Abstract
Objective
There is limited research investigating the possible mechanisms of how starting combination antiretroviral therapy (cART) at a higher CD4+ cell count decreases mortality. This study investigated the association between initiating cART with short-term and long-term achievement of viral suppression; emergence of any drug resistance and of an AIDS-defining illness (ADI); long-term treatment adherence; and all-cause mortality.
Methods
This retrospective cohort study included 4120 naive patients who initiated cART between 2000 and 2012. Patients were followed until 2013, death or until the last contact date (varied by outcome). The main exposure was the interaction between period of cART initiation (2000–2006 and 2007–2012) and CD4+ cell count at cART initiation (<500 versus ≥500 cells/μl). We considered both baseline and longitudinal covariates. We fitted different multivariable models using cross-sectional and longitudinal statistical methods, depending on the outcome.
Results
Patients who initiated cART with a CD4+ cell count at least 500cells/μl in 2007–2012 had an increased likelihood of achieving viral suppression at 9 months and of maintaining an adherence level of at least 95% overtime, and the lowest probability of developing any resistance and an ADI during follow-up. These patients were not the ones with the highest likelihood of maintaining viral suppression over time, most likely due to viral load blips experienced during the follow-up.
Conclusion
The outcomes in this study likely play an important role in explaining the positive impact of early cART initiation on mortality. These results should alleviate some of the concerns clinicians may have when initiating cART in patients with high CD4+s as recommended by current treatment guidelines.
Keywords: adherence, AIDS-defining illness, antiretroviral therapy, combination antiretroviral therapy, CD4+ cell count, drug resistance, loss to follow-up, mortality, viral load, viral suppression
Introduction
With global estimates of around 35 million people living with HIV [1], the HIV/AIDS epidemic continues to be one of the world's most pressing public health challenges. In the last decade, there has been marked progress in combination antiretroviral treatment (cART), with more therapeutic options, safer and better-tolerated drugs, and simpler dosing regimens [2–8]. This has facilitated a shift towards earlier cART initiation to promote immune restoration [9,10] and prevent AIDS-related morbidity, as well as premature mortality [11]. More recently, the use of cART to prevent HIV transmission has fuelled enthusiasm for the implementation of HIV Treatment as Prevention programmes to control the HIV/AIDS pandemic [12–15]. In the summer of 2014, The Joint United Nations Programme on HIV/AIDS (UNAIDS) released a new target for antiretroviral cART roll out by 2020, calling for 90% of HIV-infected individuals to be diagnosed worldwide, 90% of diagnosed individuals to receive cART and 90% of those on cART to achieve sustained viral suppression [16]. The 90–90–90 target is expected to dramatically alter the course of the HIV/AIDS pandemic, transforming it into a sporadic endemic condition by 2030 [16].
Early initiation of cART has been shown to have a consistent beneficial impact on morbidity and mortality [17–24]. Conversely, there is limited research investigating the possible mechanisms of how starting cART at higher CD4+ cell counts (from this point forward referred to just as CD4+) decreases mortality. These mechanisms may include improved long-term treatment adherence and virologic suppression, and decreased incidence of viral resistance. In the existing literature, there are reports that have evaluated the association between CD4+ at cART initiation and loss to follow-up (LTFU). However, these results are conflicting [25–28] and are less relevant, as treatment guidelines have evolved substantially regarding cART initiation eligibility [17,29]. Some studies, although few, have looked at other mechanisms that may be related to mortality outcomes by CD4+ at cART initiation [30–36]. In 2007, Phillips et al. [33] found an increase in triple class failure among people who initiated cART at low a CD4+ (<200 cells/μl). More recently, Lodi et al. [34] reported a decrease in virologic failure and drug resistance in patients who initiated cART early. In 2008, Geretti et al. [30] found no association between CD4+ at cART initiation and risk of virologic failure. Therefore, we conducted this study aiming to investigate the association between initiating cART at different CD4+ cutoffs with short-term and long-term achievement of viral suppression; the emergence of drug resistance; long-term treatment adherence; LTFU; the emergence of an AIDS-defining illness (ADI); and all-cause mortality.
Materials and methods
Data
In British Columbia, Canada, cART, HIV medical care and laboratory monitoring are fully subsidized (free, without copayments or deductibles) by the provincial government since 1992 under the aegis of the BC Centre for Excellence in HIV/AIDS (BC-CfE)'s Drug Treatment Programme (DTP). cART is distributed to eligible patients on the basis of BC-CfE's HIV therapeutic guidelines, which have remained consistent with those put forward by the International Antiviral Society-USA (IAS-USA) since 1996 [17,29,37].
In this analysis, patients were cART naive, aged at least 19 years old, and enrolled in the DTP between 1 January 2000 and 31 December 2012. Within this cohort, cART was initiated with two nucleoside reverse transcriptase inhibitors as backbone, combined with either a non-nucleoside reverse transcriptase inhibitor (efavirenz or nevirapine) or a ritonavir-boosted protease inhibitor (lopinavir or atazanavir), given that these were the most prescribed therapies during the study period. Participants must also have had a CD4+ and a viral load measurement within 6 months of the antiretroviral initiation date. CD4+s are measured by flow cytometry, followed by fluorescent mAb analysis (Beckman Coulter Canada, LP, Mississauga, Ontario, Canada). The CD4+ data come from different laboratories across British Columbia, and the DTP captures approximately 85% of all CD4+ tests done in the province, as these results are not automatically uploaded in our database. All viral load tests in British Columbia are performed at the virology laboratory at St Paul's hospital and are uploaded automatically in the DTP database. As the quantification range of viral load assays has evolved over time, for analytical purposes, we truncated our measurements to range from less than 50 (coded as 49) to more than 100000 (coded as 100010) copies/ml [38–41]. HIV drug resistance genotyping is routinely performed at the BC-CfE virology laboratory on samples with viral loads of at least 250 copies/ml upon physician request. Methods for HIV-1 RNA extraction and drug resistance analysis have been described in detail elsewhere [42]. Mortality data for all causes were provided by the BC Vital Statistics Agency and linked monthly to the DTP. ADI case reports were obtained from the BC-CfE, enriched with clinical records from St Paul's Hospital, the BC Cancer Agency and the BC Vital Statistics Agency.
Statistical analyses
In this study, the main exposure variable included the interaction between the period of cART initiation (2000–2006 and 2007–2012) and CD4+ at cART initiation categorized as less than 500 versus at least 500 cells/μl. In a subanalysis, we also categorized CD4+ as less than 350 versus at least 350 cells/μl. We decided to classify the years of cART initiation into these groups to ensure that we control for any differences that may exist regarding prescribing practices in those years.
