did not react with any human blood groups when screened against human RBC panels I, II, and C. The Alvedia anti-DEA 1 monoclonal antibody was not tested in these human antigen screening experiments.
In contrast, some human typing reagents produced reactions in tube agglutination experiments against DEA 1+ and DEA 1− canine RBCs. Human polyclonal Anti-Fya and Anti-Fyb (Immucor) with anti-human globulin (anti-IgG; polyspecific, Gamma-clone) were used to detect Duffy-like antigens on the surface of canine RBCs. Although pre-treatment of RBCs with papain eliminates the Duffy reaction with human RBCs, pre-treatment of canine RBCs with papain did not change the agglutination reactions seen in less than half of initially untreated DEA 1+ canine RBCs showing moderate to strong agglutination with human anti-Fya antisera (Table 3). The untreated DEA 1+ samples that did not initially react with anti-Fya antisera, reacted with weak to strong agglutination following pre-treatment with papain. All of the untreated and papain-treated DEA 1− RBC samples showed moderate to strong agglutination with anti-Fya antisera except for one sample which did not show agglutination when untreated, but a weak reaction occurred when those RBCs were pre-treated with papain (Table 3).
Table 3.
Agglutination reaction strengths of Immucor human polyclonal Anti-Fya and Anti-Fyb used to detect Duffy-like antigens on the surface of Dog Erythrocyte Antigen (DEA) 1+ and DEA 1− canine RBC that were either treated with papain (P) or left untreated (U). Gamma-clone Anti-Human Globulin (anti-IgG; polyspecific) was added for the reaction to occur.
| DEA 1+ | Anti-Fya | Anti-Fyb | |||
|---|---|---|---|---|---|
| Untreated | Papain | Untreated | Papain | ||
| A0 | 4+ | 0 | 2+ | 4+ | 3+ |
| A1 | 4+ | 0 | 1+ | 4+ | 4+ |
| A2 | 4+ | 3+ | 3+ | 4+ | 3+ |
| A3 | 3+ | 0 | 2+ | 4+ | 4+ |
| A4 | 3+ | 2+ | 3+ | 2+ | 3+ |
| A5 | 3+ | 0 | 1+ | 3+ | 3+ |
| A6 | 3+ | 3+ | 3+ | 2+ | 3+ |
| DEA 1− | |||||
| B1 | 0 | 2+ | 3+ | 3+ | 3+ |
| B2 | 0 | 3+ | 3+ | 2+ | 3+ |
| B3 | 0 | 3+ | 4+ | 3+ | 3+ |
| B4 | 0 | 2+ | 3+ | 0 | 4+ |
| B5 | 0 | 0+ | 1+ | 0 | 3+ |
| B6 | 0 | 3+ | 3+ | 0 | 3+ |
| B7 | 0 | 3+ | 2+ | 0 | 2+ |
All DEA 1+ RBC samples, both untreated and treated with papain, showed moderate to strong agglutination with anti-Fyb sera. Half of the DEA 1− samples did not agglutinate with anti-Fyb sera, but did so strongly when RBCs were pre-treated with papain. The other half of DEA 1− RBCs showed moderate to strong agglutination with anti-Fyb sera (Table 3). Thus, the DEA 1 antigen does not appear to be a Duffy-like antigen since papain pre-treatment did not eliminate the reaction.
Incubating canine RBCs with monoclonal antibodies used for the human Rh groups E, C, e, c, and D did not result in agglutination of any untreated DEA 1+ or DEA 1− samples. Even after papain treatment of canine RBCs, agglutination was still not observed between human anti-C or anti-E reagents (except one very weak anti-E) and either DEA 1+ or DEA 1− canine RBCs. All DEA 1+ RBCs treated with papain did show enhanced agglutination reactions with the human Rh anti-c, anti-e, and both series 4 and 5 anti-D reagents. The Anti-D series are monoclonal blends that contain both monoclonal IgM anti-D and monoclonal IgG anti-D antibodies. All DEA 1− samples treated with papain showed weak agglutination reactions with series 4 anti-D antibody. One DEA 1− sample had additional weak agglutination reactions with all antibodies except for anti-E. Three out of four DEA 1− papain treated RBCs did not agglutinate with anti-c and anti-D series 5 antibodies, whereas all papain treated DEA 1+ RBCs did (Table 4).
