Abstract
Technological advances now enable routine measurement of mRNA and protein abundances, and estimates of their rates of synthesis and degradation that inform on their values and the degree of change in response to stimuli. Importantly, more and more data on time-series experiments are emerging, e.g. of cells responding to stress, enabling first insights into a new dimension of gene expression regulation - its dynamics and how it allows for very different response signals across genes. This review discusses recently published methods and datasets, their impact on what we now know about the relationships between concentrations and synthesis rates of mRNAs and proteins in yeast and mammalian cells, their evolution, and new hypotheses on translation regulatory mechanisms generated by approaches that involve ribosome footprinting.
The different stages of protein expression regulation
The seemingly simple task of producing a protein molecule from its gene is in fact highly complex. Protein production is regulated in multiple, diverse ways which all act in a controlled, but stochastic and highly dynamic manner in what we collectively call ‘gene expression regulation’. Gene expression regulation involves synthesis of mRNA and protein via transcription and translation, respectively, and degradation of the molecules. Both transcription and translation are coordinated by many participating factors and pathways. Roughly 2,000 of the ~20,000 protein-coding genes in the human genome encode are transcription factors1. A similar fraction of the genome appears to regulate the second stage of protein synthesis: the human genome may encode as many as ~1,000 RNA-binding proteins and ~1,000 miRNAs which putatively regulate both RNA degradation and translation2-4.
Many additional processes add further complexity to gene expression regulation. Alternative pre-mRNA splicing generates an average of four transcript variants per human gene5-7. Alternative translation initiation and termination can create additional variants. Once a protein is made, ~200 unique post-translational modifications, including phosphorylation, acetylation, ubiquitination, and SUMOylation, can be attached to target it for degradation, change its localization, interactions, and functions. Consequently, the Uniprot sequence database comprises >68,000 human protein variants, produced from just over 20,000 genes8.
While sometimes overlooked, the degradation of mRNA and protein molecules is as much regulated as is their synthesis. mRNA turnover regulation is highly complex, occurring through two major pathways. In rapidly growing cells, most mRNA decay is initiated by removal of the m7G cap found on the 5’ end. However, in some cases decay is initiated by removal of the polyA tail – a process called deadenylation 9. Furthermore, the vast majority of protein degradation in eukaryotic cells is managed by the proteasome which itself consists of a protease core and regulatory caps. Proteasomal degradation is initiated by lysine-48-linked polyubiquitination of the target protein – a process regulated by more than 100 ubiquitinating and deubiquitinating enzymes in yeast, and hundreds in mammalian cells8, 10, 11. The targets and condition-specific activities of these enzymes are only known for a small subset.
These regulatory processes are further complicated by feedback mechanisms and coupling between individual processes12. For example, mRNA degradation has been reported to be coupled to both transcription 13 and translation 9, 14. Other work suggests that RNA-binding proteins and miRNAs, two entirely different regulators of RNA translation and degradation, can jointly regulate the same pathway 15. Therefore, the ‘one gene – one protein’ hypothesis is far from describing gene expression regulation in its entirety, ignoring the plethora of different protein products, their interactions, combinatorial regulation, and changes in response to stimuli.
This review first outlines recent methods that enable large-scale measurements of concentrations and rates. We place special emphasis on an approach called ribosome footprinting, which provides estimates of translation efficiency and has received much attention with respect to both the insights it provides and its limitations. We then discuss new insights into the principles and evolution of gene expression regulation from studies using these techniques on yeast and mammalian cells. We finish by describing our view of where the field of systems biology of gene regulation is headed and what questions are likely to be addressed in the near future.
Experimental approaches to characterize gene expression regulation
Excitingly, the last decade has seen enormous technological and methodological advances that enable large-scale measurements of the above-described multiple dimensions of gene expression regulation – both with respect to measurements of concentrations and rates (Table S1). While modifications and interactions can also be measured, they are not the focus of this review. For comprehensive reviews of other ‘dimensions’ of gene expression dynamics, see refs. 16, 17.
Measuring genome-wide mRNA and protein concentrations
Standard methods to estimate mRNA and protein concentrations are high-throughput RNA sequencing and shotgun proteomics, respectively (Table S1). In most cases, molecular concentrations of genes are estimated relative to each other. However, both approaches can be used with spike-in reference samples if absolute copy numbers per cell are desired. While RNA-seq is truly genome-wide, shotgun proteomics has yet to cross that threshold. Individual proteomics efforts from laboratories with highest-end instrumentation are now able to identify up to ~12,000 proteins in mammalian cells, e.g. ref. 18, but routine measurements identify fewer proteins. However, compared to just a few years ago, proteomics has advanced enough to allow time-series experiments, measuring the abundance of hundreds to thousands of proteins across multiple time points. As a result, the first integrative studies that combine mRNA and matching protein concentration measurements have recently been published (Table 1).
Table 1. Example datasets on dynamic gene expression regulation.
Examples of recently published datasets providing first insights into the dynamics of eukaryotic gene expression regulation are shown below. While attempting to cover a range of published datasets, this collection may not be comprehensive.
