Abstract
Purpose
Radiation-induced fibrosis (RIF) is a long-term side effect of external beam radiation therapy for the treatment of cancer. It results in a multitude of symptoms that significantly impact quality of life. Understanding the mechanisms of RIF-induced changes is essential to developing effective strategies to prevent long-term disability and discomfort following radiation therapy. In this review, we describe the current understanding of the etiology, clinical presentation, pathogenesis, treatment, and directions of future therapy for this condition.
Methods
A literature review of publications describing mechanisms or treatments of RIF was performed. Specific databases utilized included PubMed and clinicaltrials.gov, using keywords “Radiation-Induced Fibrosis,” “Radiotherapy Complications,” “Fibrosis Therapy,” and other closely related terms.
Results
RIF is the result of a misguided wound healing response. In addition to causing direct DNA damage, ionizing radiation generates reactive oxygen and nitrogen species that lead to localized inflammation. This inflammatory process ultimately evolves into a fibrotic one characterized by increased collagen deposition, poor vascularity, and scarring. Tumor growth factor beta serves as the primary mediator in this response along with a host of other cytokines and growth factors. Current therapies have largely been directed toward these molecular targets and their associated signaling pathways.
Conclusion
Although RIF is widely prevalent among patients undergoing radiation therapy and significantly impacts quality of life, there is still much to learn about its pathogenesis and mechanisms. Current treatments have stemmed from this understanding, and it is anticipated that further elucidation will be essential for the development of more effective therapies.
Keywords: Cancer, Radiation, Fibrosis, Fibroblast, Inflammation, TGF-β, Therapy
Introduction
Patients with cancer often receive external beam ionizing radiation therapy either alone or in combination with surgery and/or chemotherapy. Ionizing radiation induces damage not only in rapidly proliferating tumor cells but also in normal tissue in the radiation field. Much of the immediate effect in response to irradiation of normal tissue is influenced by the radiosensitivity of individual patients. For instance, patients with ataxia-telangiectasia carry a mutation in the ataxia-telangiectasia mutated (ATM) DNA repair gene that mitigates the ability of cells to repair radiation-induced DNA damage, conferring high radiosensitivity. Comparatively, the majority of the late effects of radiation vary in severity depending on the radiation dose, fraction size, and volume treated.
One important late effect that is a significant contributor to patient morbidity is radiation-induced fibrosis (RIF), which may occur in the skin and subcutaneous tissue, lungs, gastrointestinal and genitourinary tracts, as well as any other organs in the treatment field. Radiation injury triggers inflammation and ultimately stimulates transdifferentiation of fibroblasts into myofibroblasts. In addition to their excessive proliferation, these myofibroblasts produce excess collagen and other extracellular matrix (ECM) components, which is compounded by a reduction in remodeling enzymes. Subsequent fibrosis reduces tissue compliance and—in a majority of cancer patients and particularly those with head and neck cancer—causes cosmetic and functional impairment that significantly impacts quality of life. With this in mind, the following review will present a comprehensive discussion of the etiology, presentation, pathogenesis, and therapy for RIF.
Methods
A thorough literature search was performed using the PubMed database and, in parts, clinicaltrials.gov. Keywords “Radiation-Induced Fibrosis,” “Radiotherapy Complications,” “Fibrosis Therapy,” and other closely related terms were utilized, yielding an abundance of results of which primarily those of the last decade were considered. Approximately 150 of these were subsequently reviewed, excluding around 30 due to quality or findings non-contributory to the goals of the review. About ten additional articles were supplied by local experts in the field.
Etiology
A number of factors increase the risk of RIF. The primary treatment-related factors are the total dose of radiotherapy and dose per fraction, the volume of tissue treated, and the time course of treatment delivery. More specifically, the degree of RIF directly correlates with increased radiation dose and hypofractionation (fewer fractions require greater doses), increased field size, and prolongation of therapy (Borger et al. 1994; Davis et al. 2005; Geara et al. 1998; Graham et al. 1999; Johansson et al. 2002). Other treatment-related factors known to play a role include concurrent use of chemotherapy (Kirwan et al. 2003; Toledano et al. 2006) as well as incorporation of surgical management pre- or post-radiotherapy (Machtay et al. 2008). Patient-related factors like preexisting connective tissue disease (Holscher et al. 2006) may also contribute to RIF. In particular, patients with systemic scleroderma, systemic lupus erythematosus (SLE), or Marfan syndrome are more susceptible to developing severe RIF (Gold et al. 2007; Suarez et al. 2014).
More recently, genetics have been found to play a part in the predisposition to RIF. In breast cancer, for example, increased risk of RIF has been associated with a genetic variant in the ATM (ataxia-telangiectasia mutated) gene, which is responsible for the repair of DNA double-strand breaks (Andreassen et al. 2006; Edvardsen et al. 2007). Other single-nucleotide polymorphisms (SNPs) have been identified in genes encoding proteins including superoxide dismutase 2 (SOD2), X-ray repair cross-complementing proteins 1 and 3 (XRCC1 and XRCC3), transforming growth factor beta 1 (TGFβ1), and double-strand-break repair protein rad21 homolog (RAD21; Azria et al. 2004, 2008; Cheuk et al. 2014). Several different loci like CADM1 (cell adhesion molecule 1), SLAMF6 (signaling lymphocyte activation molecule family member 6), and CDK1NA (cyclin-dependent kinase inhibitor 1) have also been implicated (Ao et al. 2009). Further, a quantitative trait locus on chromosome 17 has been found to determine the pulmonary fibrotic response not only to radiation but also to many other forms of injury (Haston et al. 2002), suggesting the presence of a universal lung injury gene (Haston and Travis 1997; Madani et al. 2007). Additional genes like CAP1 (adenylyl cyclase-associated protein 1), IL18 (interleukin 18), MMP12 (matrix metalloproteinase 12), PER3 (period circadian protein homolog 3 protein), LTF (lactoferrin), Ifi202a (p202), and RAD51AP1 (RAD51-associated protein 1) play a role in the degradation of post-radiation extracellular matrix (ECM) (Iwakawa et al. 2004). Mitochondrial DNA has been examined as well, and a genetic variant in TXNRD2 (thioredoxin reductase 2), which encodes a mitochondrial enzyme involved in the removal of reactive oxygen species (ROS), has been connected to rates of subcutaneous fibrosis (Edvardsen et al. 2013). Lastly, epigenetic modifications to DNA and histones have been associated with RIF (Weigel et al. 2014) as evidenced by the suppression of cutaneous radiation syndrome by histone deacetylase inhibitors (HDACs; Chung et al. 2004). These types of DNA alterations are long term, and as such they likely play a significant role in the development of the chronic fibrotic response to radiation injury that persists even after the initial insult is no longer present.