Table 4.
Agglutination reaction strengths of Immucor monoclonal antibodies against the human Rh groups e, E, c, C, and D used to detect Rh-like antigens on the surface of Dog Erythrocyte Antigen (DEA) 1+ and DEA 1− canine RBC that were either treated with papain (P) or left untreated (U). DEA 1+ strength of the DEA 1+ samples indicated in parenthesis next to sample names.
| Sample (DEA 1+ strength) | Anti-E | Anti-e | Anti-C | Anti-c | Anti-D Series 4 |
Anti-D Series 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U | P | U | P | U | P | U | P | U | P | U | P | |
| DEA 1+ | ||||||||||||
| A0 (4+) | 0 | 0 | 0 | 1+ | 0 | 0 | 0 | 3+ | 0 | 2+ | 0 | 2+ |
| A1 (4+) | 0 | 1+ | 0 | 3+ | 0 | 0 | 0 | 3+ | 0 | 3+ | 0 | 2+ |
| A2 (4+) | 0 | 0 | 0 | 1+ | 0 | 0 | 0 | 2+ | 0 | 1+ | 0 | 2+ |
| A3 (3+) | 0 | 0 | 0 | 2+ | 0 | 0 | 0 | 2+ | 0 | 3+ | 0 | 3+ |
| DEA 1− | ||||||||||||
| B1 | 0 | 0 | 0 | 2+ | 0 | 0 | 0 | 2+ | 0 | 2+ | 0 | 1+ |
| B2 | 0 | 0 | 0 | 1+ | 0 | 0 | 0 | 0 | 0 | 1+ | 0 | 0 |
| B5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1+ | 0 | 0 |
| B7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1+ | 0 | 0 |
Membrane Protein Studies
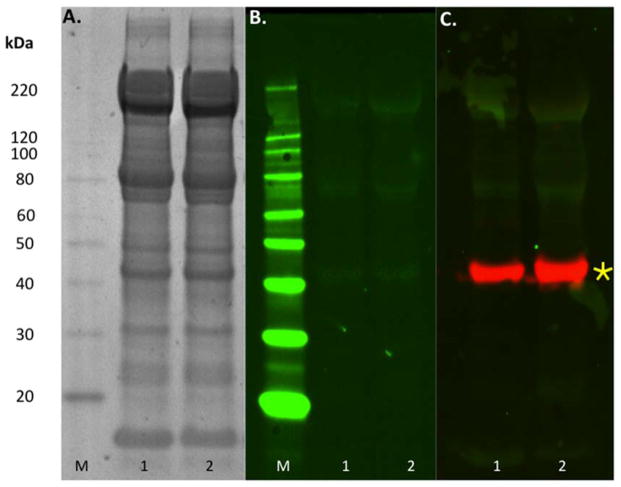
Denaturing SDS-PAGE of canine RBC ghost lysates showed the expected RBC membrane protein band pattern11 for reduced (not shown) and non-reduced samples (Figure 2A). There were no obvious protein band pattern differences between DEA 1+ and DEA 1− lysates. Immunoblotting with both the Alvedia IgG and DMS IgM (not shown) anti-DEA 1 monoclonal antibodies revealed multiple faint non-specific bands which appeared similar in reduced and non-reduced DEA 1+ and DEA 1− lysates using varying dilutions (Figure 2B). In contrast the control protein beta-actin probed with an anti-beta-actin antibody produced the expected bright 43 kDa band (Figure 2C). No bands were present on the native gel immunoblot (not shown).
Figure 2.
Protein studies on Dog Erythrocyte Antigen (DEA) 1− and DEA 1+ RBC membrane lysates.
Lane 1 contains DEA 1+ (4+ strength) lysate and lane 2 contains DEA 1− lysate. The ladder is marked “M”. A) SDS-PAGE of separated erythrocyte surface membrane proteins from DEA 1+ and DEA 1− ghost membrane samples stained with Coomassie SafeStain (BioRad). B) Western blot of canine erythrocyte membranes probed with monoclonal anti-DEA 1 antibody (Alvedia) at a 1:3,000 dilution. C) Western blot of canine erythrocyte membranes probed with monoclonal anti-DEA 1 antibody (green bands in lanes 1 and 2) and polyclonal goat anti-rabbit anti-beta actin antibody as the loading control (red bands marked with yellow asterisk). Non-specific binding seen with the monoclonal anti-DEA 1 antibody (green bands in lanes 1 and 2).