| mRNA and matching protein time-series analysis |
Organism | Condition | Number of time points | Approx. #genes |
Ref |
|---|---|---|---|---|---|
| Fournier, Mol Cell Proteomics 2009 |
Yeast | Rapamycin treatment |
7 | 6,000 | 85 |
| Vogel, Mol Cell Proteomics 2011 |
Yeast | Oxidative stress | 8 | 800 | 44 |
| Lee, Mol Sys Bio 2011 | Yeast | Osmotic stress | 6 | 2,500 | 22 |
| Lackner, Genome Biology 2012 |
Yeast (S. pombe) | Oxidative stress | 5 | 2,100 | 86 |
| Gruen, Cell Reports 2014 | Nematode worms | Development | 7 | 3,000 | 87 |
| Ly, eLIFE 2014 | Mammalian cells | Cell cycle | 3-6 | 6,000 | 88 |
| Eichelbaum, Mol Cell Prot 2014 |
Mammalian cells | LPS treatment | 3-4 | 4,800 | 89 |
| Robles, PLoS Gen 2014 | Mammalian cells | Circadian rhythm | 16 | 3,000 | 80 |
| Kristensen, Mol Sys Bio 2013 |
Mammalian cells | Differentiation | 3-5 | 1,900 | 90 |
| Jovanovic, Science 2015 | Mammalian cells | LPS treatment | 6 | 2,300 | 21 |
| Transcription rates |
Rate (median or
typical range) |
||||
| Pelechano, PLoS One, 2010 |
Yeast | Normal | 2 to 30 mRNAs/hr | 4,700 | 26 |
| Miller, Mol Sys Bio 2011 | Yeast | Normal and osmotic stress |
1 to 600 mRNAs/cell cycle |
5,200 | 29 |
| Schwanhaeusser, Nature 2011 |
Mammalian cells | Normal | 2 mRNA/hr | 5,000 | 23 |
| RNA degradation |
Half-life (median or
typical range) |
||||
| Wang, PNAS, 2002 | Yeast | Normal | 20 min (3 to 90 min) | 4,700 | 91 |
| Neymotin, RNA, 2004 | Yeast | Normal | 15 min | 5,200 | 30 |
| Miller, Mol Sys Bio, 2011 | Yeast | Normal and osmotic stress |
11 min | 5,200 | 29 |
| Munchel, Mol Sys Bio, 2011 |
Yeast | Normal and stress | 20 min | 5,200 | 92 |
| Yang, Genome Res, 2003 | Mammalian cells | Normal | 2 hrs | 1,000s | 93 |
| Dolken, RNA, 2008 | Mammalian cells | Normal | 20 min to 48 hrs | 1,000s | 94 |
| Friedel, Nucl Acid Res, 2009 |
Mammalian cells | Normal | 4.5 to 5.1 hrs | 8,000 | 95 |
| Schwanhaeusser, Nature 2011 |
Mammalian cells | Normal | 7.6 to 9 hrs | 5,000 | 23 |
| Translation rates |
Rate (median or
typical range) |
||||
| Schwanhaeusser, Nature 2011 |
Mammalian cells | Normal | 140 proteins/(mRNA*hr) |
5,000 | 23 |
| Ingolia, Cell 2011 | Mammalian cells | Normal | 5.6 codons/sec | 20,000 | 34 |
| Protein degradation |
Half-life (median or
typical range) |
||||
| Belle, PNAS 2006 | Yeast | Normal | 4 to 161 min | 3,750 | 96 |
| Christiano, Cell Reports 2014 |
Yeast | Normal | 8.8 to 12.0 hrs | 4,000 | 97 |
| Yen, Science 2008 | Mammalian cells | Normal | 0.5 to 2 hrs | 8,000 | 98 |
| Doherty, J Proteome Res 2009 |
Mammalian cells | Normal | 6 min to 10s of hrs | 600 | 31 |
| Price, PNAS 2010 | Mammalian cells | Normal | 72 to 216 hrs | 2,500 | 99 |
| Cambridge, J Prot Res 2011 |
Mammalian cells | Normal | - | 4,100 | 67 |
| Schwanhaeusser, Nature 2011 |
Mammalian cells | Normal | 46 hrs | 5,000 | 23 |
| Boisvert, Mol Cell Proteomics 2012 |
Mammalian cells | Normal | 20 hrs | 8,000 | 100 |
Similarly, new computational tools have emerged that enable statistical significance analysis of these time course experiments to extract regulatory information (for focused reviews, see 19, 20). For example, a recent study by Jovanovic et al. used a mathematical model involving differential equations to estimate translation and protein degradation rates from time-series, pulsed labeling data 21. Earlier work in yeast 22 and human 23 applied similar approaches: the change of protein concentration over time is modeled as a linear function of protein synthesis based on the mRNA concentration and a translation rate, and degradation, based on the existing protein concentration and a degradation rate. While a linear model is a very simple approach, it can still reproduce comparatively complex concentration changes for a large fraction of observed patterns 24. To quantify the contributions of the different regulatory levels and identify genes and time points at which these significant changes occur, we recently developed a statistical framework called Protein Expression Control Analysis (PECA). PECA transforms time-course mRNA and matching protein expression data into significance measures of regulation at both the RNA or protein level, resolved at a per-time-point basis 25.
Such computational analyses of time-series data are vital to progress to the next stage of gene expression analysis: the dynamics of regulatory systems. They provide highly specific types of information at the level of individual genes, but also, in conjunction with other, orthogonal information, describe emerging properties of the networks that regulate gene expression. For example, time series data can verify the functional interactions between regulators and their targets and is instrumental to identification of causal relationships 20. Further, when we applied PECA to various yeast time series datasets 25, we detected significantly changing genes at a per-time-point basis and some cases of regulatory ‘buffering’, i.e. synthesis of mRNA molecules that were counteracted by degradation of proteins (and vice versa) – observations that would be hidden in static data.
Measuring rates of synthesis and degradation
Recent technological developments now allow researchers to move beyond descriptions of concentrations to experimental measurements of molecular synthesis and degradation rates (Table S1). Classic approaches to estimate rates of mRNA degradation involve shutting off transcription with either drugs (e.g. actinomycin and thiolutin) or temperature sensitive yeast mutants of RNA polymerase II (rpb1-1)9(Table S1). After inhibiting synthesis, the decreasing molecule concentrations are fit to a decay function to estimate degradation rates. However, these approaches have two main disadvantages. First, degradation may not follow the assumed decay function. Second, these approaches severely disrupt cellular homeostasis, and therefore provide poor estimates of the actual degradation rates. For example, thiolutin has been shown to inhibit both mRNA synthesis and degradation in yeast26, 27. Even the well-regarded rpb1-1 mutation system in yeast appears to decrease transcription only 3-fold at the non-permissive temperature, with rates recovering after about one hour 28. As a result, there is little concordance among mRNA degradation rates measured in different studies.
To circumvent these problems, methods have been developed to measure degradation rates in the absence of inhibitors. Instead, pulsed labeling is used to mark preexisting and newly synthesized mRNAs or proteins. In the case of RNA, several techniques pulse-label RNA with 4-thio-uridine, a non-disruptive analog that can be biochemically enriched after RNA preparation 29, 30(Table S1). A time-resolved comparison of the labeled and unlabeled molecules can then be used to estimate both transcription and RNA degradation rates. Datasets first emerged for yeast, but are also now available for mammalian cells 23.
Similar logic applies to measuring protein synthesis and degradation. Time-resolved proteomics measurements of differently labeled amino acids, in approaches such as pulsed- and dynamic-SILAC31, 32, have provided rate estimates for both yeast and mammalian cells (Table S1). One challenge with proteomics-based measurements is insufficient coverage, which is further decreased when several time points are required. Another challenge lies in the rate of label incorporation into the proteins, which can lead to small mass spectrometric peaks below the detection limit. Therefore it is very difficult to measure synthesis rates for proteins that are rarely translated. To circumvent this challenge, novel approaches have been developed which use a methionine-analog or a tagged puromycin translation inhibitor to specifically enrich for newly synthesized proteins (Table S1). Such approaches, increase sensitivity and coverage of the translation rate measurements – but have they have their own disadvantages by the methionine analog affecting cellular homeostasis and not easily penetrating thick cell walls such as those of yeast.