Clinical presentation
RIF usually occurs 4–12 months after radiation therapy and progresses over several years. It affects almost every part of the body that is exposed to radiation. The clinical presentation depends on the type of tissue exposed to irradiation. In general, RIF may manifest as skin induration and thickening, muscle shortening and atrophy, limited joint mobility, lymphedema, mucosal fibrosis, ulceration, fistula, hollow organ stenosis, and pain (Dorr and Hendry 2001). More regionally specific manifestations include trismus, xerostomia, decreased vocal quality, osteoradionecrosis, dysphagia, and aspiration in patients with head and neck malignancy (Delanian et al. 2005; Delanian and Lefaix 2002; Gupta et al. 2012; Jones et al. 2006; Rosenthal et al. 2006; Sonis and Fey 2002; Vainshtein et al. 2014); cervical plexopathy, brachial plexopathy, interstitial fibrosis, dyspnea, and oxygen requirement in patients with breast or lung malignancy (Abratt et al. 2004; Delanian et al. 1999; Gross 1977); and urinary urgency, increased urinary frequency, diarrhea, loss of reproductive function, and dyspareunia in patients with abdominopelvic malignancy (Coia et al. 1995; Marks et al. 1995; Potter et al. 2000). Diagnosis of RIF is likewise dependent on the site affected. In the skin or subcutaneous tissue, for instance, it may be done by palpation; in the muscle, by Young’s modulus measurements (tensile or stiffness), using ultrasound to provide more quantitative measurements (Leung et al. 2002). As it stands, there remains no uniform consensus with respect to objectively quantifying the degree of fibrosis, and there is inconsistency among grading scales like the Radiation Therapy Oncology Group (RTOG) criteria and version 4.0 of the Common Terminology Criteria for Adverse Events, the former of which does not specifically address RIF in assessing overall radiation toxicity (Deng et al. 2014; Radiation Therapy Oncology Group 2014).
Pathogenesis
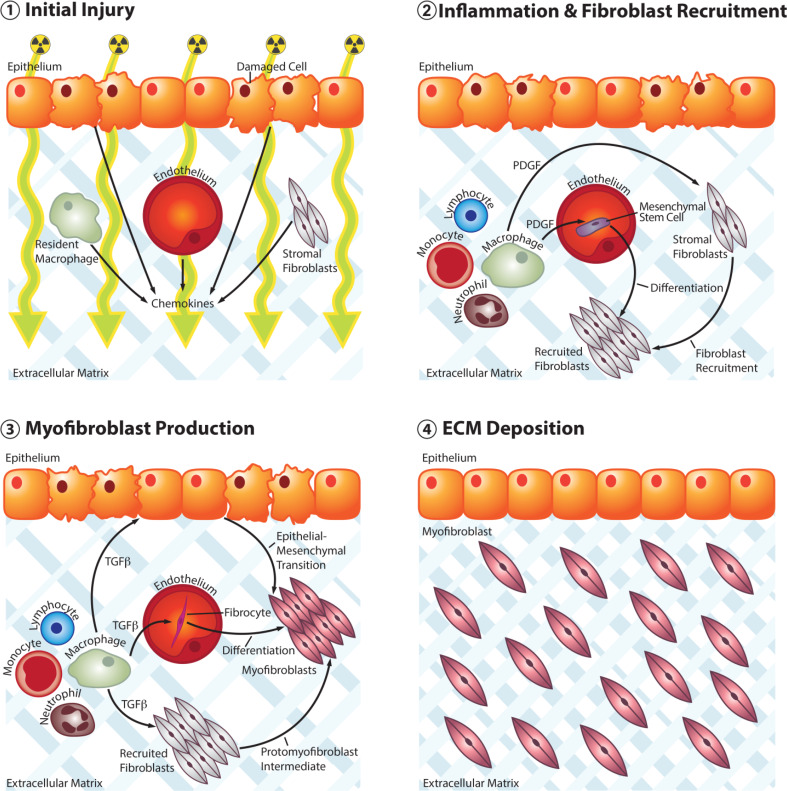
The mechanism of RIF is similar to that of any chronic wound healing process. An initial injury incites an acute response that leads to inflammation, followed by fibroblast recruitment and activation with extracellular matrix deposition (Fig. 1). Radiation is energy in the form of waves or high-speed particles. The term “ionizing” indicates that said energy is strong enough to displace bound electrons. Ionizing radiation refers to three types of emissions—alpha, beta, and gamma—with therapeutic radiation being predominantly gamma (Harrison and Stather 1996). Radiation injury results from two primary mechanisms: direct DNA damage and the generation of reactive oxygen species (ROS; Travis 2001). The latter is more prominent in RIF and involves the interaction of ionizing radiation with water molecules to form free radicals, including superoxide, hydrogen peroxide, and hydroxyl radical (Tak and Park 2009), the last of which accounts for 60–70 % of the total damage (Terasaki et al. 2011; Zhao and Robbins 2009). Reactive nitrogen species (RNS) also likely play a role in radiation injury, as treatment with the inducible nitric oxide synthase (iNOS) inhibitor, L-nitroarginine methyl ester (L-NAME), prevented acute lung injury in rats (Khan et al. 2003). Free radicals damage all components of cells, including proteins, nucleic acids, and lipids (Terasaki et al. 2011; Zhao and Robbins 2009). Superoxide dismutase, catalase, and glutathione peroxidase are responsible for controlling free radical damage (Greenberger and Epperly 2007). A deficiency in these enzymes or excess ROS/RNS leads to oxidative stress in tissues (Chaudiere and Ferrari-Iliou 1999; Darley-Usmar and Halliwell 1996; Evans and Halliwell 1999). Injured cells release chemoattractant molecules that trigger nonspecific inflammation (Denham and Hauer-Jensen 2002; Travis 2001; Williams et al. 2010) [Fig. 1(1)]. Furthermore, thrombosis and ischemia exacerbate local injury leading to further release of inflammatory chemokines and cytokines (Boerma and Hauer-Jensen 2010; Lefaix and Daburon 1998).