Discussion
DEA 1 is the clinically most important RBC antigen, causing acute hemolytic transfusion reactions in previously sensitized DEA 1− dogs given DEA 1+ blood.8 However, despite its importance, there is little known about this canine blood group system. Following our recent discovery of varied strengths of DEA 1 expression among dogs7, we now show an autosomal dominant mode of inheritance of at least 4 alleles of DEA 1, with strongly to weakly DEA 1+ alleles dominant over the DEA 1− allele and no direct correlation between the historical DEA 1.2+ and strength of DEA 1 reaction. Using a monoclonal anti-DEA 1 antibody that is commonly used in blood-typing and flow cytometry studies of canine RBCs, we were unable to detect polypeptide bands in Western blot experiments, despite various optimization efforts. Thus the anti-DEA 1 antibody may be specific for conformational or combinatorial epitopes lost during SDS-PAGE. Additionally, screenings of DEA 1+ and DEA 1− cells with human blood group typing reagents showed reactivity with antibodies against some human Rh blood group antigens, but the specificity of such binding is currently unclear. Considering the difficulties in biochemical characterization of the DEA 1 antigen, a molecular genetic approach using families with well-defined blood types seems most promising in the future.
Based upon studies carried out in the 1960s with polyclonal antibodies (sera from sensitized dogs), the DEA 1 system was thought to contain 3 alleles, with DEA 1.1 dominant over DEA 1.2 and possibly DEA 1.3.5 Erythrocytes from individual dogs were thought to be either positive for one type, or negative for all. Sera for the detection of blood samples from DEA 1.3+ and DEA 1.3− dogs have not been available since that initial report. Notably, all 6 dogs that were previously typed as DEA 1.2+ elsewhere (ABRI and HemoSolutions) were found to be DEA 1.1+ with the polyclonal reagents used here, and gave DEA 1− to moderately to strong DEA 1+ typing results with a monoclonal DEA 1 antibody (Table 3). This is consistent with our recently reported results from a few DEA 1.2+ dogs.7 In the aforementioned study, the DEA 1.2+ samples were weakly to moderately DEA 1+ but in this current study we also had some moderately strong DEA 1+ and DEA 1− test results among the DEA 1.2+ dogs. Different samples, dogs and reagents were used which may explain the varied results. Furthermore, the 1.1 and 1.X reagents used for the typing of DEA 1.2+ dogs made interpretation of agglutination results challenging, even for trained personnel, and the only laboratory (ARBI) that had offered routine typing for DEA 1.2 has recently stopped doing so. As the clinical importance of the DEA 1.2 type had not been established, we conclude that the previously typed DEA 1.2+ dogs are most likely DEA 1+, thus supporting our previous study.7
To elucidate the mode of DEA 1 inheritance, we normalized the blood PCV (20%) in the samples for use in both strip chromatography/densitometry and flow cytometry typing analyses using one monoclonal anti-DEA 1 antibody (Alvedia), thus permitting standardized comparative typing of families. Consistent with prior studies4, we found that the DEA 1 blood group system is an autosomal trait, with both male and female dogs being either DEA 1− or to varying degrees DEA 1+. As expected, matings between DEA 1− dogs strictly produced DEA 1− offspring. Independent of strength of DEA 1 reaction, matings between DEA 1− and DEA 1+ dogs resulted in either all DEA 1+ or both DEA 1+ and DEA 1− offspring. Moreover, matings between DEA 1+ dogs primarily resulted in DEA 1+ dogs, with an occasional DEA 1− dog. Finally, the strength of the DEA 1 reaction in offspring was frequently the same as that of one parent or weaker if one of the parents carried a weaker DEA 1 allele. These observations are most consistent with the conclusion that DEA 1 is encoded by a single gene locus with multiple alleles and that the stronger DEA 1 alleles are dominant to the weaker DEA 1+ and DEA 1− alleles. The pedigree analyses indicate at least 4 alleles (DEA 1−, weakly, moderately and strongly DEA 1+). However, the MFI values of DEA 1+ samples with strengths of 3+ and 4+ are very close together and make it difficult to distinguish between these 2 phenotypes. It is also possible that there may be more alleles, as we only evaluated a relatively small number of families. Blood group systems with a null allele and different strength of the same or similar antigens have been reported in other species.5
We investigated the mode of inheritance of the DEA 1+ and DEA 1− phenotype because understanding the genetic basis of the DEA 1 blood group will help veterinary blood banks and clinics select blood donor dogs more efficiently. Typing not only for DEA 1+ or DEA 1−, but for the degree of DEA 1 positivity may become helpful in better matching of recipients and donors and avoiding hemolytic transfusion reactions. The effect of transfusing blood from a weakly DEA 1+ to a DEA 1− dog or blood from a strongly DEA 1+ dog to a recipient with weak DEA 1+ blood is unknown. Thus, transfusion studies would be beneficial in determining whether the difference in DEA 1+ antigenicity strength results in different sensitization and possibly causes hemolytic transfusion reactions in previously sensitized dogs.