The possible explanatory variables varied by study outcome and could include as measured at baseline: age (continuous), sex (male/female), history of injection drug use (no/yes/unknown) and viral load (log10 transformed); measured at 6 or at 12 months since cART initiation: adherence level (0% to <40%, 40% to <80%, 80% to <95% and ≥95%); and measured longitudinally at every 6 months until the end of study follow-up -adherence level (0% to <40%, 40% to <80%, 80% to <95% and ≥95%), viral load (log10 transformed) and follow-up time (defined on the basis of each outcome). Adherence was assessed using a validated refill compliance measurement and defined as the number of days of antiretroviral drugs dispensed divided by the number of days between refills (expressed as a percentage) [43].
Patients LTFU were censored at the last contact date (i.e. the date for a laboratory test, a prescription refill or a physician visit) prior to any of the following events: if they moved outside of British Columbia; if the last contact date was longer than 18 months, if they started cART before 30 June 2012; if the last contact date was before 31 December 2013, if they started cART after 30 June 2012; if they enrolled in a blinded trial involving receiving placebo medication; or if they had a scheduled treatment interruption, given that information on the return date of the patient to the programme is unclear.
Longitudinal outcomes
All outcomes were assessed every 6 months from the date of cART initiation to the end of follow-up and modelled via a multivariable generalized estimating equations explanatory model assuming a binomial distribution, a logit link function and an autoregressive correlation structure of order one [44].
Combination antiretroviral therapy adherence
We modelled the probability of maintaining an adherence of at least 95% over time.
Explanatory variables included age, sex, history of injection drug use, longitudinal viral load and follow-up time. Follow-up time was measured from cART initiation until 31 December 2013 (if alive), to the last contact date (if LTFU), or to the death date.
Viral suppression
We modelled the probability of maintaining viral suppression (a viral load <50 copies/ml) over time.
Explanatory variables included age, sex, history of injection drug use, longitudinal adherence level and follow-up time defined the same way as per the adherence analysis.
HIV drug resistance
Resistant samples were assigned to one of the four classes on the basis of a modification of the 2014 IAS-USA list of mutations [45], as lamivudine/emtricitabine resistance (M184V/I); any other nucleoside reverse transcriptase inhibitor (NRTI) resistance (41L, 62V, 65R, 67N, 69D or insertion, 70E/R, 74V, 75I, 77L, 115F, 116Y, 151M, 210W, 215F/Y or 219E/Q); any nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance (100I, 101E/H/P, 103N, 106A/M, 108I, 138A/G/K/Q/R, 181C/I/V, 188C/H/L, 190A/S, 225H, 230L or 236L); and any protease inhibitor resistance (30N, 32I, 33F, 46I/L, 47A/V, 48V, 50L/V, 54V/L/M, 58E, 74P, 76V, 82A/F/L/S/T, 84V, 88S or 90M). We assumed that samples with a viral load less than 250 copies/ml harboured no resistance. We restricted this analysis to include patients with no resistance prior to starting cART.
We modelled the probability of developing resistance to any class (yes/no) over time, defined by any combination of the above resistance classes.
Explanatory variables included age, sex, history of injection drug use, longitudinal adherence level and viral load, and follow-up time. Follow-up time was measured from cART initiation until 31 December 2013 (if no resistance was detected), to the last contact date (if LTFU before detecting any resistance), to the death date (if deceased before detecting any resistance), or to the date when any resistance was detected.
All-cause mortality
We modelled the probability of being dead by the end of follow-up.
Explanatory variables as per the resistance analysis and follow-up time were defined as per the adherence analysis.
Emergence of an AIDS-defining illness after combination antiretroviral therapy initiation
We modelled the probability of developing an ADI by the end of follow-up.
Explanatory variables are as per the resistance analysis. Follow-up time was measured from cART initiation to 31 December 2013 (if no ADI was reported), to the last contact date (if LTFU before any ADI was reported), to the death date (if deceased before an ADI was reported) or to the date when an ADI was reported.
Cross-sectional outcomes
All outcomes were modelled via a multivariable logistic regression explanatory model.
Loss to follow-up
Analysis was restricted to patients alive at the end of follow-up or those LTFU.
We modelled the probability of being LTFU (yes/no).
Explanatory variables included age, sex, history of injection drug use, baseline viral load, adherence level measured at 12 months since cART initiation and follow-up time. Follow-up time was measured from cART initiation until 31 December 2013 (if alive) or to the last contact date (if LTFU).
Viral suppression within 9 months after combination antiretroviral therapy initiation
We modelled the probability of achieving viral suppression within 9 months after initiating cART (yes/no), defined by two consecutive viral loads less than 50 copies/ml.
Explanatory variables included age, sex, history of injection drug use, baseline viral load, adherence level measured at 6 months since cART initiation and follow-up time. Follow-up time was measured from cART initiation until 31 December 2013 (if no viral suppression), to the last contact date (if LTFU before viral suppression), to the death date (if deceased before viral suppression) or to the viral suppression date.
In both longitudinal and cross-sectional multivariable models, a modified backward stepwise technique, based on Akaike Information Criterion (AIC), or the quasi-AIC for the generalized estimating equation models (QIC), and Type III P values, was used in the selection of explanatory variables [43]. Categorical variables were compared using the Fisher's exact test (for 2 × 2 tables) or the Cochran–Mantel–Haenszel test (for other table sizes), and continuous variables were compared using the Wilcoxon rank-sum test [46]. All analyses were performed using SAS, version 9.3 (SAS, Cary North Carolina, USA).
Results
Table 1 presents the characteristics at cART initiation and treatment outcomes of 4120 patients included in our analyses. The median age was 42 years [25th–75th percentile (Q1–Q3): 35–49], 80% were male, 36% had a history of injection drug use and patients were followed for a median of 60 months (Q1–Q3: 34–95). By the end of the follow-up, we observed that 492 (12%; rate 1.77 per 1000 person-months) patients were deceased and 735 (18%; rate 2.65 per 1000 person-months) were LTFU (Table 1). The distribution of CD4+ at the start of cART based on the strata less than 200, 200–349, 350–499 and at least 500 cells/μl was 44%, 32%, 14% and 10%, respectively. The median viral load at the start of cART was 4.90 log10copies/ml (Q1–Q3: 4.38–5.00); 78% of patients had at least 95% adherence during the first 6 months on cART, 71% achieved viral suppression at 9 months and 7% developed an ADI during follow-up. Baseline resistance was detected in 112 (3%) of patients. Of the 4008 patients without baseline resistance, 448 (11%) developed resistance to any drug class during follow-up.