Ribosome profiling – generating hypotheses on translation regulation
Often, it is not the actual rate that is most interesting in an experiment, but an estimate of the efficiency of the process – it may be more informative to learn how a rate is regulated in the cell rather than to compare two rate values. Recent years have seen much excitement about a new method that combines the resolution and deep coverage of next-generation sequencing with measurements of translation efficiency – and to generate hypotheses on the mechanisms underlying translation regulation. The method is interchangeably called ribosome profiling, ribosome footprinting, or Ribo-seq (Table S1) 33.
The basic approach is outlined in Table S1 and Figure 2. The data generated by ribosome profiling can provide both bulk translation efficiency estimates per gene and nuanced mechanistic details of translation with respect to regulatory sequence elements in the mRNA. While standardized computational methods for data analysis are still under development, the relative number of ribosomes translating an open reading frame (ORF) can be estimated by tallying the number of reads that cover each ORF. By comparing these estimates of ribosome load with RNA-abundance estimates, the relative efficiency of translation (RPF/mRNA) can be measured. No other method provides this information simultaneously at system-wide scale.
Figure 2. Ribosome profiling.
Cell cultures are treated with cycloheximide and lysed in a buffer that maintains ribosome/mRNA associated polysomes. Polysomes are split into two fractions. Nuclease digestion of one fraction removes mRNA fragments not protected by ribosomes. Ribosome protected fragments (RPFs) are then purified and cloned into high-throughput sequencing libraries (left). mRNA is purified from the second fraction, fragmented by base hydrolysis, and cloned into sequencing libraries (right). Libraries are then sequenced to deep coverage.
However, ribosome footprinting also has some disadvantages. First, it is one of the most difficult experimental methods in RNA biology, involving many different steps. Second, unless used in combination with different translation inhibitors and time-resolved measurements34, it does not provide actual rate estimates. Furthermore, ribosome footprinting assumes that the number of mRNA-bound ribosomes correlates with translation efficiencies. Although this is likely to be a reasonable assumption, a direct comparison of ribosome footprinting and proteomics-based identification of newly synthesized proteins is still needed. Finally, the slow uptake of translation inhibitors (which may be an essential part of the method) can complicate studies in yeast 35.
New insights into gene expression regulation
For many years, comparisons of protein and mRNA concentrations have been limited to steady-state measurements. Under these conditions, the population averages of protein and mRNA concentrations in unperturbed cells do not vary over time, with the molecules produced and degraded simultaneously at equilibrium rates. Excitingly, for both baker’s and fission yeast and mammalian cells, several time-series datasets of protein and mRNA expression are now available, complemented by a few measurements of synthesis and degradation rates (Table 1). These data allow for early insights into the principles governing gene regulation in dynamic systems, i.e. cells responding to a stimulus. The following sections highlight what we think might be general trends and future perspectives in light of these recent advances.
Relationships between concentrations and rates
The first question that has been asked for many years addresses the correlation between mRNA and protein concentrations within one organism growing at steady state36. A perfect correlation between mRNA and protein concentrations would suggest no gene-specific differences in translation or protein degradation – but reality is far from that. By 2009, several estimates for the protein-mRNA correlation under steady state were available36, but no common trend across bacteria and yeast was observed. For mammalian cells, both a computational study in 2010 and an experimental approach in 2011 concluded that “transcription is only half the story”37 and protein-level regulation may be as important as that of the RNA-level23, 38. Similarly, in synchronized cells at different cell cycle stages, post-transcriptional regulation has been observed for much of the proteome39. However, these findings have been disputed, and reanalysis of the 2011 data showed that transcription may indeed do the majority of the regulatory workload, accounting for 56 to 81% of the overall variation in gene expression40.
Moving from steady-state to dynamic systems, an experimental study in dendritic cells responding to lipopolysaccharide (LPS) treatment estimated that mRNA levels (set by both transcription and degradation) explain 59 to 68% of the variance in protein levels21 – again placing the main workload in gene expression regulation on transcription. The authors find that RNA-level regulation governs the response of newly synthesized proteins, while protein-level regulation is more important for concentration adjustments of preexisting proteins with basic cellular functions 21. During the LPS response, mRNA changes drive the overall expression response, and even for down-regulated mRNAs the discrepancy to changes in protein concentrations can be explained by a combination of slow and delayed protein degradation and translation21. The study partially contrasts earlier findings in yeast responding to osmotic stress, where the correlation between protein and mRNA abundances was stronger for up-regulated than for down-regulated genes, suggesting that protein-level regulation is dominant with respect to protein removal22. One of the reasons for these drastic reprogramming choices might lie in the fact that these studies subjected cells to very large and rapid perturbations. Further nuances and implications of the relationship between protein and mRNA concentrations in the cell are discussed in an excellent review in ref. 41.
A second, very basic question involves the ranges of concentrations and rates that were found – and again a somewhat surprising picture emerged. For example, although RNA-seq is arguably more sensitive compared to proteomics – collecting millions of reads compared to tens of thousands of spectra – the dynamic range of mRNA concentrations seems consistently smaller than that of protein concentrations 23, 42. For example, RNA concentrations in mammalian cells vary over four to five orders of magnitude, starting from 0.1 molecule per mammalian cell on average – while protein concentrations have been found to cover up to six or even up to 12 orders of magnitude 18, 42. For example, ribosomes each contain dozens of ribosomal proteins and exist in copy numbers as high as ten million per mammalian cell 43.
Similarly, the rates of synthesis and degradation vary enormously between genes, but are in general much larger at the protein level compared to the RNA level (Figure 1). For example, yeast transcription rates range between 0.03 and 0.5 mRNA copies per minute and RNA half-lives range from 2 to 60 minutes, but vary under different conditions (Table S1)26, 28, 30. Transcription rates in mammalian cells range between 0.1 and 100 mRNA copies per cell per hour 23. In comparison, proteins are much more stable than mRNA, with median half-lives of hours if not days, and mammalian translation rates vary from 0.1 to 105 proteins per mRNA per hour across genes (Table 1). However, estimates gained from ribosome profiling analyses placed this range much smaller, spanning only two orders of magnitude33 – a finding that might be due to technical limitations. In sum, proteins, compared to mRNA, have a larger dynamic range in rates of synthesis and degradation, delivering one explanation for the fact that overall protein concentrations are much larger than mRNA concentrations.
