Abstract
Phosphorous-31 magnetic resonance spectroscopy (31P-MRS) provides a unique noninvasive window into myocardial energy homeostasis. Mouse models of cardiac disease are widely used in preclinical studies, but application of 31P-MRS in the in vivo mouse heart has been limited. The small-sized, fast-beating mouse heart imposes challenges regarding localized signal acquisition devoid of contamination with signal originating from surrounding tissues. Here, we report the implementation and validation of 3D Image Selected In vivo Spectroscopy (ISIS) for localized 31P-MRS of the in vivo mouse heart at 9.4 T.
Cardiac 31P MR spectra were acquired in vivo in healthy mice (n = 9) and in transverse aortic constricted (TAC) mice (n = 8) using respiratory-gated, cardiac-triggered 3D ISIS. Localization and potential signal contamination were assessed with 31P-MRS experiments in the anterior myocardial wall, liver, skeletal muscle and blood. For healthy hearts, results were validated against ex vivo biochemical assays. Effects of isoflurane anesthesia were assessed by measuring in vivo hemodynamics and blood gasses.
The myocardial energy status, assessed via the phosphocreatine (PCr) to ATP ratio, was ~25% lower in TAC mice compared to controls (0.76 ± 0.13 vs. 1.00 ± 0.15; P < 0.01). Localization with 1D ISIS resulted in two-fold higher PCr/ATP ratios than measured with 3D ISIS, due to high PCr levels of chest skeletal muscle that contaminate the 1D ISIS measurements. Ex vivo determinations of the myocardial PCr/ATP ratio (0.94 ± 0.24; n = 8) confirmed in vivo observations in control mice. Heart rate (497 ± 76 beats/min), mean arterial pressure (90 ± 3.3 mmHg), and blood oxygen saturation (96.2 ± 0.6 %) during the experimental conditions of in vivo 31P-MRS were within the normal physiological range.
Our results show that respiratory-gated, cardiac-triggered 3D ISIS allows for noninvasive assessments of in vivo mouse myocardial energy homeostasis with 31P-MRS under physiological conditions.
Keywords: energy metabolism, heart, ISIS, mouse, 31P-MRS, transverse aortic constriction (TAC)

Introduction
Mouse models are widely used in preclinical studies on the pathogenesis of cardiomyopathies. Disturbed myocardial energy metabolism has been identified as an important contributor to the development of cardiomyopathy (1). Assessment of the myocardial energy status is therefore instrumental in characterizing disease progression or treatment response. The high-energy phosphates adenosine 5’-triphosphate (ATP) and phosphocreatine (PCr) are essential for providing energy for cellular processes such as sarcomere contraction in cardiomyocytes, and for energy transport and buffering. The inherent instability of high-energy phosphates compromises accurate assessment of the myocardial energy status with biochemical techniques, which require disruptive or terminal biopsies, precluding longitudinal in vivo investigations. Many magnetic resonance imaging (MRI) and, to a lesser extent, spectroscopy (MRS) methods have been introduced to study the in vivo mouse heart noninvasively (2–4). These methods allow for longitudinal studies of disease progression and assessments of the effects of therapeutic strategies.
Phosphorous-31 MRS (31P-MRS) is the only method that provides a noninvasive window into in vivo high-energy phosphates (1). Localized signal acquisition is essential to restrict the obtained spectrum to the heart, excluding signal from nearby liver tissue or chest skeletal muscle. Localization methods for 31P-MRS include single-voxel as well as chemical shift imaging (CSI) approaches. 31P-CSI allows for an assessment of regional differences in myocardial energy status (5), but is susceptible to intervoxel signal contamination due to Fourier bleeding (6). In contrast, single-voxel localization with 3D Image Selected In vivo Spectroscopy (ISIS) (7) leads to a better defined voxel shape (8), but voxel size is typically much larger compared to CSI, and commonly includes the whole left ventricle (LV) (9).
Localized 31P-MRS of the in vivo mouse heart is very challenging due the small organ size (~100 mg), the high heart rate (~500 beats/min), and the intrinsically low sensitivity of 31P-MRS. Methods for in vivo 1D and 2D 31P-CSI of the mouse heart were initially demonstrated in healthy mice (10) and in a transgenic mouse model for cardiomyopathy (11). These experiments were performed at a constant TR, which is essential for a reliable signal quantification. None of these methods used cardiac triggering or respiratory gating to account for physiological motion of the tissue of interest. Together with the effects of Fourier bleeding in CSI methods, tissue displacement could lead to contamination of the spectrum with signal from the liver, blood, and/or chest skeletal muscle.
One early study describes the application of cardiac-triggered 3D ISIS for in vivo 31P-MRS of the mouse heart (9). While cardiac triggering was used in those experiments to synchronize the acquisitions with the cardiac cycle, no measures were taken to ensure a constant TR. When applying ISIS under partially saturated conditions (i.e., if TR < 5 × T1), variations in TR can lead to signal contamination (12,13) as well as modulations of signal amplitudes due to T1-dependent partial saturation effects. Those early experiments were performed at 2.35 T and required a lengthy experimental time of nearly 3 hours (9). At higher field strengths, scan time can possibly be reduced to acceptable values while maintaining sufficient SNR for signal quantification.
Here, we report the implementation of 3D ISIS for single-voxel localized 31P-MRS of the in vivo mouse heart at 9.4 T. Because 3D ISIS is a multi-shot localization method and hence particularly sensitive to motion artifacts, we employed both respiratory gating and cardiac triggering whilst maintaining a steady state of magnetization with dummy excitations during respiratory gates to ensure a constant TR (14). Results were validated against ex vivo biochemical assays of myocardial PCr and ATP concentrations. Hemodynamics and blood gasses were measured to assess potential effects of isoflurane anesthesia on the cardiovascular physiology. To demonstrate suitability for cardiac applications, the method was applied to a well-characterized mouse model of heart failure (15,16).
Methods
Animals
All procedures were approved by the Animal Ethics Committee of Maastricht University (Maastricht, The Netherlands). Male C57BL/6 mice (n = 8; body weight = 26.2 ± 2.6 g) underwent transverse aortic constriction (TAC) surgery as described previously (17). Anesthesia was induced using 2-3% isoflurane in 0.4 L/min flow of 1:1 O2:medical air, after which mice were intubated for mechanical ventilation. Analgesia was provided using buprenorphine (0.1 mg/kg subcutaneous). An incision was made above the first intercostal space to gain access to the aortic arch. The aorta was tied off together with a 27G needle between the innominate artery and the left common carotid artery with a 6-0 silk suture. Subsequent needle removal left an aortic stenosis, inducing LV pressure-overload. The chest was closed and the intubation tube was removed to allow full recovery. Seven weeks after surgery, MR data were acquired as described below. Healthy mice (n = 9; body weight = 24.4 ± 2.0 g) served as controls. Following the MR measurements, anesthetized mice were sacrificed by exsanguination. Blood was collected in EDTA tubes for ex vivo analysis with 31P-MRS.