Table 1. Distribution of patient characteristics and outcomes.
| Covariates and outcomes | n (%) or Median (Q1–Q3) |
|---|---|
| CD4+ cell count (cells/ml) at cART initiation | |
| <200 | 1827 (44%) |
| 200–349 | 1311 (32%) |
| 350–499 | 591 (14%) |
| More than 500 | 391 (10%) |
| Sex | |
| Male | 3328 (80%) |
| Female | 792 (20%) |
| Age (years) | 42 (35–49) |
| Viral load (log10 copies/ml) at cART initiation, median (Q1-Q3) | 4.90 (4.38–5.00) |
| History of injection drug use | |
| No | 1839 (45%) |
| Yes | 1501 (36%) |
| Unknown | 780 (19%) |
| Follow-up (months) | 60 (34–95) |
| Adherence to therapy during the first 6 months on cART | |
| ≥95% | 3219 (78%) |
| 80 to <95% | 213 (5%) |
| 40 to <80% | 455 (11%) |
| 0 to <40% | 233 (6%) |
| Suppression at 9 months | |
| Yes | 2926 (71%) |
| No | 1194 (29%) |
| Drug resistance at cART initiation | |
| No | 4008 (97%) |
| Yes | 112 (3%) |
| Developing resistance to any class during follow-upa | |
| No | 3560 (89%) |
| Yes | 448 (11%) |
| Developing an AIDS-defining illness during follow-up | |
| No | 3815 (93%) |
| Yes | 305 (7%) |
| Status at the end of follow-up | |
| Alive | 2893 (70%) |
| Death | 492 (12%) |
| Loss to follow-up | 735 (18%) |
Q1: 25th percentile, Q3: 75th percentile. cART, combination antiretroviral therapy.
Denominator is the number of patients without baseline resistance.
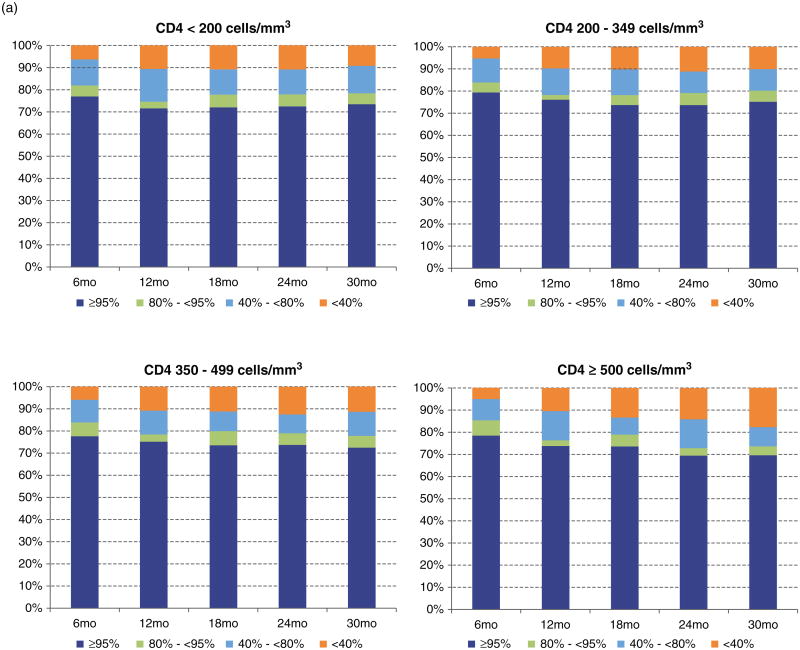
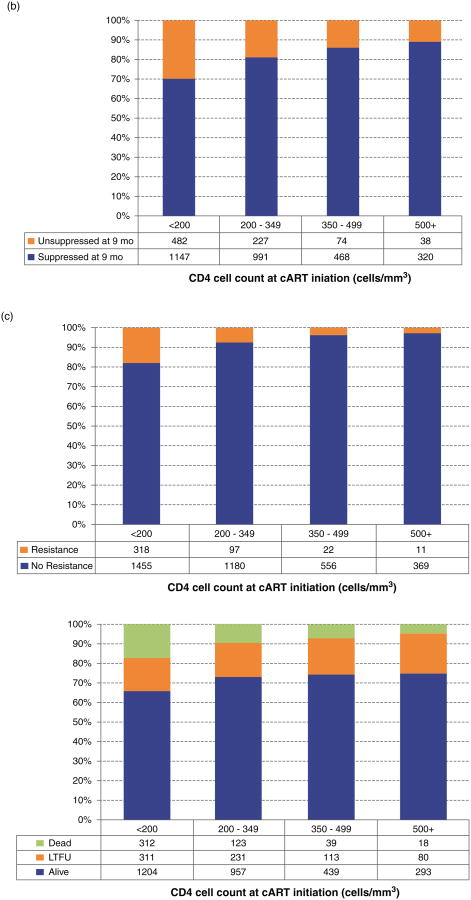
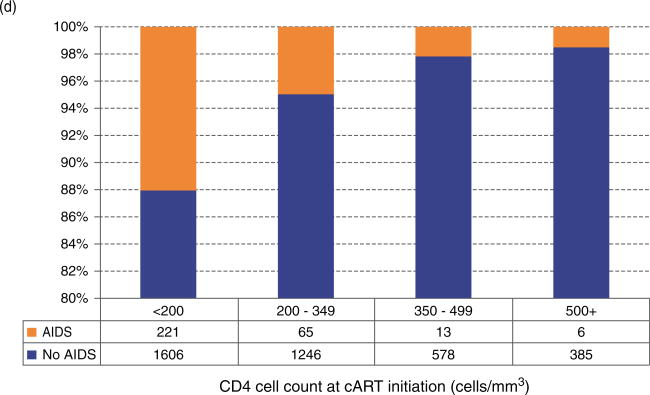
The distribution of patients on the basis of the baseline CD4+ cell count strata was examined by adherence measured over time (Fig. 1a); achievement of viral suppression at 9 months (Fig. 1b); accumulation of drug resistance to any class during follow-up (Fig. 1c); their status at the end of follow-up (Fig. 1d); and by the emergence of an ADI during follow-up (Fig. 1e). The distribution of patients by adherence level over time did not differ significantly in CD4+ strata 350–499 and at least 500 cells/μl (Fig. 1a) (P = 0.3665 and P = 0.0662, respectively). For the other outcomes, we observed that patients in the stratum at least 500 cells/μl were more likely to achieve viral suppression at 9 months, to be alive at the end of follow-up and less likely to develop drug resistance and an ADI during follow-up (P < 0.0001). Interestingly, the proportion of patients LTFU in each CD4+ strata was very similar (P = 0.1606).