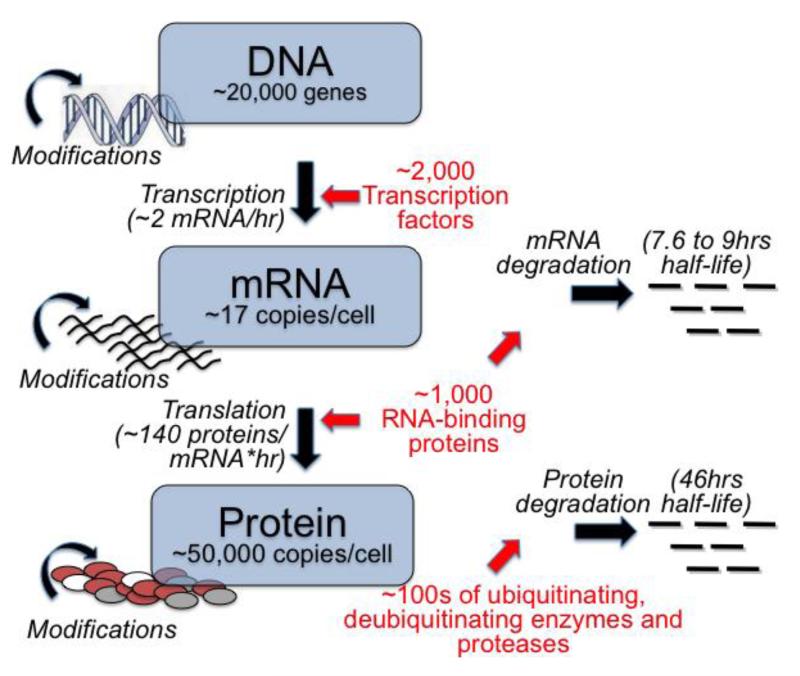
Figure 1. Annotating the Central Dogma of Molecular Biology.
An illustrated version of the Central Dogma of Molecular Biology shows that, thanks to the emergence of new technologies, we can now quantify the concentrations and rates that produce and degrade mRNAs and proteins. Estimates are for mammalian cells, taken from different sources (see text and mainly ref. 23). Numbers are for illustration purposes and represent overall estimates.
Speculations on reasons and consequences of different rates
As a refined view of gene expression regulation slowly emerges (Figure 1), we can begin to ask questions as to why rates of mRNA and protein synthesis and degradation may have evolved to their current values, and how, these rates produce very different expression response patterns to internal or environmental challenges on a per-gene basis. This question is especially interesting if one assumes that well-adapted biological systems must be capable of mounting large and rapid responses to the fluctuations found in the wild, while remaining robust to transient perturbations.
The ability to change concentrations rapidly and drastically depends greatly on the absolute concentration: it is much easier to change small concentrations than large ones. As described above, mRNAs are usually expressed at concentrations lower than those of proteins (Figure 1), which allows for very fast and large fold-changes while not requiring much absolute synthesis and degradation due to low molecule numbers. In contrast, changing the concentrations of proteins, which are often higher than those of the corresponding mRNAs, by only a small amount requires enormous efforts with respect to translation or degradation and a large energy expense. Therefore unsurprisingly, stimulus-dependent fold-changes observed at the mRNA-level are often much larger than those seen for proteins21, 22, 44. This consideration is perhaps one explanation for the small effects on translation often seen by regulators such as miRNAs and RNA-binding proteins45, 46.
Gene functions may place particular constraints on mRNA and protein synthesis and degradation rates. For example, consider the differing requirements of transcription factors (TFs) and ribosomal proteins (RPs). During vegetative growth, TFs often function with few protein copies while RPs are required at very high copy number. However, the two classes of genes may have similar mRNA concentrations (Figure 3, example I). Upon a stimulus, transcription factors are often the first responders to cellular signals, requiring rapid production and fold changes in a switch-like manner. Such a fast response could be enabled by rapid translation or slow protein degradation. Therefore, it is unsurprising that ribosomes and other highly expressed proteins have large translation rates which change drastically during a stimulus to enable changes in absolute protein copy numbers34, 47. Similarly, the temporal response patterns might differ based on gene function. As a first line of response to signals, transcription factors often require rapid production and large fold-changes in a switch-like manner, while house-keeping genes such as ribosomes or enzymes may not need such a fast response. The low total concentrations of TFs also enables their rapid degradation, leading to pulse-like responses.
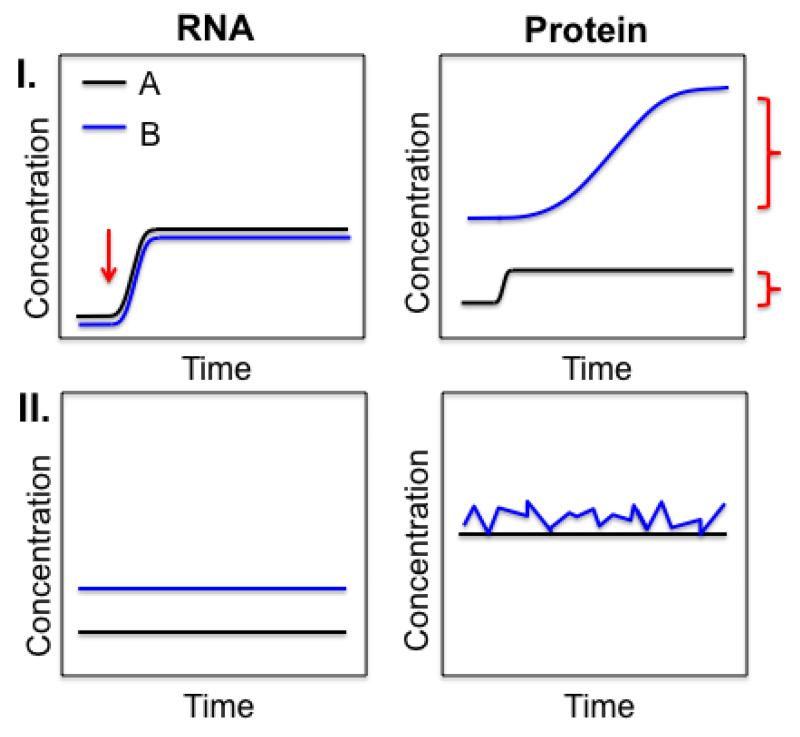
Figure 3. Possible outcomes of different rates of synthesis and degradation.
Different rates of synthesis and degradation can result in different concentrations and concentration changes over time. Example I. While having similar RNA concentrations, protein B (e.g. a transcription factor) is much more abundant than protein A (e.g. a ribosomal protein). Upon a stimulus (red arrow), RNA concentrations switch to a new steady-state through increased transcription. For protein B, despite large changes in translation rate, the response time to double the protein concentration (red bracket) is much slower compared to protein A. Example II. At steady-state, transcription for protein B is faster than for protein A which, at similar RNA degradation rates, results in B’s RNA being more abundant than that for A. The resulting protein concentrations might be very similar. However, due to comparatively slow translation, B’s protein concentration is much noisier over time than that for A.