MR protocol
Mice were anesthetized with 2-3% isoflurane in 0.4 L/min flow of medical air and positioned prone in a purpose-built support cradle above a custom-built, actively decoupled, two-turn 31P surface coil (Ø 15 mm) for signal reception. Anesthesia was maintained with 1.2-1.6% isoflurane in a continuous flow of 0.4 L/min medical air. The front paws were taped onto gold-coated ECG electrodes integrated in the anesthesia mask. A respiratory balloon was positioned underneath the lower abdomen. Vital signs were monitored and used for MR gating and triggering by the SA Monitoring and Gating System 1025 (Small Animal Instruments, Stony Brook, NY, USA). Mouse body temperature was maintained using a heating pad with integrated warm water flow, and monitored with an external abdominal fiber optic probe. The setup was inserted into a horizontal-bore 9.4 T magnet (Magnex Scientific, Oxon, UK), interfaced to a Bruker Avance III console (Bruker Biospin MRI, Ettlingen, Germany), and controlled by the ParaVision 5.0 software package (Bruker Biospin). The system was equipped with a 740 mT/m gradient set, and a volume coil (Ø 54 mm) composed of a quadrature 1H birdcage resonator and a linear 31P birdcage resonator (RAPID Biomedical, Rimpar, Germany), used for 1H MRI and shimming, and for radiofrequency transmission for 31P-MRS, respectively.
Scout 1H MR images were acquired to confirm positioning of the heart within the sensitive area of the 31P surface coil. A segmented, prospectively cardiac-triggered, respiratory-gated fast low-angle shot sequence was used to acquire cine 1H MR image series of 16-18 frames per cardiac cycle. Four 1-mm LV short-axis slices were complemented with 4- and 2-chamber long-axis views, and used for quantification of LV function and morphology as well as for anatomical reference during 3D ISIS voxel planning for localized 31P-MRS. Imaging parameters were: field of view 30 × 30 mm2; matrix = 128 × 128; TE = 1.8 ms; TR = 7 ms; flip angle = 15°; number of averages (NA) = 4. Total acquisition time was ≈ 20 minutes.
Subsequently, an 11 × 11 × 11 mm3 voxel in the sensitive area of the surface coil was shimmed manually by minimizing the 1H2O line width, acquired with a respiratory-gated, cardiac-triggered point resolved spectroscopy sequence (18). Calibration of the 31P sinc excitation pulse (pulse length = 1.2 ms; bandwidth = 32.0 ppm) was performed by varying pulse power to achieve maximal signal from a spherical phantom (Ø 5 mm; 15 M phosphoric acid) positioned underneath the 31P surface coil. After removal of the phantom, unlocalized pulse-acquire 31P MR spectra were obtained from a subset of animals (n = 5 per group) to assess metabolite T1 values using conventional saturation-recovery experiments. Parameters were: 1.2 ms 90° sinc excitation pulse; bandwidth = 32.0 ppm; γ-ATP on resonance; TR = 500, 1000, 2000, 4000, 6000, and 15000 ms; NA = 1024 - 32.
Next, a respiratory-gated, cardiac-triggered 3D ISIS sequence was used for localized cardiac 31P-MRS. Dependent on heart size, a 3D ISIS voxel (TAC: 326 ± 43 μL vs. control: 175 ± 8.8 μL) was positioned to enclose the end-diastolic LV, carefully excluding the liver and chest skeletal muscle (Figure 1A-B). 3D ISIS parameters were: TR ≈ 2 seconds; NA = 768 (96 3D ISIS cycles) preceded by 1 dummy cycle; 6.25 ms 180° adiabatic hyperbolic secant inversion pulses (bandwidth = 37.5 ppm); 1.2 ms 90° sinc excitation pulse (bandwidth = 32.0 ppm); γ-ATP on resonance; 2048 acquisition points. Although the excitation pulse was calibrated, the potential effect of spatial contamination by so-called ‘T1 smearing’ due to inhomogeneous excitation pulses (19) was further minimized by choosing the least-optimal inversion order in the left-right orientation. This avoided contamination of the spectra with signal from chest skeletal muscle (anterior-posterior orientation) or the liver (head-feet orientation). Triggering was timed at ECG R-wave upslope detection. Respiratory gating causes fluctuations in effective TR, leading to variations in longitudinal magnetization between subsequent acquisitions. If longitudinal magnetization is not equal at the start of all eight acquisitions within one 3D ISIS cycle, cancellation of unwanted signals in the addition/subtraction scheme is incomplete. Thus, when measuring at TR < 5 × T1, a constant TR is required to minimize signal contamination. Moreover, measuring at constant TR prevents complex modulations of signal amplitudes that hamper corrections for partial saturation effects. Therefore, we performed unlocalized dummy excitation pulses during respiratory gates to achieve an essentially constant TR of ≈ 2 seconds. Acquisition time was < 40 minutes. In a subset of healthy mice (n = 6), 3D ISIS was also performed at TR ≈ 15 seconds; NA = 192 (24 cycles). These measurements were used to verify the partial saturation correction factor obtained with unlocalized saturation-recovery experiments.
Figure 1.
End-diastolic left ventricular (LV) MR images obtained from a control mouse (A) and a mouse with a transverse aortic constriction (TAC) (B). The constriction is indicated by the arrow. Dilated hypertrophic cardiomyopathy is evidenced by increased LV wall thickness and LV cavity volume in the TAC mouse. Rectangles indicate the voxels selected for localized 31P-MRS with 3D ISIS. Panels C and D display 31P MR spectra acquired in vivo with 3D ISIS in a healthy mouse heart and a TAC heart, respectively. Myocardial PCr/γ-ATP, corrected for partial saturation, was lower in TAC mice (n = 8) compared to healthy controls (n = 9) (E). Data are expressed as mean ± SD. **, P < 0.01. α-, β-, γ-ATP, α-, β-, and γ-phosphate groups in ATP; 2,3-DPG, 2,3-diphosphoglycerate; PCr, phosphocreatine; Pi, inorganic phosphate.