The biochemical and molecular characterization of the DEA 1 system has been challenging. Earlier limited immunoblotting studies of RBC membranes probing one DEA 1.1+ dog sample using a monoclonal antibody (DMS) suggested that DEA 1.1 was composed of 2 proteins of 50 and 200 kDa12, whereas another study using anti-DEA 1.1 antisera identified a protein band at 85 kDa in a DEA 1.2+ dog which lacked comparison to DEA 1.1− and DEA 1.1+ dogs.13 These studies were neither extended to determine the nature of these proteins nor confirmed by others. Using the same immunoblotting techniques on RBC ghost membrane samples from DEA 1+ and DEA 1− dogs, we did not detect any specific binding to membrane proteins with either anti-DEA 1 monoclonal antibodies used in our laboratory or polyclonal anti-DEA 1 and 1.1 and 1.X antibodies. In our study, only multiple weak bands were detected and no differences between DEA 1+ and DEA 1− samples were observed, despite optimization of the loaded sample protein concentration and volume, reduction with DTT, and variations in primary and secondary antibody dilutions. Since the monoclonal antibody binds to the native antigen expressed on an intact RBC during flow cytometry studies, but not to denatured protein during immunoblotting, it most likely recognizes either a conformational epitope on DEA 1 or a combinatorial epitope involving DEA 1 and an additional RBC membrane component that dissociates under denaturing conditions. Analogous conformational epitopes are found in human anti-Wra or –Wrb, which recognize a complex formed between band 3 and glycophorin A.14
Challenges in biochemical characterization of complex blood group systems are also faced in human transfusion medicine. The Rh (Rhesus) blood group system is the most polymorphic of the human blood groups, consisting of over 50 antigens, and along with the ABO blood group is clinically most important in human medicine.15 Only one monoclonal anti-RhD antibody has been found to react strongly by immunoblotting, detecting a protein at 33 kDa.16 However, characterization of the Rh system in people requires special immunoblotting conditions, including a specific pH, temperature, ionic strength, and antibody concentration; and properly stored RBCs and ghost membrane lysates.15 These conditions may be applicable to more advanced immunoprecipitation experiments to biochemically define DEA 1 antigens in the future.
The only human blood group antibodies in our comparative screening that reacted with canine RBCs were those against the human Duffy and Rh blood groups. The DEA 1 monoclonal antibody did not react with any human cells on the panel. DEA 1+ RBC treated with papain, a proteolytic enzyme derived from Carica papaya that cleaves and degrades negatively charged sialic acid-bearing glycoproteins17, showed moderately enhanced agglutination reactions using the anti-c and series 5 anti-D reagents, as would be expected to occur with Rh+ human RBCs containing the D antigen. In contrast, only one DEA 1− papain treated canine RBC samples showed enhanced agglutination when incubated with these reagents. Additionally, DEA 1− RBC showed only a very weak agglutination with series 4 anti-D reagent after papain treatment. The reaction pattern of DEA 1+ RBCs with the anti-D human antibodies was of interest, since human RBC can exhibit so called Weak D expression of the RhD antigen. Similar to what we found with DEA 1+ surface protein expression variability, Rh+ people with the Weak D phenotype have varying reduced levels of D antigen on their RBCs. Notably, low level D antigen expression results from single point mutations in the RHD gene that cause amino acid changes in the intracellular or transmembrane regions of the RhD protein.17, 18 These mutations affect the attachment of the antigen to the cell membrane, thereby affecting the quantity of the RhD antigen on the surface.19 Despite this similarity, we cannot draw any conclusions regarding the potential homology between the canine and human antigens without further investigation, since polyclonal and monoclonal Rh-specific antibodies may not cross-react specifically with RBC from nonprimate animals.20 However, a study reporting that Rh-like proteins can be isolated from RBC of nonprimate mammals in which Rh protein cannot be detected serologically suggest that a potential homology in dog RBC may still be worth investigating.21 In contrast, screening of canine RBCs with human anti-Duffy (Fya and Fyb) antibodies gave some positive reactions, but treatment with papain did not weaken the reaction as it does for human cells, making a Duffy antigen-specific reaction unlikely.