In addition to the desired signal shape, concentration, and response time, different genes may differ in their requirements for accuracy and robustness to variation in gene expression levels – which can be achieved by specific combinations of transcription and translation rates (Figure 3, example II). For some genes, the accuracy in producing the correct amount might be crucial for their function, while others function well even if their cell-to-cell concentration varies. Therefore, the concentrations of some proteins may change stochastically over time more than those for others. A computational study in yeast suggested such a scenario: high transcription rates combined with low translation rates lead to less noise in final protein concentrations than the opposite case48.
Interestingly, recent work suggests that gene expression noise is subject to purifying selection for at least one yeast gene 49. Thus, “Nothing in Biology Makes Sense Except in the Light of Evolution” (Theodosius Dobzhansky, 1973) may also apply to the evolution of different rates of synthesis and degradation – and the time is ripe for models and hypotheses that explain these selection processes.
Ribosome profiling glances into mechanisms of translation regulation
Ribosome profiling reports ribosome positions at the level of codons or even nucleotides – providing unique insights into possible mechanisms that affect translation efficiency of genes, for example with respect to codon usage. Although many codons can be used to specify the same amino acid, most organisms show clear usage preferences. Genes with abundant mRNA generally use codons that are decoded by abundant tRNA, suggesting that this codon usage bias contributes to increased translation efficiency of abundant mRNA transcripts. To examine this relationship, many researchers have attempted to extract codon-specific translation elongation rates from ribosome profiling data (Table 1), however these studies resulted in markedly varying conclusions. Some suggest that rare codons stall translation50, but this effect is not seen after correcting for amplification and sequencing biases present in ribosome profiling datasets51. However, all of this work was done with datasets from yeast grown in log-phase at steady-state, and, similar to the discussions above, it is possible that the codon usage bias becomes more important during dynamic gene expression regulation, e.g. during meiosis or mating.
Further, mapping the genomic locations of ribosome profiling reads has revealed that ribosomes often bind to unexpected regions in the RNA. The most prominent of these include eukaryotic mRNA transcript leader sequences (TLSs, also known as 5’ UTRs). Translation within TLSs often occurs in regions termed upstream ORFs (uORFs). While uORF translation has been known for decades anecdotally52, ribosome profiling suggested that it may be much more widespread than previously appreciated and that uORFs often make use of start codons differing from the canonical AUG sequence 53. In fact, roughly ~10,000 uORFs are proposed to function in translation regulation during yeast meiosis54.
Other ribosome profiling experiments revealed that ribosomes often associate with RNAs thought to be non-coding. For example, Ingolia and colleagues reported that candidate long non-coding RNAs (lncRNAs) from mouse embryonic stem cells were often bound by ribosomes34. Other data support translation of short ORFs in non-coding RNAs 55, 56, however further evidence indicates that most ncRNAs do not encode functional proteins57, 58 – raising the question whether binding by ribosomes indeed leads to translation of the mRNA or not. One possibility is that ribosome association may function as a surveillance mechanism to degrade improperly localized ncRNA via nonsense mediated decay.
Conservation and divergence of concentrations and rates
Above we discussed the impact and consequences of the variation in mRNA and protein concentrations across genes within one organism. We now examine the role of these processes across organisms, during evolution (Figure 4). For example, mRNA abundances of orthologous genes vary greatly between species. This variation has been observed in comparisons across kingdoms, including yeast59, Drosophila60, 61, mice62, and humans63. Despite this widespread variation in mRNA abundance, more recent work suggests that protein abundance is less divergent. In a comparison of D. melanogaster and C. elegans proteome and transcriptome expression data, Schrimpf and colleagues discovered that the correlation between these species’ protein abundance was higher than that seen for their mRNA abundance64. Soon after, Laurent et al. expanded this analysis to eight organisms and reported that increased conservation of protein abundance could be found in comparisons across all domains of life, including, E. coli, S. cerevisiae, and humans65. More recently, the same phenomenon was observed in comparisons of lymphoblastoid cell lines derived from humans, chimpanzees, and rhesus macaques66: selection pressures to conserve protein concentrations across organisms appear to be higher than those on mRNAs. A study in mouse and human cells showed that strong conservation may also apply to protein degradation rates 67 – although rates appear to vary across subcellular localizations68.
Figure 4. Comparisons of mRNA and protein concentrations among and between species.
Shown are simple comparisons of mRNA and protein concentrations, which revealed a surprising observation when compared across organisms (see text, ref. 84). A. mRNA and protein levels are positively correlated in both C. elegans and H. sapiens. B. Interspecies comparisons show higher conservation of protein levels than of mRNA. As more datasets become available, we are beginning to understand how mRNA and protein synthesis and degradation rates contribute to the evolution of gene expression.
The fact that variation in mRNA abundance between species is not necessarily mirrored at the protein level suggests that post-transcriptional processes act to buffer evolutionary changes in expression regulation69. Indeed, comparisons of mRNA abundance and turnover rates in yeast revealed that mRNA degradation often offsets evolutionary differences in mRNA levels70. Other work has found that translation regulation buffers species differences in mRNA abundance71, 72. These studies compared translation efficiency and mRNA abundance in S. cerevisae and S. paradoxus using ribosome profiling and found that roughly a quarter of the transcriptome exhibited changes in translation efficiency that were biased toward reducing interspecies differences in protein production. While the observed extent of this “translational buffering” varies between studies73, a more recent comparison of S. cerevisiae and S. uvarum (bayanus) identified even more translational buffering than earlier studies74, potentially due to precise control of environmental differences by co-culturing the two species.
The molecular mechanisms underlying post-transcriptional buffering remain unclear. Interestingly, many trans-acting factors have been found responsible for buffering via mRNA turnover70 and translation72. In many of these cases, buffering is likely mediated by proteins that function at multiple levels of gene expression. For example, the yeast RNA binding protein Rpb4 appear to affect both transcription and translation, as do the CCR4/Not complex and Not5ref.75. In other cases, buffering of species differences in mRNA levels was mediated by cis-acting factors. For example, swapping promoter elements between S. cerevisiae and S. paradoxus was sufficient to reproduce species differences in mRNA turnover76. Interestingly, promoter sequences have also been shown to determine the subcellular localization and translation efficiency of mRNAs induced during glucose starvation in yeast77. Regardless of the exact mechanisms responsible, it appears that transcription, mRNA turnover, and translation are intimately coupled in yeast in a manner that generally increases the robustness of gene expression, i.e. the conservation of protein expression levels across organisms.