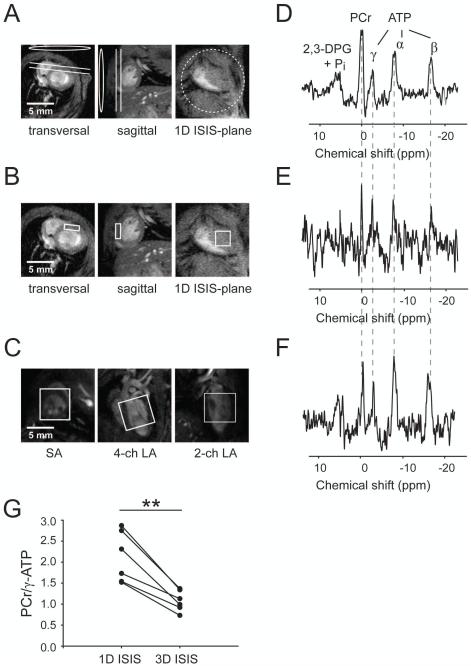
To compare 3D ISIS localization with 1D localization, we acquired spectra using 1D ISIS of the anterior myocardial wall in a slice essentially parallel to the surface coil (Figure 2A) in a subset of mice (n = 6). Acquisition parameters were kept similar to the 3D ISIS approach, except for NA = 384 (192 1D ISIS cycles) and slice thickness = 1 mm. Furthermore, to obtain spectra solely from the myocardium and to rule out any contamination with signal from LV cavity blood, 3D ISIS was performed with a small voxel (1 × 3 × 3 mm3) positioned within the anterior myocardial wall (Figure 2B). Acquisition time was ≈ 2.5 hours with NA = 3072 (384 cycles).
Figure 2.
Comparisons of 1D ISIS and 3D ISIS localization for cardiac 31P-MRS. Geometrical localization for (A) 1D ISIS in the anterior myocardial wall (slice thickness = 1 mm), indicated in transversal and sagittal reference images. Circle and ellipses indicate the position of the 31P surface coil. 3D ISIS voxel localization in (B) the anterior myocardial wall (1 × 3 × 3 mm3) and (C) enclosing the whole LV (6 × 6 × 6 mm3). The corresponding 31P MR spectra from (D) 1D ISIS (NA = 384), (E) 3D ISIS in the myocardium (NA = 3072), and (F) 3D ISIS of the whole LV (NA = 384). All spectra were acquired in the same mouse at TR = 2 seconds. (G) PCr/γ-ATP ratio assessed via 1D ISIS (as in A) and 3D ISIS of the whole LV (as in C). **, P < 0.01 (two-sided paired t-test, n = 6). α-, β-, γ-ATP, α-, β-, and γ-phosphate groups in ATP; 2,3-DPG, 2,3-diphosphoglycerate; PCr, phosphocreatine; Pi, inorganic phosphate. SA: short axis, 4-ch LA: 4-chamber long-axis, 2-ch LA: 2-chamber long-axis.
Additionally, spectra from liver and hind limb skeletal muscle (n = 3) were acquired in vivo with respiratory-gated and cardiac-triggered 3D ISIS (Figure 3) to evaluate the performance of 3D ISIS localization with respect to in vivo 31P-MRS of these tissues as reported in the literature (20,21).
Figure 3.
Voxel positioning for 3D ISIS in (A) the liver (5 × 5 × 5 mm3) and in (B) hind limb skeletal muscle (4 × 4 × 4 mm3) in the mouse, and the corresponding 31P MR spectra for (C) the liver (NA = 768) and (D) skeletal muscle (NA = 384). Note the prominent resonance of PCr detected in skeletal muscle, whereas it is absent in liver tissue. α-, β-, γ-ATP, α-, β-, and γ-phosphate groups in ATP; PCr, phosphocreatine; Pi, inorganic phosphate.
Finally, to investigate the contribution of blood metabolites to the cardiac 31P MR spectra acquired in vivo, spectra of fresh blood were measured ex vivo using a pulse-acquire sequence. A vial (n = 6) with ~1 mL of blood in EDTA was positioned just over the surface coil and maintained at 37°C by a heating pad. Parameters were: 1.2 ms 90° sinc excitation pulse; γ-ATP on resonance; TR = 2000 ms; NA = 512.
Image analysis
LV cavity and myocardial wall volumes were quantified by semiautomatic segmentation of the cine images (Pie Medical Imaging, Maastricht, The Netherlands) as described previously (22), yielding LV end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), ejection fraction (EF), and LV myocardial mass.
31P-MRS data analysis
Fitting of the metabolite signals to Lorentzian line shapes was performed in the time domain using AMARES in jMRUI (23). The PCr resonance at 0.00 ppm was used as an internal chemical shift reference. The ATP resonances at −2.48 ppm (γ; doublet), −7.52 ppm (α; doublet) and −16.26 (β; triplet) were fitted with equal amplitudes and line widths within each multiplet, and a J-coupling constant of 17 Hz. The γ-ATP line widths (LWγ-ATP) were constrained relative to the PCr line width (LWPCr) according to an empirically determined relation: LWγ-ATP = LWPCr + 14.85 Hz (n = 63; r = 0.78; P < 0.001). Blood 2,3-diphosphoglycerate (2,3-DPG) resonances obscured the inorganic phosphate (Pi) resonance. Therefore, these signals were fitted with two peaks: one for 2,3-DPG5.4 ppm and Pi at 5.4 ppm, and one for 2,3-DPG6.3 ppm at 6.3 ppm.
Saturation-recovery curves of PCr, γ-ATP and α-ATP were fitted by a mono-exponential function to estimate the corresponding longitudinal relaxation rate constants R1. Mean R1 values were used to determine metabolite T1 values via R1 = 1/T1.
The in vivo myocardial energy status was expressed as the PCr/γ-ATP ratio, corrected for partial saturation.
Because blood contains ATP, but no PCr (24), signal contamination from blood in in vivo cardiac 31P MR spectra may lead to underestimation of the myocardial PCr/γ-ATP ratio. Therefore, we evaluated whether the PCr/γ-ATP ratio in the current work could be affected by blood contamination. The [γ-ATP/2,3-DPG6.3 ppm]blood ratio in spectra acquired in fresh blood was determined as a measure for ATP content in blood. The contribution of resonances from blood metabolites to in vivo spectra was assessed by quantifying the [2,3-DPG6.3 ppm/γ-ATP]LV ratio in the cardiac 3D ISIS spectra. Next, the relative contribution of signal from ATP in the blood to the ATP signal obtained with in vivo 3D ISIS was calculated as: [γ-ATP/2,3-DPG6.3 ppm]blood × [2,3-DPG6.3 ppm/γ-ATP]LV × 100%.
Validations
To test our in vivo 31P-MRS method against conventional biochemical assays in ex vivo tissue samples, validation measurements were conducted in a separate cohort of healthy mice (n = 4). Following the acquisition of 3D ISIS-localized myocardial 31P MR spectra as described above, the heart was immediately snap frozen upon thoracotomy. Myocardial high-energy phosphate concentrations were determined spectrophotometrically as described previously (25).