We hope to further define the structure and function of the DEA 1 canine blood antigen in the future. Understanding the molecular basis can also open doors to deciphering disease pathogenesis as is seen with human blood groups. In people, Plasmodium vivax invades RBC by using the Duffy blood-group antigen (Fy) as a receptor and is a major cause of malaria.22 Additionally, the human Rh RBC antigen is an ammonia transporter and despite its popularity in the RBC field, the Rh factor is also found in cells of the kidney, liver, gastrointestinal tract, testes, and other organs.23, 24, 25, 26 Disruption of function can have severe implications on cellular or organ function, which can manifest in tissue damage and disease.27
In conclusion, we demonstrated the inheritance pattern of DEA 1− and weakly to strongly DEA 1+ dogs is a multiallelic autosomal dominant blood system. Like many of the human blood groups, including Rh, we hypothesize that the DEA 1 system may be more complicated than initially thought. As such, it will require both genetics and more advanced biochemical studies to further define the proteins involved.
Acknowledgments
This study was supported in part by NIH OD 010939 and the veterinary scholars program from NIH 2T35 OD 010919 and Merial. The monoclonal DEA 1 antibody and typing kits were kindly provided by Alvedia, Lyon, France and DMS Laboratories, Inc, Flemington, NJ. The assistance with blood samples by Animal Blood Resources International (ABRI), Dixon, CA, Covance, Cumberland, VA, HemoSolutions, Colorado Springs, CO, and Marshall, North Rose, NY and the staff in the Clinical Pathology Laboratory and canine research colony at the University of Pennsylvania are also thanked.
Footnotes
Conflict of Interest Declaration: The PennGen Laboratories offer blood typing. Urs Giger has been a scientific advisor to Alvedia, DMS, Covance, and Marshall. However, the design and execution of the study and writing of the manuscript have been done entirely independently.
References
- 1.Bull RW. Animal blood groups. In: Smith JS, Westphal RG, editors. American Association of Blood Banks Technical Workshop on Veterinary Transfusion Medicine. Bethesda: American Association of Blood Banks; 1989. pp. 1–2. [Google Scholar]
- 2.Vriesendorp HM, Westbroek DL, D’Amaro J, et al. Joint report of 1st International Workshop on Canine Immunogenetics. Tissue Antigens. 1973;3:145–63. [PubMed] [Google Scholar]
- 3.Blais MC, Berman L, Oakley DA, Giger U. Canine Dal blood type: A red cell antigen lacking in some Dalmatians. J Vet Intern Med. 2007;21:281–6. doi: 10.1892/0891-6640(2007)21[281:cdbtar]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohenhaus AE. Importance of blood groups and blood group antibodies in companion animals. Transfus Med Rev. 2004;18:117–26. doi: 10.1016/j.tmrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Bell K. Blood groups of domestic animals. In: Agar NS, Board DG, editors. Red Blood Cells of Domestic Mammals. Amsterdam: Elsevier Press; 1983. pp. 137–64. [Google Scholar]
- 6.Hale AS. Canine blood groups and their importance in veterinary transfusion medicine. Vet Clin North Am Small Anim Pract. 1995;25:1323–32. doi: 10.1016/s0195-5616(95)50157-3. [DOI] [PubMed] [Google Scholar]
- 7.Acierno MM, Raj K, Giger U. DEA 1 expression on dog erythrocytes analyzed by immunochromatographic and flow cytometric techniques. J Vet Intern Med. 2014;28:592–8. doi: 10.1111/jvim.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giger U, Gelens CJ, Callan MB, Oakley DA. An acute hemolytic transfusion reaction caused by dog erythrocyte antigen 1.1. incompatibility in a previously sensitized dog. J Am Vet Med Assoc. 1995;206:1358–62. [PubMed] [Google Scholar]