Fig. 1.
Schematic depicting four broad stages in the pathogenesis of RIF. 1 Ionizing radiation damages cells in the exposed field and leads to the production of proinflammatory cytokines. 2 Neutrophils, lymphocytes, and monocytes arrive at the site of injury while resultant M2 macrophages produce PDGF, leading to recruitment of stromal fibroblasts as well as differentiation of circulating mesenchymal stem cells. 3 Subsequent TGF-β production by M2 macrophages promotes the development of myofibroblasts from recruited stromal fibroblasts through a protomyofibroblast intermediate as well as through epithelial–mesenchymal transition and differentiation of circulating fibrocytes. 4 Over time, myofibroblast proliferation along with excess deposition and decreased degradation of extracellular matrix leads to fibrosis with reduced vascularity and a paucity of cells
Neutrophils are the first inflammatory cells to arrive at the site of injury (Abreu et al. 2005). Increased expression of intercellular adhesion molecule 1 (ICAM-1) (Hallahan et al. 2002) and platelet endothelial cell adhesion molecule 1 (PECAM-1) (Quarmby et al. 1999) on disrupted endothelial surfaces contributes to neutrophil extravasation and transmigration into tissues (Lefaix and Daburon 1998). When these cells come into contact with collagen fragments and fibronectin, they release proinflammatory cytokines like tumor necrosis factor alpha (TNF-α), IL-1, and IL-6 (Calveley et al. 2005; Finkelstein et al. 1997; Olman et al. 2002; Porter et al. 2002; Sedgwick et al. 2002) that perpetuate the development of ROS and lead to even greater local inflammation. The next cells to arrive are the monocytes and lymphocytes (Haston et al. 2007; Sharplin and Franko 1989), which interact with each other to lead to the differentiation of monocytes into two subsets of macrophages (Gordon and Martinez 2010; Sica and Mantovani 2012; Varin and Gordon 2009): classically activated pro-inflammatory M1 or alternatively activated anti-inflammatory M2 (Ford et al. 2012; Zhang et al. 2011). Platelet-derived growth factor (PDGF) secreted from the M2 subset promotes neoangiogenesis and stimulates the migration of fibroblasts into the injured tissue (Li et al. 2007) from either the surrounding stroma or from circulating mesenchymal stem cells (Mathew and Thomas 2012) [Fig. 1(2)]. They also secrete TGF-β, which is heavily implicated in RIF (Li et al. 2006). Indeed, TGF-β is responsible for a number of functions that contribute to the pathogenesis of this condition, including the production of fibroblasts from bone marrow progenitors (Campana et al. 2004; Rodemann and Bamberg 1995) and the differentiation of fibroblasts into myofibroblasts (Yarnold and Brotons 2010), whereby a phenotypic change in the fibroblasts results in increased expression of alpha-smooth muscle actin (α-SMA), followed by subsequent transformation into protomyofibroblasts and eventual maturation into myofibroblasts (Tomasek et al. 2002). These myofibroblasts may also derive from circulating bone marrow-derived progenitor cells known as fibrocytes or from epithelial cells undergoing epithelial–mesenchymal transition (EMT) (Darby and Hewitson 2007) [Fig. 1(3)]. In response to TGF-β, myofibroblasts secrete excess collagen, fibronectin, and proteoglycans (Chithra et al. 1998), and in doing so they are responsible for the increased stiffness and thickening of the tissue (Lefaix and Daburon 1998; Martin et al. 2000). Furthermore, TGF-β promotes decreased matrix metalloproteinase (MMP) activity (especially MMP-2 and MMP-9) and increased activity of tissue inhibitors of metalloproteinases (TIMPs), compounding the already excessive ECM deposition (Pardo and Selman 2006). Lastly, although myofibroblasts promote endothelial cell proliferation and angiogenesis through the secretion of basic fibroblast growth factor (bFGF) (Finlay et al. 2000), excess collagen reduces vascularity over time (Lefaix and Daburon 1998) [Fig. 1(4)]. This makes fibrotic areas susceptible to physical trauma and gradual ischemia, which may lead to loss of function, tissue atrophy, reduction in the number fibroblasts, or necrosis (Burger et al. 1998; Delanian et al. 1998, 2001; Denham and Hauer-Jensen 2002; Rudolph et al. 1988; Toussaint et al. 2002). Interestingly, no correlation has been found between the severity of early fibrotic lesions and the development of late effects of RIF (Bentzen and Overgaard 1991; Bentzen et al. 1993, 1989; Bourhis et al. 2006).
Implications for therapy
Prevention is the first step in managing RIF, and, since the dose of radiation and the volume of tissue irradiated are the most significant risk factors, limitation of these parameters is usually the first consideration. With modern conformal radiation techniques, most of the radiation therapy is directed to the tumor rather than the surrounding tissue, as in sparing of the salivary glands in irradiated head and neck tissue (Eisbruch et al. 2003). Likewise, decreases in breast induration, telangiectasia, lung fibrosis, and xerostomia were seen with intensity-modulated radiotherapy (IMRT; Barnett et al. 2012; Donovan et al. 2007; Gupta et al. 2012; Jiang et al. 2012). With more technical advances, an expected reduction in RIF is likely; even so, current modalities continue to cause injury, necessitating subsequent medical interventions to control fibrosis.