To monitor the effects of anesthesia on hemodynamics and blood gasses, healthy mice (n = 5) underwent cannulation of the carotid artery after induction of isoflurane anesthesia. Mean arterial pressure (MAP) and heart rate were monitored using a heparinized saline-filled catheter connected to a blood pressure transducer (Baxter TruWave PX600F, Edwards, Irvine, CA, USA), and recorded using LabVIEW 5.1 (National Instruments, Austin, TX, USA) as described previously (26). Each experiment was continued for 1.5 hours, and mimicked the experimental conditions of the in vivo 31P-MRS acquisitions in terms of mouse body temperature, heart and respiratory rates, and anesthesia maintenance as described above. After 1.5 hours, a 100-μL arterial blood sample was obtained via the cannula and subsequently analyzed using a blood gas analyzer (RAPIDPoint 500, Siemens Healthcare, Erlangen, Germany). Immediately thereafter, the anesthetized mice were sacrificed by snap freezing the heart upon thoracotomy for ex vivo spectrophotometric assay of PCr and ATP concentrations (25).
Statistical analyses
Data are reported as mean ± SD. The statistical significance of differences was analyzed using two-sided paired or unpaired t-tests, as appropriate. The level of significance was set at P < 0.05.
Results
MRI: LV hypertrophy in TAC mice
We assessed in vivo LV morphology and function from cine MR images to confirm the hypertrophic phenotype and impaired cardiac performance in TAC mice (Table 1, Figure 1A-B). LV mass was 95% higher in TAC mice compared to healthy mice (P < 0.001), indicating LV hypertrophy in TAC mice. Concomitantly, EDV (P < 0.001) and ESV (P < 0.001) were higher in TAC mice compared to controls. This translated in a lower SV (−33%, P < 0.001) and EF (−65%, P < 0.001) in TAC mice. Combined, these data illustrate the development of dilated hypertrophic cardiomyopathy with severe systolic dysfunction after 7 weeks of LV pressure-overload in TAC mice.
Table 1.
LV morphology and functional parameters obtained with MRI in control mice and TAC mice.
| Control (n = 9) | TAC (n = 8) | P | |
|---|---|---|---|
| LV mass (mg) | 90.0 ± 14.9 | 176 ± 19.1 | *** |
| LV mass/body weight (mg/g) | 3.7 ± 0.6 | 6.0 ± 0.9 | *** |
| Heart rate (beats/min) | 495 ± 50 | 539 ± 31 | NS |
| End-diastolic volume (μL) | 63.7 ± 10.8 | 123 ± 26.1 | *** |
| End-systolic volume (μL) | 20.8 ± 5.4 | 94.1 ± 24.8 | *** |
| Stroke volume (μL) | 43.0 ± 7.3 | 28.7 ± 6.6 | *** |
| Ejection fraction (%) | 67.6 ± 5.6 | 23.9 ± 5.2 | *** |
Data are expressed as mean ± SD. Effect of TAC (two-sided unpaired t-test):
P < 0.001; NS, not significant.
31P-MRS: in vivo myocardial energy status
Typical 31P MR spectra acquired with 3D ISIS in a healthy mouse heart and a TAC heart are shown in Figure 1C-D. Resonances of PCr, and ATP are indicated. Inorganic phosphate (Pi, ~5 ppm) was obscured by 2,3-DPG arising from blood. Spectral line width for PCr was 0.37 ± 0.15 ppm.
Conventional pulse-acquire 31P MR saturation-recovery experiments of the mouse chest were performed in order to estimate the high-energy phosphate metabolite T1 relaxation times at 9.4 T. T1 values did not differ between control mice and TAC mice, and were 2.54 ± 0.41 s for PCr, 1.45 ± 0.25 s for γ-ATP, and 1.09 ± 0.31 s for α-ATP. Given a TR of 2 seconds, this resulted in a partial saturation correction factor for PCr/γ-ATP of 1.37 in 3D ISIS experiments. In healthy mice (n = 6), 3D ISIS was also performed under fully relaxed conditions. These localized acquisitions yielded a partial saturation correction factor for myocardial PCr/γ-ATP of 1.38 ± 0.28 for spectra acquired at TR = 2 seconds, which is in good agreement with the value derived from the unlocalized pulse-acquire saturation-recovery experiments. Myocardial PCr/γ-ATP, corrected for partial saturation, was lower in TAC mice compared to healthy controls (0.76 ± 0.13 vs. 1.00 ± 0.15; P < 0.01, Figure 1E), which is indicative of a compromised myocardial energy homeostasis in TAC mice.
Localization of a slice containing anterior myocardial wall with 1D ISIS (Figure 2A) systematically resulted in higher PCr/γ-ATP ratios than those obtained with 3D ISIS of the whole LV (2.12 ± 0.61 vs. 1.08 ± 0.25; P < 0.01, Figure 2G). Skeletal muscle tissue surrounding the anterior myocardial wall within the sensitive area of the surface coil (Figure 2A) likely contributed to the higher PCr/γ-ATP ratio observed with 1D ISIS. This was corroborated by spectra (Figure 2E) obtained with a very small 9-μL 3D ISIS voxel positioned in the anterior myocardial wall (Figure 2B), which qualitatively confirm the PCr/γ-ATP ratio of ~1 for healthy myocardium.
31P-MRS of liver, skeletal muscle, and blood
Using the respiratory-gated and cardiac-triggered 3D ISIS approach, we acquired 31P MR spectra from in vivo mouse liver and hind limb skeletal muscle. Absence of PCr in liver tissue was confirmed (Figure 3C), illustrating that essentially no signal from PCr in adjacent skeletal muscle contaminated the spectra that were obtained from the liver with 3D ISIS. Spectra obtained from hind limb skeletal muscle yielded a PCr/γ-ATP ratio of 3.59 ± 0.58 (Figure 3D), which is typical for healthy mouse skeletal muscle in resting conditions (21,27).
In spectra obtained from fresh blood (Figure 4), the blood ATP content was estimated via the [γ-ATP/2,3-DPG6.3 ppm]blood ratio, which was 0.22 ± 0.14. Contribution of signal from metabolites in the blood to the cardiac spectra, estimated via [2,3-DPG6.3 ppm/γ-ATP]LV, was similar for controls and TAC mice, and was 0.18 ± 0.10. The relative contribution of signal from ATP in the blood to the ATP signal in cardiac 3D ISIS spectra was therefore approximately 4%. These results show that contamination of the 3D ISIS spectra with signal from ATP in LV blood is marginal, and only minimally affects the myocardial PCr/γ-ATP ratio.
Figure 4.
31P MR spectrum obtained from fresh blood. 2,3-DPG, 2,3-diphosphoglycerate; PDE, phosphodiesters; α-, β-, γ-ATP, α-, β-, and γ-phosphate groups in ATP.