Outlook
Large-scale methods are now in place to measure both mRNA and protein concentrations and their rates of synthesis and degradation (Table S1), and first datasets have become available that describe these aspects of gene expression regulation in dynamic systems (Table 1)16. After some earlier insights, we may wonder where the field might be headed. One of the next goals should be to obtain more time-series datasets from different organisms and tissue types under a variety of conditions. These data will help evaluate the general trends already observed in yeast and mammalian cells (Table 1). For example, studies in yeast have shown that under stress, the cells appear to strictly regulate either synthesis or degradation of a given protein, but not both24, and we do not know if this finding is conserved across organisms.
Additional datasets would also help inform the discussion on the relative importance of transcription versus translation. As often in biology, the answer may be a diplomatic “it depends” – on the type of stimulus, the amount of protein that is needed, the response time required, the desired signal shape, or the required accuracy in exact copy numbers (Figure 3). Different rates can achieve these scenarios. For example, in cells responding to LPS treatment, transcription plays enables rapid synthesis of functionally relevant proteins, while protein degradation acts more slowly and removes pre-existing functions21. In comparison, translation regulation plays major roles in both yeast and mammalian cell responses to environmental challenges or the circadian rhythm44, 78-80. Evidence for coupling among transcription, translation, and mRNA turnover processes further complicates this picture – and we are only beginning to understand the impact of such coupling12.
To evaluate how much these first insights apply in general – across conditions or organisms – we need not only more datasets, but also tools to analyze the data efficiently, specifically incorporating the dynamics of gene expression changes. While the quantitative analysis of RNA-sequencing and shotgun proteomics data is comparatively standardized, complex methods, such as ribosome profiling still lag behind with respect to informatics tools. The largest challenges lie in appropriate normalization and internal calibration to obtain, for example, reliable quantification of changes in ribosome binding between different regions of an mRNA or cross-normalization of transcription and translation data.
The future will likely also bring more integrative studies that combine several different techniques to examine multiple aspects of gene expression within one system, e.g. proteomics and transcriptomics measurements combined with translation and degradation assays. Importantly, theses studies need to carefully consider which type of measurement fits the biological question best and the relative sources of bias and error inherent to each method. While providing essential information on the mechanisms of translation regulation, ribosome profiling does not necessarily provide translation rates; a type of information gained from pulsed-labeling proteomics techniques (Table S1). Future integrative studies will demand increasingly advanced analysis techniques – an area that will also see much future development. Mathematical techniques, such as higher order singular value decomposition81-83, may be used to extract patterns that are common and specific to sets of diverse data matrices. We are at the beginning of an exciting era that moves towards a new dimension of gene expression analysis: that of the dynamics of a response, and the intricate interplay between the multiple regulatory processes that control protein production.
Supplementary Material
Acknowledgements
We thank Hyungwon Choi and Justin Rendleman for valuable feedback on the manuscript. C.V. acknowledges funding by the NSF (EAGER), the NIH (Ro1 GM113237), NYU Whitehead Foundation, the NYU URCF, and the Zegar Family Foundation Fund for Genomics Research at New York University. We apologize to all authors whose work was not cited here due to length restrictions.
References
- 1.Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM. Nature Publishing Group. 2009;10(4):252–263. doi: 10.1038/nrg2538. [DOI] [PubMed] [Google Scholar]
- 2.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, Wyler E, Bonneau R, Selbach M, Dieterich C, Landthaler M. Mol Cell. 2012;46(5):674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Davey NE, Humphreys DT, Preiss T, Steinmetz LM. Cell. 2012 [Google Scholar]
- 4.Friedländer MR, Lizano E, Houben AJS, Bezdan D, Báñez-Coronel M, Kudla G, Mateu-Huertas E, Kagerbauer B, González J, Chen KC, Leproust EM, Martí E, Estivill X. Genome biology. 2014;15(4):R57. doi: 10.1186/gb-2014-15-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komander D, Clague MJ, Urbe S. Nat Rev Mol Cell Biol. 2009;10(8):550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 6.Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, Barnes I, Bignell A, Boychenko V, Hunt T, Kay M, Mukherjee G, Rajan J, Despacio-Reyes G, Saunders G, Steward C, Harte R, Lin M, Howald C, Tanzer A, Derrien T, Chrast J, Walters N, Balasubramanian S, Pei B, Tress M, Rodriguez JM, Ezkurdia I, van Baren J, Brent M, Haussler D, Kellis M, Valencia A, Reymond A, Gerstein M, Guigo R, Hubbard TJ. Genome Research. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, Slobodeniuc V, Kutter C, Watt S, Colak R, Kim T, Misquitta-Ali CM, Wilson MD, Kim PM, Odom DT, Frey BJ, Blencowe BJ. Science. 2012;338(6114):1587–1593. doi: 10.1126/science.1230612. [DOI] [PubMed] [Google Scholar]
- 8.T.U. Consortium Nucleic acids research. 2015;43(D1):D204–D212. [Google Scholar]
- 9.Parker R. Genetics. 2012;191(3):671–702. doi: 10.1534/genetics.111.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komander D. Biochem Soc Trans. 2009;37(Pt 5):937–953. doi: 10.1042/BST0370937. [DOI] [PubMed] [Google Scholar]
- 11.Komander D. Sub-cellular biochemistry. 2010;54:69–87. doi: 10.1007/978-1-4419-6676-6_6. [DOI] [PubMed] [Google Scholar]
- 12.Dahan O, Gingold H, Pilpel Y. Trends Genet. 2011;27(8):316–322. doi: 10.1016/j.tig.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Braun KA, Young ET. Molecular and cellular biology. 2014 [Google Scholar]
- 14.Presnyak V, Alhusaini N, Chen Y-H, Martin S, Morris N, Kline N, Olson S, Weinberg D, Baker KE, Graveley BR, Coller J. Cell. 2015;160(6):1111–1124. doi: 10.1016/j.cell.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi A, Van de Peer Y, Michoel T. Nucleic Acids Res. 2011;39(21):9108–9117. doi: 10.1093/nar/gkr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larance M, Lamond AI. Nat Rev Mol Cell Biol. 2015;16(5):269–280. doi: 10.1038/nrm3970. [DOI] [PubMed] [Google Scholar]