Inhibition of matrix synthesis and reduction in inflammation have served as the primary aims of therapeutic development in RIF. Several preclinical models have been tested in sites including lung, skin, breast, and intestinal tissue using techniques ranging from small molecule inhibition to cell transplantation (Table 1). Due to its crucial role in the pathogenesis of RIF, TGF-β and its associated signaling molecules have been examined as therapeutic targets. More specifically, the small molecule inhibitor, LY2109761, natural compound derivatives (halofuginone and quercetin), and siRNA have been used to target various components of the TGF-β pathway to mitigate inflammation, matrix deposition, and fibrosis. Integrin receptors also play an important role in cell–matrix interactions, and inhibition of α5β6 integrin with a specific antibody prevented fibrosis in a mouse model (Flechsig et al. 2012; Horton et al. 2013c; Lemos and Andrade 2010; Li et al. 2006; Xavier et al. 2004). Apart from TGF-β and its downstream effectors, targeting other signaling pathways has also yielded promising results in preclinical models. This includes the use of the sphingosine-1-phosphate (S1P) receptor agonists, SEW2871 and (s)-TFY720-phosphonate (fTyS0), a serine palmitoyltransferase (SPT) inhibitor (myriocin), an anti-CXCR4 compound (MSK-122), and a Rho-kinase inhibitor (Y-27632), all of which were found to mitigate fibrosis (Bourgier et al. 2005; Gorshkova et al. 2012, 2013; Shu et al. 2013). Additionally, a number of commonly used medications have been found to attenuate RIF pathology, including imatinib (tyrosine kinase inhibitor), simvastatin (HMG-CoA inhibitor), enalapril [angiotensin-converting enzyme (ACE) inhibitor], and dexamethasone (steroid) (Evans et al. 1987; Gao et al. 2013; Horton et al. 2013a; Mathew et al. 2011). Lastly, cell-based therapies have been assessed for their anti-fibrotic potential. Systemic infusion of syngeneic or allogeneic bone marrow-derived stem cells resulted in reduced skin contracture, decreased thickening, and less collagen deposition in a mouse model of RIF; there was also an increase in the immunosuppressive cytokine, IL-10, and a decrease in the proinflammatory cytokine, IL-1β (Horton et al. 2013b). This initial study highlights the potential of cell-based therapy in RIF.
Table 1.
Agents tested in preclinical models of RIF
| Molecule/approach | Antagonism | Site | Inhibition of matrix synthesis | Reduction in inflammation | References |
|---|---|---|---|---|---|
| LY2109761 | TGF-β receptor 1 | Lung | Yes | Yes | Flechsig et al. (2012) |
| Halofuginone | TGF-β receptor 2 | Breast | Not tested | Not described | Xavier et al. (2004) |
| Quercetin | Cofilin | Skin | Yes | Not tested | Horton et al. (2013c) |
| siRNA | Smad3 | Skin | Yes | Not tested | Lee et al. (2010) |
| 6.3G9 monoclonal Ab | α5β6 Integrin | Lung | Yes | Yes | Puthawala et al. (2008) |
| SEW2871, fTy50 | SPT | Heart | Yes | Yes | Gorshkova et al. (2013) |
| Myriocin | SPT | Lung | Yes | Yes | Gorshkova et al. (2012) |
| MSK-122 | CXCR4/CXCL12 | Lung | Yes | Not described | Shu et al. (2013) |
| Y-27632 | Rho kinase | Intestine | Yes | Not described | Bourgier et al. (2005) |
| Imatinib | Tyrosine kinase | Skin | Yes | Yes | Horton et al. (2013a) |
| Simvastatin | HMG-CoA reductase | Lung | Not tested | Yes | Mathew et al. (2011) |
| Enalapril | ACE | Lung | Yes | Yes | Gao et al. (2013) |
| Dexamethasone | Inflammation | Lung | Not tested | Yes | Evans et al. (1987) |
| Bone marrow-derived mesenchymal cells | Inflammation | Skin | Yes | Yes | Horton et al. (2013b) |
Several clinical trials have been carried out to determine the anti-fibrotic efficacy of biologicals and small molecule inhibitors. In breast cancer, the combination of anti-inflammatory pentoxifylline with antioxidant vitamin E has been shown to improve tissue compliance in patients with RIF (Jacobson et al. 2013), while the effect of adding hyperbaric oxygen to this regimen is still being studied (Otón 2013). Likewise in head and neck cancer, an eight-week course of pentoxifylline achieved a modest improvement in mean dental gap in 20 patients with nasopharyngeal carcinoma post-radiotherapy (Chua et al. 2001), and outcome measures of SOD administration are still being examined using a predetermined scale of fibrosis and quality-of-life impact assessment (Spanos 2013). Furthermore, two agents—the vascular endothelial growth factor (VEGF) inhibitor, bevacizumab, and the antiproliferative agent, pirfenidone—are now being tested for their efficacy in patients already suffering from RIF, with the former utilizing outcome measures of pulmonary function testing and thoracic CT assessment (Camphausen 2013; Ji 2013).
In spite of the preclinical models and clinical trials mentioned thus far, the number of approved therapies for RIF remains small. Symptomatic treatment is commonplace, and specific interventions depend on the location and severity of fibrosis. For example, physiotherapy has been shown to be effective in reducing lymphedema and preserving shoulder motion post-radiation in patients with breast cancer, and the LPG Systems mechanical massage technique has been shown to reduce RIF in breast cutaneous tissue (Bourgeois et al. 2008; Box et al. 2002). For patients with head and neck cancer that have trismus post-radiation, progressive increases in mouth opening using tongue blades, the Dynasplint Trismus System, or the TheraBite Jaw Motion Rehabilitation System have been recommended (Baranano et al. 2011; Grandi et al. 2007; Kamstra et al. 2013; Melchers et al. 2009; Sciubba and Goldenberg 2006; Shulman et al. 2008; Stubblefield et al. 2010). Coronoidectomy has been shown to be efficacious in refractory cases (Bhrany et al. 2007), although careful thought and consideration must be given to this intervention as surgery may lead to even greater fibrosis. Other modalities that have been tested in this condition include microcurrent therapy (Dijkstra et al. 2004) and botulinum toxin A injection, the latter of which improved pain and masticator spasm but did not significantly impact jaw opening (Hartl et al. 2008).
Conclusion
Although radiotherapy offers immense benefit to the patient, it still causes unwanted long-term sequelae. Not surprisingly, the dose of radiation and the amount of tissue volume exposed are the main risk factors for RIF. The disease process differs from normal wound healing by the aberrant growth of myofibroblasts and the excessive deposition of extracellular matrix proteins. More site-specific research is necessary to determine the mechanisms of RIF, as symptoms can vary widely, for example, between the oral cavity, breast, and lungs. In patients with established RIF, the treatment is primarily symptomatic, with no effective method that offers complete remission at this time. Future interventions will likely continue to focus on the molecular mechanisms of this condition to mitigate the inflammatory responses, control myofibroblast development, and reduce collagen deposition. Additionally, developing a means of grading the degree of fibrosis will go a long way toward ensuring that patients with RIF are managed appropriately with minimal treatment side effects.