Fig. 1. Distribution of CD4+ at the start of combination antiretroviral therapy by different outcomes.
Stratified by: (a) adherence level over time; (b) achievement of suppression in 9 months after combination antiretroviral therapy initiation; (c) the emergence of resistance to any antiretroviral class during follow-up; (d) LTFU: lost to follow-up; (e) The emergence of an AIDS-defining illness during follow-up.
Focusing on our main exposure, the distribution of patients was 1628 (40%) for 2000–2006 and less than 500 cells/μl, 50 (1%) for 2000–2006 and at least 500 cells/μl, 2101 (51%) for 2007–2012 and less than 500 cells/μl, and 341 (8%) for 2007–2012 and at least 500 cells/μl (Table 2a). Although the number of patients in the CD4+ stratum at least 500 cells/μl was small for both periods of time, mostly due to the treatment guidelines in those years, we still obtained highly significant results. For example, we observed that young men, patients with an unknown history of injection drug use, those who initiated cART with a lower viral load and maintained an adherence of at least 95% during the first 6 months on cART were more likely to be classified into stratum 2007–2012 and at least 500 cells/μl (P < 0.0001). In addition, patients who achieved suppression at 9 months, who did not develop an ADI or drug resistance during follow-up and were alive at the end of follow-up were also more likely to be classified into this stratum (P < 0.0001). Of note, there was no difference among exposure groups regarding drug resistance prior to the initiation of cART (P = 0.1321).
Table 2. Bivariable associations between covariates and outcomes, stratified by the period of combination antiretroviral therapy initiation (2000–2006 and 2007–2012) and CD4+ cell count level at combination antiretroviral therapy initiation.
| (a) Using the CD4+ cell count cut-off of 500 cells/ml at cART initiation | |||||
|---|---|---|---|---|---|
| Covariate | Period (years) and CD4+ cell count (cells/μl) | P | |||
|
| |||||
| 2000–2006 and <500 | 2000–2006 and ≥500 | 2007–2012 and <500 | 2007–2012 and ≥500 | ||
| Sex, n (%) | |||||
| Male | 1309 (80%) | 38 (76%) | 1683 (80%) | 298 (87%) | 0.0073 |
| Female | 319 (20%) | 12 (24%) | 418 (20%) | 43 (13%) | |
| Age (years), median (Q1–Q3) | 42 (36–48) | 39 (33–45) | 42 (35–49) | 39 (32–47) | <0.0001 |
| Viral load (log10 copies/ml) at cART initiation, median (Q1–Q3) | 5.00 (4.69–5.00) | 4.88 (4.32–5.00) | 4.80 (4.27–5.00) | 4.46 (4.02–5.00) | <0.0001 |
| History of injection drug use, n (%) | |||||
| No | 710 (44%) | 25 (50%) | 948 (45%) | 156 (46%) | <0.0001 |
| Yes | 674 (41%) | 16 (32%) | 735 (35%) | 76 (22%) | |
| Unknown | 244 (15%) | 9 (18%) | 418 (20%) | 109 (32%) | |
| Follow-up (months), median (Q1–Q3) | 104 (84–129) | 103 (77–127) | 47 (29–65) | 30 (20–42) | <0.0001 |
| Adherence to therapy during the first 6 months on cART, n (%) | |||||
| ≥95% | 1241 (76%) | 29 (58%) | 1670 (80%) | 279 (82%) | <0.0001 |
| 80 to <95% | 74 (5%) | 6 (12%) | 111 (5%) | 22 (6%) | |
| 40 to <80% | 195 (12%) | 10 (20%) | 223 (11%) | 27 (8%) | |
| 0 to <40% | 118 (7%) | 5 (10%) | 97 (5%) | 13 (4%) | |
| Suppression at 9 months, n (%) | |||||
| Yes | 1075 (66%) | 31 (62%) | 1531 (73%) | 289 (85%) | <0.0001 |
| No | 553 (34%) | 19 (38%) | 570 (27%) | 52 (15%) | |
| Drug resistance at cART initiation, n (%) | |||||
| No | 1581 (97%) | 46 (92%) | 2047 (97%) | 334 (98%) | 0.1321 |
| Yes | 47 (3%) | 4 (8%) | 54 (3%) | 7 (2%) | |
| Developing resistance to any class during follow-up, n (%)a | |||||
| No | 1278 (81%) | 42 (91%) | 1913 (94%) | 327 (98%) | <0.0001 |
| Yes | 303 (19%) | 4 (9%) | 134 (6%) | 7 (2%) | |
| Developing an AIDS-defining illness during follow-up, n (%) | |||||
| No | 1432 (88%) | 48 (96%) | 1998 (95%) | 337 (99%) | <0.0001 |
| Yes | 196 (12%) | 2 (4%) | 103 (5%) | 4 (1%) | |
| Status at the end of follow-up, n (%) | |||||
| Alive | 982 (60%) | 26 (52%) | 1618 (77%) | 267 (78%) | <0.0001 |
| Death | 339 (21%) | 11 (22%) | 135 (6%) | 7 (2%) | |
| Loss to follow-up | 307 (19%) | 13 (26%) | 348 (17%) | 67 (20%) | |
| Overall | 1628 (40%) | 50 (1%) | 2101 (51%) | 341 (8%) | |
|
| |||||
| (b) Using the CD4+ cell cut-off 350 cells/μl at cART initiation | |||||
| Period (years) and CD4+ cell count (cells/ml) | |||||
|
|
|||||
| Covariate | 2000–2006 and <350 | 2000–2006 and ≥350 | 2007–2012 and <350 | 2007–2012 and ≥350 | P |
|
| |||||
| Sex, n (%) | |||||
| Male | 1221 (80%) | 126 (78%) | 1292 (80%) | 689 (84%) | 0.0436 |
| Female | 295 (20%) | 36 (22%) | 330 (20%) | 131 (16%) | |
| Age (years), median (Q1–Q3) | 42 (36–48) | 41 (33–48) | 43 (35–50) | 41 (32–47) | <0.0001 |
| Viral load (log10 copies/ml) at cART initiation, median (Q1–Q3) | 5.00 (4.72–5.00) | 4.85 (4.20–5.00) | 4.88 (4.36–5.00) | 4.48 (3.98–4.96) | <0.0001 |
| History of injection drug use, n (%) | |||||
| No | 658 (43%) | 77 (48%) | 721 (44%) | 383 (47%) | <0.0001 |
| Yes | 633 (42%) | 57 (35%) | 613 (38%) | 198 (24%) | |
| Unknown | 225 (15%) | 28 (17%) | 288 (18%) | 239 (29%) | |
| Follow-up (months), median (Q1–Q3) | 104 (84–128) | 109 (86–140) | 51 (33–67) | 33 (22–49) | <0.0001 |
| Adherence to therapy during the first 6 months on cART, n (%) | |||||
| ≥95% | 1157 (76%) | 113 (70%) | 1294 (80%) | 655 (80%) | <0.0001 |
| 80 to <95% | 70 (5%) | 10 (6%) | 79 (5%) | 54 (7%) | |