Validations
The in vivo PCr/γ-ATP ratio measured with 3D ISIS-localized 31P-MRS in healthy control mice (1.00 ± 0.19; n = 19) matched conventional ex vivo spectrophotometric assays in myocardial tissue (0.94 ± 0.24; n = 8; Figure 5A). In a subset of healthy mice, both in vivo and ex vivo measurements were performed. Spectrophotometric assays in myocardial tissue mirrored in vivo 31P-MRS measurements of myocardial PCr/γ-ATP ratios in the same hearts (1.00 ± 0.32 vs. 0.94 ± 0.14; n = 4; Figure 5B). Concentrations of PCr and ATP in ex vivo myocardial tissue samples (n = 8) were 18.3 ± 5.7 mmol/kg dry weight and 19.3 ± 2.5 mmol/kg dry weight, respectively.
Figure 5.
Comparison (A) of in vivo 3D ISIS-localized 31P-MRS measurements (n = 19) and conventional ex vivo spectrophotometric assays (n = 8) of the myocardial PCr/ATP ratio in healthy mice. Data are expressed as mean ± SD. In a subset of healthy mice (n = 4), both 31P-MRS and biochemical assays were performed. The difference in PCr/ATP ratio between the two methods is plotted against their mean per mouse in a Bland-Altman plot (B). Dashed lines indicate the limits of agreement (mean ± 2 SD) around the mean (solid line).
Mouse hemodynamics and blood gasses were measured after 1.5 hours of anesthesia to assess the potential effects of the isoflurane anesthetic regimen on the cardiovascular physiology. Throughout these experiments, which mimicked the experimental conditions of the 31P-MRS procedure, MAP (90 ± 3.3 mmHg) and heart rate (497 ± 76 beats/min) remained well within the physiological range for healthy C57BL/6 mice (28). Moreover, after 1.5 hours of anesthesia, blood oxygen saturation and other blood gas parameters were normal (Table 2). These data illustrate that the experimental conditions of the in vivo MR protocol have no major influence on mouse hemodynamics or blood gasses.
Table 2.
Hemodynamics and blood gasses measured in control mice after 1.5 hours of anesthesia (1.2-1.6% isoflurane in a continuous flow of 0.4 L/min medical air).
| C57BL/6 (n = 5) | |
|---|---|
| Heart rate (beats/min) | 497 ± 76 |
| Mean arterial pressure (mmHg) | 90 ± 3.3 |
| pH | 7.41 ± 0.03 |
| pCO2 (mmHg) | 31.6 ± 3.5 |
| pO2 (mmHg) | 110.7 ± 4.4 |
| Total hemoglobin (mM) | 9.2 ± 0.2 |
| O2 saturation (%) | 96.2 ± 0.6 |
Data are expressed as mean ± SD.
Discussion
We describe a noninvasive method to study the in vivo myocardial energy status in healthy and diseased mice using 3D ISIS-localized 31P-MRS. Acquisitions were respiratory-gated and cardiac-triggered, whilst maintaining steady-state conditions using dummy excitations to ensure accurate localization. We validated our method against conventional biochemical assays of PCr and ATP concentrations in ex vivo myocardial tissue collected immediately after acquiring the in vivo 31P MR spectra. Using a 1H/31P MR setup, cardiac 31P-MRS with 3D ISIS was combined with cine 1H MRI to assess morphological, functional, and metabolic parameters in a single experimental session of less than 2 hours. With this approach, we identified a reduced myocardial energy status, evidenced by a ~25% lower PCr/γ-ATP ratio, accompanied by hypertrophic growth and severely impaired myocardial function in a surgical mouse model of heart failure. These pathophysiological changes are consistent with previous studies (16,29).
The myocardial PCr/ATP ratio in healthy mice was ~1, measured both in vivo with 31P-MRS and ex vivo with spectrophotometric assays in the same hearts. This value is on the lower range of the normal PCr/ATP values reported in literature for humans (overall mean: 1.72 ± 0.26) (30). In humans, heart rate and cardiac work can increase up to three-fold during dobutamine stress (31) or exercise (32). In healthy mice, dobutamine infusion induced a heart rate increase of only ~39% (27), whereas running exercise increased mouse heart rates by only 40% to 90% (33,34). Indeed, it has been suggested that mice may have a lower cardiac energy reserve (35), given the narrower dynamic range of heart rates and cardiac work in mice compared to men.
Notably, the myocardial PCr/ATP ratio for healthy mice in the current work was lower than PCr/ATP values obtained in other in vivo 31P-MRS mouse studies (9–11). With measurements of physiological hemodynamics and blood gasses during 1.5 hours of anesthesia (28,36), we have ruled out adverse effects of the experimental conditions on mouse cardiovascular physiology that may cause a decreased PCr/ATP ratio. Instead, many technical aspects of localized 31P-MRS acquisition and quantification could contribute to the variability in the MRS-derived PCr/ATP values reported in literature (30), and should be taken into consideration when comparing between different laboratories (37,38). The three main causes for apparent discrepancies in literature reports are differences in 1) correction for partial saturation, 2) contamination of the spectra by signal from the liver or skeletal muscle tissue, and 3) contaminating blood in the LV cavity (30). Below, each of these issues are addressed for the current study.
Partial saturation correction
For the correction of partial saturation effects, we used metabolite T1 values as determined by unlocalized pulse-acquire saturation-recovery experiments. Because the T1 values in chest skeletal muscle could be different from those in cardiac muscle (39), we validated the correction factor in healthy mice by acquiring 3D ISIS-localized spectra from the LV myocardium under fully relaxed conditions. Indeed, the partial saturation correction factor derived from unlocalized saturation-recovery experiments was in agreement with measurements localized to the heart. This observation validates the assumption (40) that in the healthy mouse the T1(PCr)/T1(γ-ATP) ratio is essentially the same for chest skeletal muscle and myocardium at 9.4 T (11).
Minimizing signal contamination
Because 3D ISIS requires multiple acquisitions for signal localization, the method is particularly sensitive to motion artifacts and consequential contamination from tissues surrounding the heart such as liver and chest skeletal muscle. Additional contamination (‘T1-smearing’) can be introduced by differences in longitudinal magnetization between subsequent acquisitions within one ISIS cycle due to imperfect flip angle of the excitation pulse combined with a TR < 5 × T1 (12,13,19). Similar effects occur when TR is not constant between acquisitions, while measuring at TR < 5 × T1. Previous studies in rodents (9) and in humans (41–43) using 3D ISIS for cardiac applications did not measure at constant TR for a steady state of magnetization, nor at fully relaxed conditions. The 3D ISIS sequence presented here uses a respiratory-gated and cardiac-triggered timing strategy to ensure localized inversion of the LV signal at identical cardiac and respiratory phases for all acquisitions, in combination with dummy excitations during respiratory gates to maintain a constant TR. In addition, by using the γ-ATP signal to determine the PCr/ATP ratio, we minimized the chemical shift displacement between PCr and ATP. PCr signal was absent in 3D ISIS-localized liver spectra, demonstrating that skeletal muscle located between the surface coil and the voxel of interest did not contaminate the spectra and that signal acquisition was effectively restricted to the liver. Indeed, localized 31P-MRS of the liver (20) will also benefit from the strategy proposed here (44).