In conclusion, the strengths of this review lie in its comprehensive coverage of the etiology, molecular pathology, and therapeutic developments of RIF, while its limitations are manifest by an inability to elaborate further on the variable presentations of RIF or its complex biochemical pathology. Further studies on these aspects would provide even more compelling evidence for looking to pathogenesis in developing effective therapeutic interventions for RIF.
Acknowledgments
The Department of Otolaryngology-Head and Neck Surgery at the University of Kansas Medical Center, the University of Kansas Cancer Center’s CCSG (1-P30-CA168524-02), and the Kansas Intellectual and Developmental Disabilities Center (NICHD HD00258) were the funding sources. The authors acknowledge Mr. Phil Shafer for generating the schematic diagram. We apologize to authors whose work was not cited due to space constraints.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abratt RP, Morgan GW, Silvestri G, Willcox P (2004) Pulmonary complications of radiation therapy. Clin Chest Med 25:167–177. doi:10.1016/s0272-5231(03)00126-6 [DOI] [PubMed] [Google Scholar]
- Abreu MT, Fukata M, Arditi M (2005) TLR signaling in the gut in health and disease. J Immunol 174:4453–4460 [DOI] [PubMed] [Google Scholar]
- Andreassen CN et al (2006) ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 64:776–783. doi:10.1016/j.ijrobp.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Ao X, Zhao L, Davis MA, Lubman DM, Lawrence TS, Kong FM (2009) Radiation produces differential changes in cytokine profiles in radiation lung fibrosis sensitive and resistant mice. J Hematol Oncol 2:6. doi:10.1186/1756-8722-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azria D et al (2004) Concomitant use of tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer 91:1251–1260. doi:10.1038/sj.bjc.6602146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azria D et al (2008) Single nucleotide polymorphisms, apoptosis, and the development of severe late adverse effects after radiotherapy. Clin Cancer Res 14:6284–6288. doi:10.1158/1078-0432.CCR-08-0700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranano CF, Rosenthal EL, Morgan BA, McColloch NL, Magnuson JS (2011) Dynasplint for the management of trismus after treatment of upper aerodigestive tract cancer: a retrospective study. Ear Nose Throat J 90:584–590 [DOI] [PubMed] [Google Scholar]
- Barnett GC et al (2012) Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys 82:715–723. doi:10.1016/j.ijrobp.2010.10.068 [DOI] [PubMed] [Google Scholar]
- Bentzen SM, Overgaard M (1991) Relationship between early and late normal-tissue injury after postmastectomy radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol 20:159–165 [DOI] [PubMed] [Google Scholar]
- Bentzen SM, Overgaard M, Thames HD, Christensen JJ, Overgaard J (1989) Early and late normal-tissue injury after postmastectomy radiotherapy alone or combined with chemotherapy. Int J Radiat Biol 56:711–715 [DOI] [PubMed] [Google Scholar]
- Bentzen SM, Overgaard M, Overgaard J (1993) Clinical correlations between late normal tissue endpoints after radiotherapy: implications for predictive assays of radiosensitivity. Eur J Cancer 29A:1373–1376 [DOI] [PubMed] [Google Scholar]
- Bhrany AD, Izzard M, Wood AJ, Futran ND (2007) Coronoidectomy for the treatment of trismus in head and neck cancer patients. Laryngoscope 117:1952–1956. doi:10.1097/MLG.0b013e31812eee13 [DOI] [PubMed] [Google Scholar]
- Boerma M, Hauer-Jensen M (2010) Potential targets for intervention in radiation-induced heart disease. Curr Drug Targets 11:1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger JH, Kemperman H, Smitt HS, Hart A, van Dongen J, Lebesque J, Bartelink H (1994) Dose and volume effects on fibrosis after breast conservation therapy. Int J Radiat Oncol Biol Phys 30:1073–1081 [DOI] [PubMed] [Google Scholar]
- Bourgeois JF, Gourgou S, Kramar A, Lagarde JM, Guillot B (2008) A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol 14:71–76. doi:10.1111/j.1600-0846.2007.00263.x [DOI] [PubMed] [Google Scholar]
- Bourgier C et al (2005) Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut 54:336–343. doi:10.1136/gut.2004.051169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis J et al (2006) Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. Clin Oncol 24:2873–2878. doi:10.1200/JCO.2006.08.057 [DOI] [PubMed] [Google Scholar]
- Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM (2002) Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat 75:35–50 [DOI] [PubMed] [Google Scholar]
- Burger A, Loffler H, Bamberg M, Rodemann HP (1998) Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol 73:401–408 [DOI] [PubMed] [Google Scholar]
- Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP (2005) Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol 81:887–899. doi:10.1080/09553000600568002 [DOI] [PubMed] [Google Scholar]
- Campana F et al (2004) Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J Cell Mol Med 8:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camphausen K (2013) Pirfenidone in treating patients with fibrosis caused by radiation therapy for cancer. https://clinicaltrials.gov/ct2/show/NCT00020631. Accessed 23 Oct 2014
- Chaudiere J, Ferrari-Iliou R (1999) Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol 37:949–962 [DOI] [PubMed] [Google Scholar]
- Cheuk IW, Yip SP, Kwong DL, Wu VW (2014) Association of and gene haplotypes with the development of radiation-induced fibrosis in patients with nasopharyngeal carcinoma. Mol Clin Oncol 2:553–558. doi:10.3892/mco.2014.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithra P, Sajithlal GB, Chandrakasan G (1998) Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol 59:179–186 [DOI] [PubMed] [Google Scholar]
- Chua DT, Lo C, Yuen J, Foo YC (2001) A pilot study of pentoxifylline in the treatment of radiation-induced trismus. Am J Clin Oncol 24:366–369 [DOI] [PubMed] [Google Scholar]
- Chung YL, Wang AJ, Yao LF (2004) Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther 3:317–325 [PubMed] [Google Scholar]
- Coia LR, Myerson RJ, Tepper JE (1995) Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys 31:1213–1236. doi:10.1016/0360-3016(94)00419-l [DOI] [PubMed] [Google Scholar]
- Darby IA, Hewitson TD (2007) Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257:143–179. doi:10.1016/S0074-7696(07)57004-X [DOI] [PubMed] [Google Scholar]