- 9.Kessler RJ, Reese J, Chang D, Seth M, Hale AS, Giger U. Dog erythrocyte antigens 1.1, 1.2, 3. 4. 7. And Dal blood typing and cross-matching by gel column technique. Vet Clin Pathol. 2010;39:306–16. doi: 10.1111/j.1939-165X.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steck TL, Kant JA. Preparation of impermeablc ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–80. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- 11.Barker RN. Electrophoretic analysis of erythrocyte membrane proteins and glycoproteins from different species. Comp Haemat Internat. 1991;1(3):155–60. [Google Scholar]
- 12.Andrews GA, Chavey PS, Smith JE. Production, characterization, and applications of a murine monoclonal antibody to dog erythrocyte antigen 1.1. J Am Vet Med Assoc. 1992;201(10):1549–1552. [PubMed] [Google Scholar]
- 13.Corato A, Mazza G, Hale AS, Barker RN, Day MJ. Biochemical characterization of canine blood group antigens: immunoprecipitation of DEA 1.2, 4 and 7 and identification of a dog erythrocyte membrane antigen homologous to human Rhesus. Vet Immunol Immunopathol. 1997;59:213–223. doi: 10.1016/s0165-2427(97)00080-9. [DOI] [PubMed] [Google Scholar]
- 14.Poole J. Red cell antigens on band 3 and glycophorin A. Blood Rev. 2000;14:31–43. doi: 10.1054/blre.1999.0124. [DOI] [PubMed] [Google Scholar]
- 15.Reid M, Lomas-Francis C, Olsson M. The Blood Group Antigen Facts Book. 3. New York: Elsevier; 2012. [Google Scholar]
- 16.Apoil PA, Reid ME, Halverson G, et al. A human monoclonal anti-D antibody which detects a nonconformation-dependent epitope on the RhD protein by immunoblot. Brit J of Haematology. 1997;98:365–374. doi: 10.1046/j.1365-2141.1997.2183041.x. [DOI] [PubMed] [Google Scholar]
- 17.Low B. A practical method using papain and incomplete Rh-antibodies in routine Rh blood-grouping. Vox Sanguinis. 1955;5:94. [Google Scholar]
- 18.Wagner FF, Gassner C, Muller TH, Schonitzer D, Schunter F, Flegel WA. Molecular basis of weak D phenotypes. Blood. 1999;93:385–93. [PubMed] [Google Scholar]
- 19.Westhoff CM. The structure and function of the Rh antigen complex. Seminars in Hematology. 2007;44:42–50. doi: 10.1053/j.seminhematol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westhoff CM, Wylie DE. Investigation of the human Rh blood group system in nonhuman primates and other species with serologic and Southern blot analysis. J Mol Evol. 1994;39:87–92. doi: 10.1007/BF00178253. [DOI] [PubMed] [Google Scholar]
- 21.Saboori AM, Denker BM, Agre P. Isolation of proteins related to the Rh polypeptides from nonhuman erythrocytes. J Clin Invest. 1989;83:187–91. doi: 10.1172/JCI113857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King CL, Adam JH, Xianli J, et al. Fya/Fyb antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. PNAS. 2011;108:20113–20118. doi: 10.1073/pnas.1109621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handlogten ME, Hong SP, Zhang L, et al. Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1036–47. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- 24.Lee HW, Verlander JW, Handlogten ME, Han KH, Cooke PS, Weiner ID. Expression of the rhesus glycoproteins, ammonia transporter family members, RHCG and RHBG in male reproductive organs. Reproduction. 2013;146:283–96. doi: 10.1530/REP-13-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B glycoprotein and Rh C glycoprotein, in the mouse liver. Gastroenterology. 2003;124:1432–40. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 26.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F323–37. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 27.Deschuyteneer A, Boeckstaens M, De Mees C, Van Vooren P, Wintiens R, Marini AM. SNPs Altering Ammonium Transport Activity of Human Rhesus Factors Characterized by a Yeast-Based Functional Assay. PLoS One. 2013;8:e71092. doi: 10.1371/journal.pone.0071092. [DOI] [PMC free article] [PubMed] [Google Scholar]