| 40 to <80% | 183 (12%) | 22 (14%) | 173 (11%) | 77 (9%) | |
| 0 to <40% | 106 (7%) | 17 (10%) | 76 (4%) | 34 (4%) | |
| Suppression at 9 months, n (%) | |||||
| Yes | 1000 (66%) | 106 (65%) | 1138 (70%) | 682 (83%) | <0.0001 |
| No | 516 (34%) | 56 (35%) | 484 (30%) | 138 (17%) | |
| Drug resistance at cART initiation, n (%) | |||||
| No | 1472 (97%) | 155 (96%) | 1578 (97%) | 803 (98%) | 0.3560 |
| Yes | 44 (3%) | 7 (4%) | 44 (3%) | 17 (2%) | |
| Developing resistance to any class during follow-up, n (%)a | |||||
| No | 1178 (80%) | 142 (92%) | 1457 (92%) | 783 (98%) | <0.0001 |
| Yes | 294 (20%) | 13 (8%) | 121 (8%) | 20 (2%) | |
| Developing an AIDS-defining illness during follow-up, n (%) | |||||
| No | 1329 (88%) | 151 (93%) | 1523 (94%) | 812 (99%) | <0.0001 |
| Yes | 187 (12%) | 11 (7%) | 99 (6%) | 8 (1%) | |
| Status at the end of follow-up, n (%) | |||||
| Alive | 916 (60%) | 92 (57%) | 1245 (77%) | 640 (78%) | <0.0001 |
| Death | 316 (21%) | 34 (21%) | 119 (7%) | 23 (3%) | |
| Loss to follow-up | 284 (19%) | 36 (22%) | 258 (16%) | 157 (19%) | |
| Overall | 1516 (37%) | 162 (4%) | 1622 (39%) | 820 (20%) | |
Q1: 25th percentile, Q3: 75th percentile.
Denominator is the number of patients without baseline resistance. P values should be used to compare across categories of our main exposure. Number in parenthesis for n (%) refers to column percentages.
Next, we present the results of the predicted probabilities for each of the multivariable models in our cross-sectional (Table 3) and longitudinal analyses (Table 4). On the basis of our main exposure, we observed that the probability of achieving viral suppression at 9 months and of maintaining an adherence level of at least 95% over time was highest during 2007–2012 [median across patients 0.93: (Q1–Q3: 0.90–0.96) and 0.84 (Q1–Q3: 0.75–0.88), respectively]. Focusing on the outcomes, emergence of any resistance and of an ADI during follow-up and death, patients in the 2007–2012 had by far the lowest probabilities [0.01 (Q1–Q3: 0.01–0.02), 0.01 (Q1–Q3: 0.00–0.01) and 0.01 (Q1–Q3: 0.01–0.02), respectively]. It is important to mention that, for mortality, we run a subanalysis focusing only on the period 2007–2012 and comparing the less than 500 cells/μl and at least 500 cells/μl strata, as these were the strata with the smallest sample sizes. Patients with at least 500 cells/μl CD4+ had the lowest probabilityof dying [0.01 (Q1–Q3: 0.01–0.02) versus 0.03 (Q1–Q3: 0.01–0.07)] (results not shown in Tables 3 and 4). We also observed that patients in the CD4+ stratum at least 500 cells/μl, in both periods of time, had a higher probability of being LTFU and the lowest probability of maintaining viral suppression over time. However, if we focused on comparing these outcomes among patients in the CD4+ strata at least 500 cells/μl between periods 2000–2006 and 2007–2012, we observed that patients in 2007–2012 had the lowest probability of being LTFU [0.19 (Q1–Q3: 0.16–0.23) versus 0.28 (Q1–Q3: 0.23–0.34)] and the highest probability of maintaining viral suppression over time [0.69 (Q1–Q3: 0.58–0.76) versus 0.55 (Q1–Q3: 0.19–0.81)] (results not shown in Tables 3 and 4). Similar results were obtained when looking at the same outcomes for the exposure using the 350 cells/μl cut-off (Tables 2b–4).
Table 3. Results of the cross-sectional analyses focusing on the interaction between the period of combination antiretroviral therapy initiation (2000–2006 and 2007–2012) and CD4+ cell count level at combination antiretroviral therapy initiation (<350 versus ≥350 cells/μl and <500 versus ≥500 cells/ml).
| Variable | Predicted probability of achieving viral suppression in 9 months | Predicted probability of being lost to follow-up |
|---|---|---|
| Period and CD4+ cell count (cells/μl), median (Q1–Q3) | ||
| 2000–2006 and <350 | 0.83 (0.70–0.84) | 0.18 (0.11–0.27) |
| 2000–2006 and ≥350 | 0.83 (0.62–0.87) | 0.23 (0.14–0.37) |
| 2007–2012 and <350 | 0.83 (0.70–0.87) | 0.14 (0.09–0.22) |
| 2007–2012 and ≥350 | 0.93 (0.90–0.96) | 0.19 (0.12–0.26) |
| Period and CD4+ cell count (cells/μl), median (Q1–Q3) | ||
| 2000–2006 and <500 | 0.78 (0.60–0.81) | 0.11 (0.18–0.28) |
| 2000–2006 and ≥500 | 0.70 (0.40–0.82) | 0.16 (0.22–0.41) |
| 2007–2012 and <500 | 0.82 (0.66–0.87) | 0.09 (0.15–0.23) |
| 2007–2012 and ≥500 | 0.90 (0.86–0.94) | 0.13 (0.19–0.25) |
Q1: 25th percentile, Q3: 75th percentile. Models were adjusted for predicted probability of achieving viral suppression in 9 months (for CD4+ cell count cut-off 350 cells/μl) – adherence to therapy during the first 6 months on cART, viral load at cART initiation and history of injection drug use; predicted probability of achieving viral suppression in 9 months (for CD4+ cell count cut-off 500 cells/μl) – sex, adherence to therapy during the first 6 months on cART, viral load at cART initiation and history of injection drug use; predicted probability of being lost to follow-up (for CD4+ cell count cut-off 350 cells/μl) – age, adherence to therapy during the first 12 months on cART, history of injection drug use, viral load at cART initiation and follow-up time; predicted probability of being lost to follow-up (for CD4+ cell count cut-off 500 cells/μl) – age, adherence to therapy during the first 12 months on cART, history of injection drug use and follow-up time.