- 17.Vogel C, Marcotte EM. Nat Rev Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaraj N, Wisniewski JR, Geiger T, Cox J, Kircher M, Kelso J, Paabo S, Mann M. Mol Syst Biol. 2011;7:548. doi: 10.1038/msb.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bar-Joseph Z, Gitter A, Simon I. Nat Rev Genet. 2012;13(8):552–564. doi: 10.1038/nrg3244. [DOI] [PubMed] [Google Scholar]
- 20.Bonneau R. Nat Chem Biol. 2008;4(11):658–664. doi: 10.1038/nchembio.122. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic M, Rooney MS, Mertins P, Przybylski D, Chevrier N, Satija R, Rodriguez EH, Fields AP, Schwartz S, Raychowdhury R, Mumbach MR, Eisenhaure T, Rabani M, Gennert D, Lu D, Delorey T, Weissman JS, Carr SA, Hacohen N, Regev A. Science (New York, NY) 2015;347(6226):1259038. doi: 10.1126/science.1259038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MV, Topper SE, Hubler SL, Hose J, Wenger CD, Coon JJ, Gasch AP. Mol Syst Biol. 2011;7:514. doi: 10.1038/msb.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Nature. 2011 doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 24.Tchourine K, Poultney CS, Wang L, Silva GM, Manohar S, Mueller CL, Bonneau R, Vogel C. Mol Biosyst. 2014;10(11):2850–2862. doi: 10.1039/c4mb00358f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teo G, Vogel C, Ghosh D, Kim S, Choi H. 2013 [Google Scholar]
- 26.Pelechano V, Chávez S, Pérez-Ortín JE. PLoS ONE. 2010;5(11):e15442. doi: 10.1371/journal.pone.0015442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelechano V, Pérez-Ortín JE. Yeast. 2008;25(2):85–92. doi: 10.1002/yea.1548. [DOI] [PubMed] [Google Scholar]
- 28.Sun M, Schwalb B, Schulz D, Pirkl N, Etzold S, Lariviére L, Maier KC, Seizl M, Tresch A, Cramer P. Genome Research. 2012;22(7):1350–1359. doi: 10.1101/gr.130161.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller C, Schwalb B, Maier K, Schulz D, Dümcke S, Zacher B, Mayer A, Sydow J, Marcinowski L, Dölken L, Martin DE, Tresch A, Cramer P. Molecular Systems Biology. 2011;7:458. doi: 10.1038/msb.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neymotin B, Athanasiadou R, Gresham D. RNA (New York, NY) 2014;20(10):1645–1652. doi: 10.1261/rna.045104.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doherty MK, Hammond DE, Clague MJ, Gaskell SJ, Beynon RJ. J Proteome Res. 2009;8(1):104–112. doi: 10.1021/pr800641v. [DOI] [PubMed] [Google Scholar]
- 32.Schwanhausser B, Gossen M, Dittmar G, Selbach M. Proteomics. 2009;9(1):205–209. doi: 10.1002/pmic.200800275. [DOI] [PubMed] [Google Scholar]
- 33.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. Science (New York, NY) 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingolia NT, Lareau LF, Weissman JS. Cell. 2011;147(4):789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerashchenko MV, Gladyshev VN. Nucleic Acids Res. 2014;42(17):e134. doi: 10.1093/nar/gku671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Sousa Abreu R, Penalva LO, Marcotte E, Vogel C. Mol Biosyst. 2009;5(12):1512. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plotkin JB. Mol Syst Biol. 2010;6:406. doi: 10.1038/msb.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel C, de Sousa Abreu R, Ko D, Le S-Y, Shapiro BA, Burns SC, Sandhu D, Boutz DR, Marcotte EM, Penalva LO. Molecular Systems Biology. 2010;6:1–9. doi: 10.1038/msb.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aviner R, Geiger T, Elroy-Stein O. Genes & Development. 2013;27(16):1834–1844. doi: 10.1101/gad.219105.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JJ, Bickel PJ, Biggin MD. PeerJ. 2014;2:e270. doi: 10.7717/peerj.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payne SH. Trends Biochem Sci. 2015;40(1):1–3. doi: 10.1016/j.tibs.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marguerat S, Schmidt A, Codlin S, Chen W, Aebersold R, Bahler J. Cell. 2012;151(3):671–683. doi: 10.1016/j.cell.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.B.S.f.C. Biology.
- 44.Vogel C, Silva GM, Marcotte EM. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M111.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 46.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraser HB, Hirsh AE, Giaever G, Kumm J, Eisen MB. PLoS Biol. 2004;2(6):e137. doi: 10.1371/journal.pbio.0020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzger BP, Yuan DC, Gruber JD, Duveau F, Wittkopp PJ. Nature. 2015;521(7552):344–347. doi: 10.1038/nature14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuller T, Veksler-Lublinsky I, Gazit N, Kupiec M, Ruppin E, Ziv-Ukelson M. Genome biology. 2011;12(11):R110. doi: 10.1186/gb-2011-12-11-r110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artieri CG, Fraser HB. Genome Research. 2014;24(12):2011–2021. doi: 10.1101/gr.175893.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinnebusch AG. CRC critical reviews in biochemistry. 1986;21(3):277–317. doi: 10.3109/10409238609113614. [DOI] [PubMed] [Google Scholar]
- 53.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Science. 2009 doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. Science (New York, NY) 2011;335(6068):552–557. doi: 10.1126/science.1215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingolia NT. Nature Publishing Group. 2014;15(3):205–213. doi: 10.1038/nrg3645. [DOI] [PubMed] [Google Scholar]
- 56.Smith JE, Alvarez-Dominguez JR, Kline N, Huynh NJ, Geisler S, Hu W, Coller J, Baker KE. CellReports. 2014;7(6):1858–1866. doi: 10.1016/j.celrep.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guttman M, Russell P, Ingolia NT, Weissman JS, Lander ES. Cell. 2013;154(1):240–251. doi: 10.1016/j.cell.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bánfai B, Jia H, Khatun J, Wood E, Risk B, Gundling WE, Kundaje A, Gunawardena HP, Yu Y, Xie L, Krajewski K, Strahl BD, Chen X, Bickel P, Giddings MC, Brown JB, Lipovich L. Genome Research. 2012;22(9):1646–1657. doi: 10.1101/gr.134767.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brem RB, Yvert G, Clinton R, Kruglyak L. Science (New York, NY) 2002;296(5568):752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- 60.Ranz JM, Castillo-Davis CI, Meiklejohn CD, Hartl DL. Science. 2003;300(5626):1742–1745. doi: 10.1126/science.1085881. [DOI] [PubMed] [Google Scholar]
- 61.Rifkin SA, Kim J, White KP. Nature Genetics. 2003;33(2):138–144. doi: 10.1038/ng1086. [DOI] [PubMed] [Google Scholar]