Moreover, we demonstrated the effect of potential contamination of the spectra with signal from chest skeletal muscle by comparing 3D ISIS of the whole LV with 1D ISIS of a slice containing the anterior myocardial wall, similar to 1D CSI approaches reported previously (10,45). Localization within the selected slice is realized by surface coil positioning (Figure 2A), which may introduce signal from the surrounding tissue to the resultant spectrum. The PCr/γ-ATP ratio obtained with 1D ISIS was consistent with values observed with 1D CSI (10), but two-fold higher than the myocardial PCr/γ-ATP ratio measured with 3D ISIS.
Blood contamination
Blood in the LV cavity contains ATP, but no PCr. If signal from ATP in the blood contributes to the signal acquired from the voxel of interest, the myocardial PCr/ATP ratio may be underestimated (46). A correction factor can be applied to account for signal contribution from ATP in the blood to the spectra, which depends on both the amount of ATP in the blood and the amount of blood contributing to the spectrum. We showed that the amount of ATP signal from fresh blood, in terms of [γ-ATP/2,3-DPG6.3 ppm]blood, was approximately 20%, which is consistent with previous reports of 31P-MRS studies of blood in humans (24) and mice (9,47). The contribution of signal from metabolites in blood to the in vivo 3D ISIS spectra of the whole LV, estimated via [2,3-DPG6.3 ppm/γ-ATP]LV, was less than 20%, and was not different between healthy and TAC mice. The high velocity of flowing blood during acquisition may attenuate the peaks from blood metabolites in spectra obtained in vivo (48). Based on these observations, we estimated that the contribution of signal from ATP in the blood to the ATP signal in in vivo 3D ISIS spectra was only 4%, and concluded that correcting for blood contamination was not necessary in this study. Moreover, spectra obtained with 3D ISIS selecting a small voxel confined to the anterior myocardial wall were similar to those obtained from the whole LV, confirming minimal contribution of signal from blood to the latter.
Study limitations
With our current approach of determining the PCr/ATP ratio, it is not possible to detect similar decreases in both PCr and ATP concentrations. Absolute quantification of metabolite concentration will therefore be more sensitive to changes in cardiac energy metabolism. Indeed, it has been shown that in pressure-overload mouse hearts, not only myocardial PCr levels drop, but also ATP concentrations decrease, illustrating the added value of absolute quantification over the PCr/ATP ratio (45,49). Nonetheless, the PCr/ATP ratio has been used to identify perturbations in myocardial energy homeostasis in various mouse models of disease (16,50,51). Furthermore, a reduced PCr/ATP ratio has been shown to be an important indicator of disease severity (16).
A drawback of using single-voxel localized 31P-MRS of the entire LV is that myocardial energy status cannot be assessed at a regional scale, which is of importance in investigations of myocardial ischemia. In vivo 2D 31P-CSI of the mouse heart (11) would allow for mapping of myocardial energetics to assess differences between infarcted and remote regions in mouse models of myocardial ischemic insult. Nonetheless, because many preclinical animal studies focus on pathologies that have a global effect on the heart, such as aortic stenosis, diabetes, and inborn errors of metabolism, the current 3D ISIS approach for localized 31P-MRS can be a valuable addition to the toolbox of mouse cardiac MR methods (4).
Even at the high magnetic field strength of 9.4 T, we were not able to unambiguously detect and quantify myocardial Pi, because the signal was obscured by signal from 2,3-DPG in the blood. Potentially, reliable detection of Pi would provide additional metabolic insights, as decreasing PCr levels may be mirrored by increasing Pi levels. Additionally, the chemical shift of Pi could potentially be used to quantify in vivo myocardial pH. Notably, even in the human heart Pi is often undetectable, suggesting that myocardial Pi may only be partially MR visible (30).
In conclusion, the present work describes a noninvasive approach to assess myocardial energy status in the in vivo mouse using single-voxel 3D ISIS-localized 31P-MRS, which was validated by conventional biochemical assays in ex vivo myocardial tissue samples from the same hearts. The method encompasses a respiratory-gated, cardiac-triggered 3D ISIS sequence with dummy excitations during respiratory gates, to ensure a well-defined localization. This method identified differences in high-energy phosphate metabolism between the healthy mouse heart and a widely used model for heart failure, the TAC mouse. Importantly, results were obtained under physiological conditions, which are difficult or impossible to achieve with disruptive methods such as open-thorax protocols or isolated perfused-heart setups. Furthermore, the 3D ISIS-localized spectra can be obtained within 40 minutes, leaving room for measurements of cardiac function with MRI during the same experimental session. We anticipate that localized 31P-MRS will provide valuable contributions to preclinical investigations of cardiac disease progression and therapeutic intervention efficacy.
Acknowledgements
We thank Larry de Graaf and Tom R. Geraedts for the design of dedicated hardware, Esther C.M. Kneepkens for contributions to pulse sequence design, and Leonie B.P. Niesen for biotechnical assistance. Dr. Jeroen A.L. Jeneson is acknowledged for helpful discussions.
This work was supported by the Center for Translational Molecular Medicine, project TRIUMPH (grant number 01C-103) with funding from the Dutch Heart Foundation. S.M.H. and J.J.P. are supported by VIDI grants (project numbers 016.086.336 and 700.58.421, respectively) from the Netherlands Organisation for Scientific Research (NWO). Part of this work was supported by the National Institutes of Health (A.J.B. and G.J.S.; NIH grant HL072011).