Table 4. Results of the longitudinal analyses focusing on the interaction between the period of combination antiretroviral therapy initiation (2000–2006 and 2007–2012) and CD4+ cell count level at combination antiretroviral therapy initiation (<350 versus ≥350 cells/ml and <500 versus ≥500 cells/ml).
| Variable | Predicted probability of maintaining adherence ≥95% over time | Predicted probability of maintaining viral suppression over time | Predicted probability of developing resistance to any drug class over time | Predicted probability of developing an AIDS-defining illness by the end of follow-up | Predicted probability of being dead by the end of follow-up |
|---|---|---|---|---|---|
| Period and CD4+ cell count (cells/μl), median (Q1–Q3) | |||||
| 2000–2006 and <350 | 0.79 (0.67–0.86) | 0.77 (0.58–0.90) | 0.11 (0.07–0.18) | 0.14 (0.08–0.22) | 0.14 (0.08–0.22) |
| 2000–2006 and ≥350 | 0.72 (0.56–0.81) | 0.70 (0.45–0.88) | 0.04 (0.03–0.08) | 0.13 (0.08–0.22) | 0.14 (0.09–0.22) |
| 2007–2012 and <350 | 0.83 (0.73–0.88) | 0.68 (0.57–0.79) | 0.05 (0.03–0.08) | 0.05 (0.03–0.08) | 0.05 (0.03–0.08) |
| 2007–2012 and ≥350 | 0.83 (0.74–0.87) | 0.68 (0.58–0.77) | 0.02 (0.01–0.02) | 0.03 (0.01–0.05) | 0.02 (0.01–0.04) |
| Period and CD4+ cell count (cells/μl), median (Q1–Q3) | |||||
| 2000–2006 and <500 | 0.75 (0.62–0.84) | 0.77 (0.58–0.90) | 0.11 (0.07–0.18) | 0.04 (0.02–0.08) | 0.13 (0.08–0.22) |
| 2000–2006 and ≥500 | 0.55 (0.36–0.70) | 0.51 (0.23–0.79) | 0.04 (0.03–0.08) | 0.02 (0.01–0.03) | 0.16 (0.10–0.24) |
| 2007–2012 and <500 | 0.82 (0.73–0.87) | 0.68 (0.58–0.78) | 0.04 (0.03–0.07) | 0.03 (0.01–0.04) | 0.05 (0.03–0.08) |
| 2007–2012 and ≥500 | 0.84 (0.75–0.88) | 0.65 (0.55–0.74) | 0.01 (0.01–0.02) | 0.01 (0.00–0.01) | 0.01 (0.01–0.02) |
Q1: 25th percentile, Q3: 75th percentile. Models were adjusted for predicted probability of maintaining adherence ≥95% over time (for both CD4+ cell count cut-offs) – age, sex, history of injection drug use, longitudinal viral load and follow-up time; predicted probability of maintaining viral suppression over time (for both CD4+ cell count cut-offs) – age, sex, history of injection drug use, longitudinal adherence to cART and follow-up time; predicted probability of developing resistance to any drug class over time (for both CD4+ cell count cut-offs) – age, sex, history of injection drug use, longitudinal adherence to cART, longitudinal viral load and follow-up time; predicted probability of developing an AIDS-defining illness by the end of follow-up (for both CD4+ cell count cut-offs) – history of injection drug use, longitudinal adherence to cART, longitudinal viral load and follow-up time; predicted probability of being dead by the end of follow-up (for both CD4+ cell count cut-offs) – age, sex, history of injection drug use, longitudinal adherence to cART, longitudinal viral load and follow-up time.
Discussion
Our results showed a strong association between CD4+ at cART initiation and all outcomes investigated in this study. Patients who initiated cART with a CD4+ at least 500 cells/mm3 in the most recent period (2007–2012) had an increased likelihood of achieving viral suppression at 9 months and of maintaining an adherence level of at least 95% over time than the other exposure groups. These individuals were also the ones with the lowest probability of developing any resistance and an ADI during follow-up. Interestingly, although these individuals had the lowest follow-up, comparing with patients in the same period with a CD4+ less than 500 cells/μl, they had the lowest probability of mortality. These analyses also indicated that, while patients with a CD4+ at least 500 cells/μl had a lower likelihood of being LTFU in 2007–2012 than in 2000–2006, they unfortunately did poorly regarding this outcome when compared with patients with a CD4+ less than 500 cells/μl in both periods. It is important to highlight that patients who initiated cART with a CD4+ at least 500 cells/μl in the most recent period were not the ones with the highest likelihood of maintaining viral suppression over time. On the basis of our definition for this latest outcome, we believe that this result was influenced by viral load blips that have happened during the follow-up time. Despite these viral load blips resulting in a perceived lower probability of maintaining viral suppression over time, we showed that this outcome did not negatively influence the mortality of these patients.
One of the concerns often raised in the literature around early cART initiation is that people with a high CD4+ may be at risk of poor adherence because they are asymptomatic and, as such, less motivated to take cART [47]. In a Soweto cohort, Katz et al. [48] found that ‘feeling healthy’ was the number one reason why people stopped taking their treatment. In this context, our results regarding improved adherence and increased likelihood of viral suppression in the CD4+ at least 500 cells/μl stratum in recent years are noteworthy. Our results also suggest that this issue is evolving favourably over time probably due to enhanced programmatic features, including patient support and education, low threshold services and patient navigators, as well as the availability of simpler, more effective, safer and better tolerated regimens.