- Darley-Usmar V, Halliwell B (1996) Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res 13:649–662 [DOI] [PubMed] [Google Scholar]
- Davis AM et al (2005) Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol J Eur Soc Ther Radiol Oncol 75:48–53 [DOI] [PubMed] [Google Scholar]
- Delanian S, Lefaix JL (2002) Complete healing of severe osteoradionecrosis with treatment combining pentoxifylline, tocopherol and clodronate. Br J Radiol 75:467–469 [DOI] [PubMed] [Google Scholar]
- Delanian S, Martin M, Bravard A, Luccioni C, Lefaix JL (1998) Abnormal phenotype of cultured fibroblasts in human skin with chronic radiotherapy damage. Radiother Oncol J Eur Soc Ther Radiol Oncol 47:255–261 [DOI] [PubMed] [Google Scholar]
- Delanian S, Balla-Mekias S, Lefaix JL (1999) Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol 17:3283–3290 [DOI] [PubMed] [Google Scholar]
- Delanian S, Martin M, Bravard A, Luccioni C, Lefaix JL (2001) Cu/Zn superoxide dismutase modulates phenotypic changes in cultured fibroblasts from human skin with chronic radiotherapy damage. Radiother Oncol J Eur Soc Ther Radiol Oncol 58:325–331 [DOI] [PubMed] [Google Scholar]
- Delanian S, Depondt J, Lefaix JL (2005) Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck 27:114–123. doi:10.1002/hed.20121 [DOI] [PubMed] [Google Scholar]
- Deng J, Ridner SH, Wells N, Dietrich MS, Murphy BA (2014) Development and preliminary testing of head and neck cancer related external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. doi:10.1016/j.ejon.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Denham JW, Hauer-Jensen M (2002) The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol J Eur Soc Ther Radiol Oncol 63:129–145 [DOI] [PubMed] [Google Scholar]
- Dijkstra PU, Kalk WW, Roodenburg JL (2004) Trismus in head and neck oncology: a systematic review. Oral Oncol 40:879–889. doi:10.1016/j.oraloncology.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Donovan E et al (2007) Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol 82:254–264. doi:10.1016/j.radonc.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Dorr W, Hendry JH (2001) Consequential late effects in normal tissues. Radiother Oncol J Eur Soc Ther Radiol Oncol 61:223–231 [DOI] [PubMed] [Google Scholar]
- Edvardsen H et al (2007) Linkage disequilibrium pattern of the ATM gene in breast cancer patients and controls; association of SNPs and haplotypes to radio-sensitivity and post-lumpectomy local recurrence. Radiat Oncol 2:25. doi:10.1186/1748-717X-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsen H et al (2013) SNP in TXNRD2 associated with radiation-induced fibrosis: a study of genetic variation in reactive oxygen species metabolism and signaling. Int J Radiat Oncol Biol Phys 86:791–799. doi:10.1016/j.ijrobp.2013.02.025 [DOI] [PubMed] [Google Scholar]
- Eisbruch A et al (2003) Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg 27:832–837 [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B (1999) Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci 884:19–40 [DOI] [PubMed] [Google Scholar]
- Evans ML, Graham MM, Mahler PA, Rasey JS (1987) Use of steroids to suppress vascular response to radiation. Int J Radiat Oncol Biol Phys 13:563–567 [DOI] [PubMed] [Google Scholar]
- Finkelstein JN, Johnston C, Barrett T, Oberdorster G (1997) Particulate-cell interactions and pulmonary cytokine expression. Environ Health Perspect 105(Suppl 5):1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay GA, Thannickal VJ, Fanburg BL, Paulson KE (2000) Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem 275:27650–27656. doi:10.1074/jbc.M000893200 [DOI] [PubMed] [Google Scholar]
- Flechsig P et al (2012) LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 18:3616–3627. doi:10.1158/1078-0432.CCR-11-2855 [DOI] [PubMed] [Google Scholar]
- Ford AQ, Dasgupta P, Mikhailenko I, Smith EM, Noben-Trauth N, Keegan AD (2012) Adoptive transfer of IL-4Ralpha + macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation. BMC Immunol 13:6. doi:10.1186/1471-2172-13-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M (2013) Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res 180:546–552. doi:10.1667/RR13350.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geara FB, Komaki R, Tucker SL, Travis EL, Cox JD (1998) Factors influencing the development of lung fibrosis after chemoradiation for small cell carcinoma of the lung: evidence for inherent interindividual variation. Int J Radiat Oncol Biol Phys 41:279–286 [DOI] [PubMed] [Google Scholar]
- Gold DG, Miller RC, Petersen IA, Osborn TG (2007) Radiotherapy for malignancy in patients with scleroderma: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys 67:559–567. doi:10.1016/j.ijrobp.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604. doi:10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- Gorshkova I et al (2012) Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res 53:1553–1568. doi:10.1194/jlr.M026039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorshkova IA et al (2013) Inhibition of sphingosine-1-phosphate lyase rescues sphingosine kinase-1-knockout phenotype following murine cardiac arrest. Life Sci 93:359–366. doi:10.1016/j.lfs.2013.07.017 [DOI] [PubMed] [Google Scholar]
- Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, Perez CA (1999) Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 45:323–329 [DOI] [PubMed] [Google Scholar]
- Grandi G, Silva ML, Streit C, Wagner JC (2007) A mobilization regimen to prevent mandibular hypomobility in irradiated patients: an analysis and comparison of two techniques. Med Oral Patol Oral Cir Bucal 12:E105–E109 [PubMed] [Google Scholar]
- Greenberger JS, Epperly MW (2007) Review. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization In vivo 21:141–146 [PubMed] [Google Scholar]
- Gross NJ (1977) Pulmonary effects of radiation therapy. Ann Intern Med 86:81–92 [DOI] [PubMed] [Google Scholar]
- Gupta T et al (2012) Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol J Eur Soc Ther Radiol Oncol 104:343–348. doi:10.1016/j.radonc.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Geng L, Shyr Y (2002) Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst 94:733–741 [DOI] [PubMed] [Google Scholar]
- Harrison JD, Stather JW (1996) The assessment of doses and effects from intakes of radioactive particles. J Anat 189(Pt 3):521–530 [PMC free article] [PubMed] [Google Scholar]