List of Abbreviations
- α-, β-, γ-ATP
α-, β-, and γ-phosphate groups in adenosine 5’-triphosphate
- CSI
chemical shift imaging
- 2,3-DPG
2,3-diphosphoglycerate
- ECG
electrocardiogram
- EDV
end-diastolic volume
- EF
ejection fraction
- ESV
end-systolic volume
- ISIS
image selected in vivo spectroscopy
- LV
left ventricle
- LW
line width
- MAP
mean arterial pressure
- MRI
magnetic resonance imaging
- NA
number of averages
- PCr
phosphocreatine
- PDE
phosphodiesters
- Pi
inorganic phosphate
- 31P-MRS
phosphorous-31 magnetic resonance spectroscopy
- SNR
signal-to-noise ratio
- SV
stroke volume
- TAC
transverse aortic constriction
References
- 1.Neubauer S. The failing heart - an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 2.Epstein FH. MR in mouse models of cardiac disease. NMR Biomed. 2007;20:238–255. doi: 10.1002/nbm.1152. [DOI] [PubMed] [Google Scholar]
- 3.Kober F, Troalen T, Bernard M. Recent developments in small animal cardiovascular MRI. Curr Cardiovasc Imaging Rep. 2014;7:9249. [Google Scholar]
- 4.Bakermans AJ, Abdurrachim D, Moonen RPM, Motaal AG, Prompers JJ, Strijkers GJ, Vandoorne K, Nicolay K. Small animal cardiovascular MR imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc. 2015;88-89:1–47. doi: 10.1016/j.pnmrs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323:1593–1600. doi: 10.1056/NEJM199012063232304. [DOI] [PubMed] [Google Scholar]
- 6.Keevil SF. Spatial localization in nuclear magnetic resonance spectroscopy. Phys Med Biol. 2006;51:R579–R636. doi: 10.1088/0031-9155/51/16/R01. [DOI] [PubMed] [Google Scholar]
- 7.Ordidge RJ, Connelly A, Lohman JA. Image-selected in Vivo spectroscopy (ISIS). A new technique for spatially selective nmr spectroscopy. J Magn Reson. 1986;66:283–294. [Google Scholar]
- 8.de Graaf RA. In vivo NMR spectroscopy: Principles and techniques. John Wiley & Sons; 2007. [Google Scholar]
- 9.Omerovic E, Basetti M, Bollano E, Bohlooly-Y M, Bohlooly M, Törnell J, Isgaard J, Hjalmarson A, Soussi B, Waagstein F. In vivo metabolic imaging of cardiac bioenergetics in transgenic mice. Biochem Biophys Res Commun. 2000;271:222–228. doi: 10.1006/bbrc.2000.2518. [DOI] [PubMed] [Google Scholar]
- 10.Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol. 2000;279:H2218–H2224. doi: 10.1152/ajpheart.2000.279.5.H2218. [DOI] [PubMed] [Google Scholar]
- 11.Flögel U, Jacoby C, Gödecke A, Schrader J. In vivo 2D mapping of impaired murine cardiac energetics in NO-induced heart failure. Magn Reson Med. 2007;57:50–58. doi: 10.1002/mrm.21101. [DOI] [PubMed] [Google Scholar]
- 12.Lawry TJ, Karczmar GS, Weiner MW, Matson GB. Computer simulation of MRS localization techniques: an analysis of ISIS. Magn Reson Med. 1989;9:299–314. doi: 10.1002/mrm.1910090302. [DOI] [PubMed] [Google Scholar]
- 13.Keevil SF, Porter DA, Smith MA. Experimental characterization of the ISIS technique for volume selected NMR spectroscopy. NMR Biomed. 1992;5:200–208. doi: 10.1002/nbm.1940050407. [DOI] [PubMed] [Google Scholar]
- 14.Bakermans AJ, Abdurrachim D, Geraedts TR, Houten SM, Nicolay K, Prompers JJ. In vivo proton T1 relaxation times of mouse myocardial metabolites at 9.4 T. Magn Reson Med. 2015;73:2069–2074. doi: 10.1002/mrm.25340. [DOI] [PubMed] [Google Scholar]
- 15.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maslov MY, Chacko VP, Stuber M, Moens AL, Kass DA, Champion HC, Weiss RG. Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol. 2007;292:H387–H391. doi: 10.1152/ajpheart.00737.2006. [DOI] [PubMed] [Google Scholar]
- 17.van Nierop BJ, van Assen HC, van Deel ED, Niesen LBP, Duncker DJ, Strijkers GJ, Nicolay K. Phenotyping of left and right ventricular function in mouse models of compensated hypertrophy and heart failure with cardiac MRI. PLoS One. 2013;8:e55424. doi: 10.1371/journal.pone.0055424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakermans AJ, Geraedts TR, van Weeghel M, Denis S, João Ferraz M, Aerts JMFG, Aten J, Nicolay K, Houten SM, Prompers JJ. Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging. 2011;4:558–565. doi: 10.1161/CIRCIMAGING.111.963751. [DOI] [PubMed] [Google Scholar]
- 19.Burger C, Buchli R, McKinnon G, Meier D, Boesiger P. The impact of the ISIS experiment order on spatial contamination. Magn Reson Med. 1992;26:218–230. doi: 10.1002/mrm.1910260204. [DOI] [PubMed] [Google Scholar]
- 20.Landis CS, Yamanouchi K, Zhou H, Mohan S, Roy-Chowdhury N, Shafritz DA, Koretsky A, Roy-Chowdhury J, Hetherington HP, Guha C. Noninvasive evaluation of liver repopulation by transplanted hepatocytes using 31P MRS imaging in mice. Hepatology. 2006;44:1250–1258. doi: 10.1002/hep.21382. [DOI] [PubMed] [Google Scholar]
- 21.Kan HE, Veltien A, Arnts H, Nabuurs CIHC, Luijten B, de Haan A, Wieringa B, Heerschap A. Gated dynamic 31P MRS shows reduced contractile phosphocreatine breakdown in mice deficient in cytosolic creatine kinase and adenylate kinase. NMR Biomed. 2009;22:523–531. doi: 10.1002/nbm.1364. [DOI] [PubMed] [Google Scholar]
- 22.Heijman E, Aben J-P, Penners C, Niessen P, Guillaume R, Eys G, van Nicolay K, Strijkers GJ. Evaluation of manual and automatic segmentation of the mouse heart from CINE MR images. J Magn Reson Imaging. 2008;27:86–93. doi: 10.1002/jmri.21236. [DOI] [PubMed] [Google Scholar]
- 23.Vanhamme L, van den Boogaart A, van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 24.Horn M, Neubauer S, Bomhard M, Kadgien M, Schnackerz K, Ertl G. 31P-NMR spectroscopy of human blood and serum: first results from volunteers and patients with congestive heart failure, diabetes mellitus and hyperlipidaemia. MAGMA Magn Reson Mater Physics, Biol Med. 1993;1:55–60. [Google Scholar]
- 25.Lamprecht W, Trautschold I. ATP determination, with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Academic Press; New York: 1974. pp. 2101–2110. [Google Scholar]
- 26.Zuurbier CJ, Koeman A, Houten SM, Hollmann MW, Florijn WJ. Optimizing anesthetic regimen for surgery in mice through minimization of hemodynamic, metabolic, and inflammatory perturbations. Exp Biol Med (Maywood) 2014;239:737–746. doi: 10.1177/1535370214524877. [DOI] [PubMed] [Google Scholar]