Our study has a number of strengths. Our cohort is unique in that it involves a large sample size within a population-based programme wherein all patients had access to the same facilitated free cART options, medical care and laboratory monitoring, with no copayments or deductibles. This minimizes treatment access issues as a possible confounding factor. Our database is also comprehensive, as it captures 100% of cART refills, 100% of viral loads and approximately 85% of CD4+s done in the province. Another strength was that we adjusted each of our models for a broad number of important cross-sectional and longitudinal variables. In addition, in the analysis looking at the emergence of any drug resistance, we excluded patients with primary viral resistance, which otherwise would have introduced a significant bias in our analyses.
Our study also has a number of potential limitations. As with every cohort study, causality cannot be established. Second, we controlled our analyses for history instead of ongoing injection drug use. Thus, our results did not capture changes in drug usage over time. Third, we used pharmacy refill as a measure of adherence, which may overestimate true adherence. However, we have previously validated this measurement against other commonly used adherence metrics and found it to be highly predictive of disease progression and death, among other key clinical outcomes [43,49]. Fourth, in collecting data for resistance, we assumed serum samples with a viral load less than 250 copies/ml to be nonresistant. Although resistance is possible at these low viral levels [35], its incidence in our setting is so low that it is unlikely that this could have had any effect on the direction or significance of our results. Fifth, our sample size in the 2000–2006 and CD4+ at least 500 cells/μl group was relatively small, while the more recent group 2007–2012 and CD4+ at least 500 cells/μl had a substantially larger number of patients. These sample sizes reflect the evolution of treatment guidelines under which, unlike current trends, patients were inclined to present for treatment only when they developed symptoms [17,29,50]. Nonetheless, it is noteworthy that, even with a smaller sample in 2000–2006, the differences described here were statistically significant. Sixth, it is possible that the data density between these periods has influenced our results (i.e. frequency of laboratory monitoring, follow-up visits and clinical assessment). However, it is hard to ascertain whether these activities have changed over time, as they vary depending on each patient's response to antiretroviral therapy. Finally, it may be speculated that somehow patients entering treatment in recent years may be different than those in the earlier years. We have no evidence that this would represent a significant bias given the socioeconomic downdrift of the epidemic over time in this province. However, further analyses are necessary to explore this issue. On this note, it is important to mention that different trials are underway, which will provide additional insights on this issue: the Test and Link to Care-Plus (HPTN 065) trial in USA [51], the PopART Study (HPTN 071) in Zambia and South Africa [52], and the TasP (Treatment as Prevention) trial (ANRS 12249) in KwaZulu-Natal, South Africa [53].
In summary, we demonstrated that initiation of cART at a high CD4+ was positively associated with several treatment outcomes, suggesting that these outcomes are likely to play an important role in explaining the positive impact of early cART initiation on mortality. Therefore, our results should alleviate some of the concerns clinicians may have when initiating cART in people with CD4+ at least 500 cells/μl, as recommended by current guidelines [17], particularly where free access to modern cART and related monitoring is available. Furthermore, our results support the shift to immediate cART initiation upon testing positive for HIV, consistent with the new UNAIDS 90–90–90 target for cART rollout by 2020 [16].
Acknowledgments
The author's contributions were as follows: V.D. Lima and J.S.G. Montaner were responsible for the study concept and design; V.D. Lima, W. Chau, R.S. Hogg, J.S.G. Montaner and P.R. Harrigan were responsible for the acquisition of data; V.D. Lima was responsible for the analysis and interpretation of data; V.D. Lima and A. Reuter were responsible for the drafting of the manuscript; V.D. Lima, A. Reuter, P.R. Harrigan, L. Lourenço, W. Chau, M. Hull, L. MacKenzie, S. Guillemi, R.S. Hogg, R. Barrios and J.S.G. Montaner were responsible for the critical revision of the manuscript for important intellectual content; V.D. Lima was responsible for the statistical analysis; V.D. Lima, J.S.G. Montaner obtained funding for the study and provided administrative, technical or material support; and J.S.G. Montaner was responsible for the study supervision.
Dr Montaner has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. Dr Harrigan has received consultancy fees, paid to him, from ViiV Health Care, Tobira Therapeutics, Selah Genomics Inc and Quest Diagnostics. He also holds stocks from Merck. Dr Guillemi has received honorariums from Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. Dr Hull has received limited unrestricted funding, paid to his institution, from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Roche, Merck and ViiV Healthcare.
Dr Lima is supported by a grant from the US National Institute on Drug Abuse (R03 DA033851), a grant from the Canadian Institutes of Heath Research (CIHR; MOP-125948), by a Scholar Award from the Michael Institute for Health Research and a New Investigator award from CIHR. Dr Montaner is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (R01 DA036307). Dr MacKenzie is supported by a CTN Postdoctoral Fellowship Award from the CIHR Canadian HIV Trials Network.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References
- 1.The Joint United Nations Programme on HIV/AIDS. HIV estimates with uncertainty bounds. 2014 http://www.unaids.org/en/resources/documents/2014/HIV_estimates_with_undertainty_bounds_1990–2013.
- 2.Molina JM, Cahn P, Grinsztejn B, Lazzarin A, Mills A, Saag M, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378:238–246. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 3.Nelson M, Amaya G, Clumeck N, Arns da Cunha C, Jayaweera D, Junod P, et al. Efficacy and safety of rilpivirine in treatment-naive, HIV-1-infected patients with hepatitis B virus/hepatitis C virus coinfection enrolled in the Phase III randomized, double-blind ECHO and THRIVE trials. J Antimicrob Chemother. 2012;67:2020–2028. doi: 10.1093/jac/dks130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raffi F, Jaeger H, Quiros-Roldan E, Albrecht H, Belonosova E, Gatell JM, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13:927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 5.Raffi F, Rachlis A, Brinson C, Arasteh K, Gorgolas M, Brennan C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS. 2015;29:167–174. doi: 10.1097/QAD.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77–85. doi: 10.1097/QAI.0b013e31828ace69. [DOI] [PubMed] [Google Scholar]
- 7.Rockstroh JK, Lennox JL, Dejesus E, Saag MS, Lazzarin A, Wan H, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53:807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 8.Sax PE, Tierney C, Collier AC, Daar ES, Mollan K, Budhathoki C, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204:1191–1201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okulicz JF, Le TD, Agan BK, Camargo JF, Landrum ML, Wright E, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med. 2015;175:88–99. doi: 10.1001/jamainternmed.2014.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornhill J, Inshaw J, Oomeer S, Kaleebu P, Cooper D, Ramjee G, et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc. 2014;17:19480. doi: 10.7448/IAS.17.4.19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann CJ, Gallant JE. Rationale and evidence for human immunodeficiency virus treatment as prevention at the individual and population levels. Infect Dis Clin North Am. 2014;28:549–561. doi: 10.1016/j.idc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 14.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 15.Lima VD, Johnston K, Hogg RS, Levy AR, Harrigan PR, Anema A, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 16.The Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014 http://www.unaids.org/en/resources/documents/2014/90–90–90.