- 62.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Nature. 2003;422(6929):297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- 63.Pickrell JK, Marioni JC, Pai AA, Degner JF, Engelhardt BE, Nkadori E, Veyrieras J-B, Stephens M, Gilad Y, Pritchard JK. Nature. 2010;464(7289):768–772. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrimpf SP, Weiss M, Reiter L, Ahrens CH, Jovanovic M, Malmström J, Brunner E, Mohanty S, Lercher MJ, Hunziker PE, Aebersold R, Von Mering C, Hengartner MO. PLoS biology. 2009;7(3):e48. doi: 10.1371/journal.pbio.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laurent JM, Vogel C, Kwon T, Craig SA, Boutz DR, Huse HK, Nozue K, Walia H, Whiteley M, Ronald PC, Marcotte EM. PROTEOMICS. 2010;10(23):4209–4212. doi: 10.1002/pmic.201000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan Z, Ford MJ, Cusanovich DA, Mitrano A, Pritchard JK, Gilad Y. Science. 2013 doi: 10.1126/science.1242379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cambridge SB, Gnad F, Nguyen C, Bermejo JL, Kruger M, Mann M. J Proteome Res. 2011;10(12):5275–5284. doi: 10.1021/pr101183k. [DOI] [PubMed] [Google Scholar]
- 68.Larance M, Ahmad Y, Kirkwood KJ, Ly T, Lamond AI. Mol Cell Proteomics. 2013;12(3):638–650. doi: 10.1074/mcp.M112.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vogel C. Science. 2013;342(6162):1052–1053. doi: 10.1126/science.1247833. [DOI] [PubMed] [Google Scholar]
- 70.Dori-Bachash M, Shema E, Tirosh I. PLoS biology. 2011;9(7):e1001106. doi: 10.1371/journal.pbio.1001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Artieri CG, Fraser HB. Genome Research. 2013 [Google Scholar]
- 72.McManus J, May GE, Spealman P, Shteyman A. Genome Research. 2013 doi: 10.1101/gr.164996.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Albert FW, Muzzey D, Weissman JS, Kruglyak L. PLoS Genet. 2014;10(10):e1004692. doi: 10.1371/journal.pgen.1004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Sun X, Zhao Y, Guo X, Jiang H, Li H, Gu Z. Genome Biol Evol. 2015;7(4):1155–1167. doi: 10.1093/gbe/evv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villanyi Z, Ribaud V, Kassem S, Panasenko OO, Pahi Z, Gupta I, Steinmetz L, Boros I, Collart MA. PLoS genetics. 2014;10(10):e1004569. doi: 10.1371/journal.pgen.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dori-Bachash M, Shalem O, Manor YS, Pilpel Y, Tirosh I. Genome biology. 2012;13(12):R114. doi: 10.1186/gb-2012-13-12-r114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zid BM, O'Shea EK. Nature. 2015;514(7520):117–121. doi: 10.1038/nature13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ventoso I, Kochetov A, Montaner D, Dopazo J, Santoyo J. PLoS One. 2012;7(5):e35915. doi: 10.1371/journal.pone.0035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoeckius M, Grun D, Kirchner M, Ayoub S, Torti F, Piano F, Herzog M, Selbach M, Rajewsky N. EMBO J. 2014;33(16):1751–1766. doi: 10.15252/embj.201488769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robles MS, Cox J, Mann M. PLoS Genet. 2014;10(1):e1004047. doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alter O, Brown PO, Botstein D. Proc Natl Acad Sci U S A. 2003;100(6):3351–3356. doi: 10.1073/pnas.0530258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omberg L, Golub GH, Alter O. Proc Natl Acad Sci U S A. 2007;104(47):18371–18376. doi: 10.1073/pnas.0709146104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponnapalli SP, Saunders MA, Van Loan CF, Alter O. PLoS ONE. 2012;6(12):e28072. doi: 10.1371/journal.pone.0028072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laurent J, Vogel C, Kwon T, Craig S, Boutz DR, Huse H, Nozue K, Walia H, Whiteley M, Ronald P, Marcotte EM. Proteomics. 2010;10(23):4209–4212. doi: 10.1002/pmic.201000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, Bradford WD, Glynn E, Li H, Sardiu ME, Fleharty B, Seidel C, Florens L, Washburn MP. Mol Cell Proteomics. 2009;9(2):271–284. doi: 10.1074/mcp.M900415-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lackner DH, Schmidt MW, Wu S, Wolf DA, Bahler J. Genome Biol. 2012;13(4):R25. doi: 10.1186/gb-2012-13-4-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grun D, Kirchner M, Thierfelder N, Stoeckius M, Selbach M, Rajewsky N. Cell Rep. 2014;6(3):565–577. doi: 10.1016/j.celrep.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Ly T, Ahmad Y, Shlien A, Soroka D, Mills A, Emanuele MJ, Stratton MR, Lamond AI. eLife. 2014;3:e01630. doi: 10.7554/eLife.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eichelbaum K, Krijgsveld J. Mol Cell Proteomics. 2014;13(3):792–810. doi: 10.1074/mcp.M113.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kristensen AR, Gsponer J, Foster LJ. Mol Syst Biol. 2013;9:689. doi: 10.1038/msb.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, Liu CL, Storey JD, Tibshirani RJ, Herschlag D, Brown PO. Proc Natl Acad Sci U S A. 2002;99(9):5860–5865. doi: 10.1073/pnas.092538799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munchel SE, Shultzaberger RK, Takizawa N, Weis K. Mol Biol Cell. 2011;22(15):2787–2795. doi: 10.1091/mbc.E11-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang E, van Nimwegen E, Zavolan M, Rajewsky N, Schroeder M, Magnasco M, Darnell JE., Jr. Genome Res. 2003;13(8):1863–1872. doi: 10.1101/gr.1272403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dolken L, Ruzsics Z, Radle B, Friedel CC, Zimmer R, Mages J, Hoffmann R, Dickinson P, Forster T, Ghazal P, Koszinowski UH. RNA. 2008;14(9):1959–1972. doi: 10.1261/rna.1136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedel CC, Dolken L, Ruzsics Z, Koszinowski UH, Zimmer R. Nucleic Acids Res. 2009;37(17):e115. doi: 10.1093/nar/gkp542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Belle A, Tanay A, Bitincka L, Shamir R, O’Shea EK. Proc Natl Acad Sci U S A. 2006;103(35):13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christiano R, Nagaraj N, Frohlich F, Walther TC. Cell Rep. 2014;9(5):1959–1965. doi: 10.1016/j.celrep.2014.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yen HC, Xu Q, Chou DM, Zhao Z, Elledge SJ. Science. 2008;322(5903):918–923. doi: 10.1126/science.1160489. [DOI] [PubMed] [Google Scholar]
- 99.Price JC, Guan S, Burlingame A, Prusiner SB, Ghaemmaghami S. Proc Natl Acad Sci U S A. 2010;107(32):14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boisvert FM, Ahmad Y, Gierlinski M, Charriere F, Lamont D, Scott M, Barton G, Lamond AI. Mol Cell Proteomics. 2012;11(3) doi: 10.1074/mcp.M111.011429. M111 011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.