- 27.Naumova AV, Weiss RG, Chacko VP. Regulation of murine myocardial energy metabolism during adrenergic stress studied by in vivo 31P NMR spectroscopy. Am J Physiol Heart Circ Physiol. 2003;285:H1976–H1979. doi: 10.1152/ajpheart.00474.2003. [DOI] [PubMed] [Google Scholar]
- 28.Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 2011;52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Chacko VP, Schär M, Akki A, Weiss RG. Impaired ATP kinetics in failing in vivo mouse heart. Circ Cardiovasc Imaging. 2011;4:42–50. doi: 10.1161/CIRCIMAGING.110.959320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottomley PA. Encyclopedia of Magnetic Resonance. John Wiley & Sons; 2007. NMR spectroscopy of the human heart. [Google Scholar]
- 31.Lamb HJ, Beyerbacht HP, Ouwerkerk R, Doornbos J, Pluim BM, van der Wall EE, van der Laarse A, de Roos A. Metabolic response of normal human myocardium to high-dose atropine-dobutamine stress studied by 31P-MRS. Circulation. 1997;96:2969–2977. doi: 10.1161/01.cir.96.9.2969. [DOI] [PubMed] [Google Scholar]
- 32.La Gerche A, Claessen G, van de Bruaene A, Pattyn N, van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6:329–338. doi: 10.1161/CIRCIMAGING.112.980037. [DOI] [PubMed] [Google Scholar]
- 33.Adlam D, de Bono JP, Danson EJ, Zhang MH, Casadei B, Paterson DJ, Channon KM. Telemetric analysis of haemodynamic regulation during voluntary exercise training in mouse models. Exp Physiol. 2011;96:1118–1128. doi: 10.1113/expphysiol.2011.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lujan HL, Janbaih H, Feng H-Z, Jin J-P, DiCarlo SE. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R68–R74. doi: 10.1152/ajpregu.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. Circ Res. 1998;82:898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzkopf TM, Horn T, Lang D, Klein J. Blood gases and energy metabolites in mouse blood before and after cerebral ischemia: the effects of anesthetics. Exp Biol Med (Maywood) 2013;238:84–89. doi: 10.1258/ebm.2012.012261. [DOI] [PubMed] [Google Scholar]
- 37.Bottomley PA. The trouble with spectroscopy papers. Radiology. 1991;181:344–350. doi: 10.1148/radiology.181.2.1924769. [DOI] [PubMed] [Google Scholar]
- 38.Lamb HJ, Doornbos J, den Hollander JA, Luyten PR, Beyerbacht HP, van der Wall EE, de Roos A. Reproducibility of human cardiac 31P-NMR spectroscopy. NMR Biomed. 1996;9:217–227. doi: 10.1002/(SICI)1099-1492(199608)9:5<217::AID-NBM419>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 39.van Dobbenburgh JO, Lekkerkerk C, van Echteld CJ, de Beer R. Saturation correction in human cardiac 31P MR spectroscopy at 1.5 T. NMR Biomed. 1994;7:218–224. doi: 10.1002/nbm.1940070504. [DOI] [PubMed] [Google Scholar]
- 40.Bottomley PA, Hardy CJ, Weiss RG. Correcting human heart 31P NMR spectra for partial saturation. Evidence that saturation factors for PCr/ATP are homogeneous in normal and disease states. J Magn Reson. 1991;95:341–355. [Google Scholar]
- 41.Schaefer S, Gober J, Valenza M, Karczmar GS, Matson GB, Camacho SA, Botvinick EH, Massie B, Weiner MW. Nuclear magnetic resonance imaging-guided phosphorus-31 spectroscopy of the human heart. J Am Coll Cardiol. 1988;12:1449–1455. doi: 10.1016/s0735-1097(88)80008-1. [DOI] [PubMed] [Google Scholar]
- 42.van der Meer RW, Hammer S, Smit JWA, Frölich M, Bax JJ, Diamant M, Rijzewijk LJ, de Roos A, Romijn JA, Lamb HJ. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes. 2007;56:2849–2853. doi: 10.2337/db07-0768. [DOI] [PubMed] [Google Scholar]
- 43.Fragasso G, de Cobelli F, Spoladore R, Esposito A, Salerno A, Calori G, Montanaro C, Maranta F, Lattuada G, Margonato A, Maschio A, Del, Perseghin G. Resting cardiac energy metabolism is inversely associated with heart rate in healthy young adult men. Am Heart J. 2011;162:136–141. doi: 10.1016/j.ahj.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Solanky BS, Sanchez-Canon GJ, Cobbold JFL, Taylor-Robinson SD, Bell JD, Scudamore CL, Ross E, Holder JC, So P-W, Cox IJ. Metabolic profiling of the rat liver after chronic ingestion of alpha-naphthylisothiocyanate using in vivo and ex vivo magnetic resonance spectroscopy. Toxicol Sci. 2012;126:306–316. doi: 10.1093/toxsci/kfs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta A, Chacko VP, Weiss RG. Abnormal energetics and ATP depletion in pressure-overload mouse hearts: in vivo high-energy phosphate concentration measures by noninvasive magnetic resonance. Am J Physiol Heart Circ Physiol. 2009;297:H59–H64. doi: 10.1152/ajpheart.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801. doi: 10.1016/0002-8703(91)90527-o. [DOI] [PubMed] [Google Scholar]
- 47.Lee J, Hu Q, Nakamura Y, Wang X, Zhang X, Zhu X, Chen W, Yang Q, Zhang J. Open-chest 31P magnetic resonance spectroscopy of mouse heart at 4.7 Tesla. J Magn Reson Imaging. 2006;24:1269–1276. doi: 10.1002/jmri.20766. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Duncker DJ, Xu Y, Zhang Y, Path G, Merkle H, Hendrich K, From AH, Bache RJ, Uğurbil K. Transmural bioenergetic responses of normal myocardium to high workstates. Am J Physiol. 1995;268:H1891–H1905. doi: 10.1152/ajpheart.1995.268.5.H1891. [DOI] [PubMed] [Google Scholar]
- 49.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakermans AJ, Dodd MS, Nicolay K, Prompers JJ, Tyler DJ, Houten SM. Myocardial energy shortage and unmet anaplerotic needs in the fasted long-chain acyl-CoA dehydrogenase knockout mouse. Cardiovasc Res. 2013;100:441–449. doi: 10.1093/cvr/cvt212. [DOI] [PubMed] [Google Scholar]
- 51.Tucci S, Flögel U, Hermann S, Sturm M, Schäfers M, Spiekerkoetter U. Development and pathomechanisms of cardiomyopathy in very long-chain acyl-CoA dehydrogenase deficient (VLCAD(−/−)) mice. Biochim Biophys Acta. 2014;1842:677–685. doi: 10.1016/j.bbadis.2014.02.001. [DOI] [PubMed] [Google Scholar]