- 17.Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HIV-CAUSAL Collaboration. Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strategies for Management of Antiretroviral Therapy Study G. Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 23.When To Start C. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Writing Committee for the CC. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–1569. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berheto TM, Haile DB, Mohammed S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. N Am J Med Sci. 2014;6:453–459. doi: 10.4103/1947-2714.141636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clouse K, Pettifor A, Maskew M, Bassett J, Van Rie A, Gay C, et al. Initiating antiretroviral therapy when presenting with higher CD4 cell counts results in reduced loss to follow-up in a resource-limited setting. AIDS. 2013;27:645–650. doi: 10.1097/QAD.0b013e32835c12f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, Lawn SD, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. J Acquir Immune Defic Syndr. 2010;55:e17–e23. doi: 10.1097/QAI.0b013e3181f275fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onoka CA, Uzochukwu BS, Onwujekwe OE, Chukwuka C, Ilozumba J, Onyedum C, et al. Retention and loss to follow-up in antiretroviral treatment programmes in southeast Nigeria. Pathog Glob Health. 2012;106:46–54. doi: 10.1179/2047773211Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276:146–154. [PubMed] [Google Scholar]
- 30.Geretti AM, Smith C, Haberl A, Garcia-Diaz A, Nebbia G, Johnson M, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13:927–936. [PubMed] [Google Scholar]
- 31.Uy J, Armon C, Buchacz K, Wood K, Brooks JT HOPS Investigators. Initiation of HAART at higher CD4 cell counts is associated with a lower frequency of antiretroviral drug resistance mutations at virologic failure. J Acquir Immune Defic Syndr. 2009;51:450–453. doi: 10.1097/QAI.0b013e3181acb630. [DOI] [PubMed] [Google Scholar]
- 32.Phillips AN, Dunn D, Sabin C, Pozniak A, Matthias R, Geretti AM, et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS. 2005;19:487–494. doi: 10.1097/01.aids.0000162337.58557.3d. [DOI] [PubMed] [Google Scholar]
- 33.Phillips AN, Leen C, Wilson A, Anderson J, Dunn D, Schwenk A, et al. Risk of extensive virological failure to the three original antiretroviral drug classes over long-term follow-up from the start of therapy in patients with HIV infection: an observational cohort study. Lancet. 2007;370:1923–1928. doi: 10.1016/S0140-6736(07)61815-7. [DOI] [PubMed] [Google Scholar]
- 34.Lodi S, Phillips A, Fidler S, Hawkins D, Gilson R, McLean K, et al. Role of HIV infection duration and CD4 cell level at initiation of combination anti-retroviral therapy on risk of failure. PLoS One. 2013;8:e75608. doi: 10.1371/journal.pone.0075608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57:1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 36.Lapadula G, Cozzi-Lepri A, Marchetti G, Antinori A, Chiodera A, Nicastri E, et al. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. AIDS. 2013;27:769–779. doi: 10.1097/QAD.0b013e32835cb747. [DOI] [PubMed] [Google Scholar]
- 37.British Columbia Centre for Excellence in HIV/AIDS. [Accessed 20 January 2015];Therapeutic guidelines antiretroviral (ARV) treatment of adult HIV infection. http://www.cfenet.ubc.ca/sites/default/files/uploads/Therapeutic%20Guidelines%202013-Feb-final.pdf.
- 38.Sun R, Ku J, Jayakar H, Kuo JC, Brambilla D, Herman S, et al. Ultrasensitive reverse transcription-PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:2964–2969. doi: 10.1128/jcm.36.10.2964-2969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schumacher W, Frick E, Kauselmann M, Maier-Hoyle V, van der Vliet R, Babiel R. Fully automated quantification of human immunodeficiency virus (HIV) type 1 RNA in human plasma by the COBAS AmpliPrep/COBAS TaqMan system. J Clin Virol. 2007;38:304–312. doi: 10.1016/j.jcv.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Brumme CJ, Swenson LC, Wynhoven B, Yip B, Skinner S, Lima VD, et al. Technical and regulatory shortcomings of the TaqMan version 1 HIV viral load assay. PLoS One. 2012;7:e43882. doi: 10.1371/journal.pone.0043882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Bel A, Marissens D, Debaisieux L, Liesnard C, Van den Wijngaert S, Lauwers S, et al. Correction of underquantification of human immunodeficiency virus type 1 load with the second version of the Roche Cobas AmpliPrep/Cobas TaqMan assay. J Clin Microbiol. 2010;48:1337–1342. doi: 10.1128/JCM.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez-Serna A, Min JE, Woods C, Chan D, Lima VD, Montaner JS, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis. 2014;58:1165–1173. doi: 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima VD, Bangsberg DR, Harrigan PR, Deeks SG, Yip B, Hogg RS, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55:460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis. Hoboken N: John Wiley & Sons; 2004. [Google Scholar]
- 45.Wensing AM, Calvez V, Gunthard HF, Johnson VA, Paredes R, Pillay D, et al. 2014 Update of the drug resistance mutations in HIV-1. Top Antivir Med. 2014;22:642–650. [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald JH. Handbook of biological statistics. 3rd. Baltimore, Maryland: Sparky House Publishing; 2014. [Google Scholar]
- 47.Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles' heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis. 2014;59 Suppl 1:S21–S27. doi: 10.1093/cid/ciu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–2181. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992-2011. Clin Infect Dis. 2013;57:1027–1037. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 51.Donnell DJ, Hall HI, Gamble T, Beauchamp G, Griffin AB, Torian LV, et al. Use of HIV case surveillance system to design and evaluate site-randomized interventions in an HIV prevention study: HPTN 065. Open AIDS J. 2012;6:122–130. doi: 10.2174/1874613601206010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cori A, Ayles H, Beyers N, Schaap A, Floyd S, Sabapathy K, et al. HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model. PLoS One. 2014;9:e84511. doi: 10.1371/journal.pone.0084511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwuji CC, Orne-Gliemann J, Tanser F, Boyer S, Lessells RJ, Lert F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14:230. doi: 10.1186/1745-6215-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]