- Hartl DM, Cohen M, Julieron M, Marandas P, Janot F, Bourhis J (2008) Botulinum toxin for radiation-induced facial pain and trismus. Otolaryngol Head Neck Surg 138:459–463. doi:10.1016/j.otohns.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Haston CK, Travis EL (1997) Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res 57:5286–5291 [PubMed] [Google Scholar]
- Haston CK et al (2002) Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Res 62:3782–3788 [PubMed] [Google Scholar]
- Haston CK, Begin M, Dorion G, Cory SM (2007) Distinct loci influence radiation-induced alveolitis from fibrosing alveolitis in the mouse. Cancer Res 67:10796–10803. doi:10.1158/0008-5472.CAN-07-2733 [DOI] [PubMed] [Google Scholar]
- Holscher T, Bentzen SM, Baumann M (2006) Influence of connective tissue diseases on the expression of radiation side effects: a systematic review. Radiother Oncol J Eur Soc Ther Radiol Oncol 78:123–130. doi:10.1016/j.radonc.2005.12.013 [DOI] [PubMed] [Google Scholar]
- Horton JA et al (2013a) Inhibition of radiation-induced skin fibrosis with imatinib. Int J Radiat Biol 89:162–170. doi:10.3109/09553002.2013.741281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, Citrin DE (2013b) Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells 31:2231–2241. doi:10.1002/stem.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JA et al (2013c) Quercetin inhibits radiation-induced skin fibrosis. Radiat Res 180:205–215. doi:10.1667/RR3237.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakawa M et al (2004) Strain dependent differences in a histological study of CD44 and collagen fibers with an expression analysis of inflammatory response-related genes in irradiated murine lung. J Radiat Res 45:423–433 [DOI] [PubMed] [Google Scholar]
- Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J (2013) Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys 85:604–608. doi:10.1016/j.ijrobp.2012.06.042 [DOI] [PubMed] [Google Scholar]
- Ji Y (2013) Efficiency study for acute radiation-induced and chemotherapy-induced pulmonary fibrosis with bevasizumab. https://clinicaltrials.gov/ct2/show/NCT01917877. Accessed 23 Oct 2014
- Jiang ZQ et al (2012) Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: the MD Anderson experience. Int J Radiat Oncol Biol Phys 83:332–339. doi:10.1016/j.ijrobp.2011.06.1963 [DOI] [PubMed] [Google Scholar]
- Johansson S, Svensson H, Denekamp J (2002) Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int J Radiat Oncol Biol Phys 52:1207–1219 [DOI] [PubMed] [Google Scholar]
- Jones HA et al (2006) Preliminary investigation of symptom distress in the head and neck patient population: validation of a measurement instrument. Am J Clin Oncol 29:158–162. doi:10.1097/01.coc.0000207424.62275.9d [DOI] [PubMed] [Google Scholar]
- Kamstra JI, Roodenburg JL, Beurskens CH, Reintsema H, Dijkstra PU (2013) TheraBite exercises to treat trismus secondary to head and neck cancer. Support Care Cancer 21:951–957. doi:10.1007/s00520-012-1610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Van Dyk J, Yeung IW, Hill RP (2003) Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol J Eur Soc Ther Radiol Oncol 66:95–102 [DOI] [PubMed] [Google Scholar]
- Kirwan JM, Symonds P, Green JA, Tierney J, Collingwood M, Williams CJ (2003) A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol 68:217–226 [DOI] [PubMed] [Google Scholar]
- Lee JW et al (2010) Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg 136:714–719. doi:10.1001/archoto.2010.107 [DOI] [PubMed] [Google Scholar]
- Lefaix JL, Daburon F (1998) Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys 75:375–384 [DOI] [PubMed] [Google Scholar]
- Lemos QT, Andrade ZA (2010) Angiogenesis and experimental hepatic fibrosis. Mem Inst Oswaldo Cruz 105:611–614 [DOI] [PubMed] [Google Scholar]
- Leung SF, Zheng Y, Choi CY, Mak SS, Chiu SK, Zee B, Mak AF (2002) Quantitative measurement of post-irradiation neck fibrosis based on the young modulus: description of a new method and clinical results. Cancer 95:656–662. doi:10.1002/cncr.10700 [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24:99–146. doi:10.1146/annurev.immunol.24.021605.090737 [DOI] [PubMed] [Google Scholar]
- Li M, Jendrossek V, Belka C (2007) The role of PDGF in radiation oncology. Radiat Oncol 2:5. doi:10.1186/1748-717X-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtay M et al (2008) Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 26:3582–3589. doi:10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J (2007) Predicting risk of radiation-induced lung injury. J Thorac Oncol 2:864–874. doi:10.1097/JTO.0b013e318145b2c6 [DOI] [PubMed] [Google Scholar]
- Marks LB, Carroll PR, Dugan TC, Anscher MS (1995) The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys 31:1257–1280. doi:10.1016/0360-3016(94)00431-j [DOI] [PubMed] [Google Scholar]
- Martin M, Lefaix J, Delanian S (2000) TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 47:277–290 [DOI] [PubMed] [Google Scholar]
- Mathew M, Thomas SM (2012) The cellular microenvironment of head and neck squamous cell carcinoma. In: Li X (ed) Squamous cell carcinoma. InTech, pp 163–174. doi:10.5772/25652
- Mathew B et al (2011) Simvastatin attenuates radiation-induced murine lung injury and dysregulated lung gene expression. Am J Respir Cell Mol Biol 44:415–422. doi:10.1165/rcmb.2010-0122OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers LJ, Van Weert E, Beurskens CH, Reintsema H, Slagter AP, Roodenburg JL, Dijkstra PU (2009) Exercise adherence in patients with trismus due to head and neck oncology: a qualitative study into the use of the Therabite. Int J Oral Maxillofac Surg 38:947–954. doi:10.1016/j.ijom.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Olman MA, White KE, Ware LB, Cross MT, Zhu S, Matthay MA (2002) Microarray analysis indicates that pulmonary edema fluid from patients with acute lung injury mediates inflammation, mitogen gene expression, and fibroblast proliferation through bioactive interleukin-1. Chest 121:69S–70S [DOI] [PubMed] [Google Scholar]
- Otón C (2013) Treatment of Radiation-induced Fibrosis in the Upper Aerodigestive Tract Cancer by a Combination of Pentoxifylline-tocopherol and Hyperbaric Oxygen (ORT-OXI-2009). https://clinicaltrials.gov/ct2/show/NCT01822405. Accessed 23 Oct 2014
- Pardo A, Selman M (2006) Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proceedings of the American Thoracic Society 3:383–388. doi:10.1513/pats.200601-012TK [DOI] [PubMed] [Google Scholar]
- Porter DW et al (2002) Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhalation Toxicol 14:349–367. doi:10.1080/08958370252870998 [DOI] [PubMed] [Google Scholar]
- Potter R, Knocke TH, Fellner C, Baldass M, Reinthaller A, Kucera H (2000) Definitive radiotherapy based on HDR brachytherapy with iridium 192 in uterine cervix carcinoma: report on the Vienna University Hospital findings (1993-1997) compared to the preceding period in the context of ICRU 38 recommendations. Cancer Radiother 4:159–172. doi:10.1016/s1278-3218(00)88900-3 [DOI] [PubMed] [Google Scholar]
- Puthawala K et al (2008) Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177:82–90. doi:10.1164/rccm.200706-806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby S, Kumar P, Wang J, Macro JA, Hutchinson JJ, Hunter RD, Kumar S (1999) Irradiation induces upregulation of CD31 in human endothelial cells. Arterioscler Thromb Vasc Biol 19:588–597 [DOI] [PubMed] [Google Scholar]
- Radiation Therapy Oncology Group (2014) Cooperative group common toxicity criteria. http://www.rtog.org/ResearchAssociates/AdverseEventReporting/CooperativeGroupCommonToxicityCriteria.aspx. Accessed 23 Oct 2014
- Rodemann HP, Bamberg M (1995) Cellular basis of radiation-induced fibrosis. Radiother Oncol J Eur Soc Ther Radiol Oncol 35:83–90 [DOI] [PubMed] [Google Scholar]
- Rosenthal DI, Lewin JS, Eisbruch A (2006) Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 24:2636–2643. doi:10.1200/JCO.2006.06.0079 [DOI] [PubMed] [Google Scholar]
- Rudolph R, Vande Berg J, Schneider JA, Fisher JC, Poolman WL (1988) Slowed growth of cultured fibroblasts from human radiation wounds. Plast Reconstr Surg 82:669–677 [DOI] [PubMed] [Google Scholar]
- Sciubba JJ, Goldenberg D (2006) Oral complications of radiotherapy. Lancet Oncol 7:175–183. doi:10.1016/S1470-2045(06)70580-0 [DOI] [PubMed] [Google Scholar]
- Sedgwick JB, Menon I, Gern JE, Busse WW (2002) Effects of inflammatory cytokines on the permeability of human lung microvascular endothelial cell monolayers and differential eosinophil transmigration. J Allergy Clin Immunol 110:752–756 [DOI] [PubMed] [Google Scholar]
- Sharplin J, Franko AJ (1989) A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res 119:1–14 [PubMed] [Google Scholar]
- Shu HK et al (2013) Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PLoS ONE 8:e79768. doi:10.1371/journal.pone.0079768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman DH, Shipman B, Willis FB (2008) Treating trismus with dynamic splinting: a cohort, case series. Adv Ther 25:9–16. doi:10.1007/s12325-008-0007-0 [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Investig 122:787–795. doi:10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis ST, Fey EG (2002) Oral complications of cancer therapy Oncology (Williston Park, NY) 16:680–686; discussion 686, 691–682, 695 [PubMed]
- Spanos W (2013) Study of topical superoxide dismutase to treat radiation induced fibrosis (Sodermix). https://clinicaltrials.gov/show/NCT01771991. Accessed 23 Oct 2014
- Stubblefield MD, Manfield L, Riedel ER (2010) A preliminary report on the efficacy of a dynamic jaw opening device (dynasplint trismus system) as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil 91:1278–1282. doi:10.1016/j.apmr.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Suarez EM, Knackstedt RJ, Jenrette JM (2014) Significant fibrosis after radiation therapy in a patient with Marfan syndrome. Radiat Oncol J 32:208–212. doi:10.3857/roj.2014.32.3.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak JK, Park JW (2009) The use of ebselen for radioprotection in cultured cells and mice. Free Radic Biol Med 46:1177–1185. doi:10.1016/j.freeradbiomed.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Terasaki Y et al (2011) Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol 301:L415–L426. doi:10.1152/ajplung.00008.2011 [DOI] [PubMed] [Google Scholar]
- Toledano A et al (2006) Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys 65:324–332. doi:10.1016/j.ijrobp.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363. doi:10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Toussaint O, Remacle J, Dierick JF, Pascal T, Frippiat C, Royer V, Chainiaux F (2002) Approach of evolutionary theories of ageing, stress, senescence-like phenotypes, calorie restriction and hormesis from the view point of far-from-equilibrium thermodynamics. Mech Ageing Dev 123:937–946 [DOI] [PubMed] [Google Scholar]
- Travis EL (2001) Organizational response of normal tissues to irradiation. Seminars in radiation oncology 11:184–196 [DOI] [PubMed] [Google Scholar]
- Vainshtein JM, Griffith KA, Feng FY, Vineberg KA, Chepeha DB, Eisbruch A (2014) Patient-reported voice and speech outcomes after whole-neck intensity modulated radiation therapy and chemotherapy for oropharyngeal cancer: prospective longitudinal study. Int J Radiat Oncol Biol Phys 89:973–980. doi:10.1016/j.ijrobp.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Varin A, Gordon S (2009) Alternative activation of macrophages: immune function and cellular biology. Immunobiology 214:630–641. doi:10.1016/j.imbio.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Weigel C, Schmezer P, Plass C, Popanda O (2014) Epigenetics in radiation-induced fibrosis. Oncogene. doi:10.1038/onc.2014.145 [DOI] [PubMed] [Google Scholar]
- Williams JP, Johnston CJ, Finkelstein JN (2010) Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets 11:1386–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier S et al (2004) Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem 279:15167–15176. doi:10.1074/jbc.M309798200 [DOI] [PubMed] [Google Scholar]
- Yarnold J, Brotons MC (2010) Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol J Eur Soc Ther Radiol Oncol 97:149–161. doi:10.1016/j.radonc.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Zhang H et al (2011) The development of classically and alternatively activated macrophages has different effects on the varied stages of radiation-induced pulmonary injury in mice. J Radiat Res 52:717–726 [DOI] [PubMed] [Google Scholar]
- Zhao W, Robbins ME (2009) Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem 16:130–143 [DOI] [PubMed] [Google Scholar]