Abstract
(+/−)3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) is an abused psychostimulant producing strong monoaminergic stimulation and whole-body hyperthermia. MDMA-induced thermogenesis involves activation of uncoupling proteins (UCP), primarily a type specific to skeletal muscle (UCP-3) and which is absent in brain, although other UCP types are expressed in brain (e.g., thalamus) and might contribute to thermogenesis. Since neuroimaging of brain temperature could provide insights of MDMA action, we measured spatial distributions of systemically-administered MDMA-induced temperature changes and dynamics in rat cortex and subcortex using a novel magnetic resonance method, Biosensor Imaging of Redundant Deviation of Shifts (BIRDS), with an exogenous temperature-sensitive probe (thulium ion and macrocyclic chelate 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethyl-1,4,7,10-tetraacetate (DOTMA4−)). The MDMA-induced temperature rise in cortex was greater than in subcortex (1.6±0.4°C vs. 1.3±0.4°C) and occurred more rapidly (2.0±0.2°C/h vs. 1.5±0.2°C/h). MDMA-induced temperature changes and dynamics in cortex and body were correlated, although body temperature exceeded cortex before and after MDMA. Temperature, neuronal activity, and blood flow (CBF) were measured simultaneously in cortex and subcortex (i.e., thalamus) to investigate possible differences of MDMA-induced warming across brain regions. MDMA-induced warming correlated with increases in neuronal activity and blood flow in cortex, suggesting that the normal neurovascular response to increased neural activity was maintained. In contrast to cortex, a biphasic relationship was seen in subcortex (i.e., thalamus), with a decline in CBF as temperature and neural activity rose, transitioning to a rise in CBF for temperature >37°C, suggesting that MDMA affected CBF and neurovascular coupling differently in subcortical regions. Considering that MDMA effects on CBF and heat dissipation (as well as potential heat generation) may vary regionally, neuroprotection may require different cooling strategies.
Keywords: BIRDS, blood flow, ecstasy, heat, oxidative metabolism, TmDOTMA−
Introduction
(+/−) 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) is one of the most heavily abused psychostimulants and its misuse has seen explosive growth in the last decade (1). MDMA toxicity is mainly associated with serotonergic and sympathomimetic activation (serotonin syndrome), although additional effects on other neurotransmitters systems such as dopamine, norepinephrine or acetylcholine are also present (2). MDMA is a substrate for the serotonin transporter (3), but also for the dopamine and norepinephrine transporters (4). A very severe and potentially fatal effect of MDMA is the induction of extreme hyperthermia, which in turn can have negative consequences on various organ systems (e.g., rhabdomyolysis, coagulopathy, kidney, heart or liver failure) (2). In addition to hyperthermia, MDMA may have adverse cardiovascular effects by increasing heart rate and blood pressure, which combined with hyperthermia could result in serious, possibly even life-threatening conditions (5–8).
Pharmacological studies in rats and mice using serotonin and dopamine receptor antagonists have shown that the initial rise in the body temperature in response to MDMA involves the release of dopamine acting at D1 receptors (9), because antagonists of D1 receptors but not D2 or 5-HT receptors blocked hyperthermia. Furthermore, pre-depletion of serotonin using p-chlorophenylalanine (an inhibitor of synthesis) has no effect on the initial temperature rise in response to MDMA, but prolonged the period of hyperthermia (10,11), presumably through interactions at 5-HT1A receptors. The prolongation of hyperthermia is consistent with loss of normal serotonin-dependent mechanisms of heat dissipation, as governed by local and peripheral vasodilation and via thermoregulation centers in the hypothalamus and other brain regions (12). In muscle tissue, MDMA and methamphetamine-induced thermogenesis is due to the uncoupling of oxidative phosphorylation by activation of uncoupling proteins (UCP) (13). The brain also expresses uncoupling proteins (14) - more extensively in deep brain regions (e.g., thalamus) than the cortex (15) - which conceivably might play a direct role in local thermogenesis and brain temperature rise measured in response to MDMA. However, the impact of body thermogenesis could be a major component of MDMA-induced brain warming. Thus the magnitude, distribution, and dynamics of MDMA-induced temperature changes in the brain are of critical importance to the assessment of neurotoxicity and neuroprotective strategies. Because core body temperature is usually reported with MDMA exposure, knowledge of brain temperature distribution would add a novel dimension to investigate MDMA’s effects on this important parameter and perhaps provide alternative means for neuroprotection.
Biosensor Imaging of Redundant Deviation of Shifts (BIRDS) is a new magnetic resonance chemical shift imaging (CSI) method which uses the redundant information stored in the chemical shifts of an exogenous contrast agent composed of a lanthanide (or transition) metal ion and a macrocyclic chelate, e.g., 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethyl-1,4,7,10-tetraacetate (DOTMA4−) or 1,4,7,10-tetraazacyclododecane-1,4,7, 10-tetrakis(methylene phosphonate) (DOTP8−) where the acetate is a temperature specific probe and the phosphonate allows both temperature and pH (16–18). The paramagnetically-shifted chemical shift information detected by BIRDS is subsequently converted into information about other physicochemical parameters, such as temperature and/or pH (17,19). Recently, we demonstrated that with BIRDS temperature distributions in rat brain can be obtained within minutes using a temperature-sensitive probe consisting of a complex between thulium ion (Tm3+) and DOTMA4− (16,18). The methyl 1H chemical shift of TmDOTMA− displays high temperature sensitivity and pH-independence (16), and can be measured with high signal-to-noise ratio (SNR) (e.g., up to 50 for in vivo BIRDS experiments). TmDOTMA− was chosen over TmDOTP5− due to the higher accuracy with which temperature can be determined (16). Here we used TmDOTMA− with BIRDS to measure the effects of single-dose MDMA (22±2 mg/kg) on absolute temperature distribution and dynamics of warming of the cortex and upper (medial) regions of subcortex in anesthetized rats. Simultaneous measurements of temperature (by thermocouple), neuronal activity (by microelectrodes), and tissue perfusion (by laser-Doppler flowmetry) were made to determine the relationship between MDMA-induced warming and neurovascular coupling in cortex and subcortex (i.e., thalamus).
Materials and Methods
Animal preparation (n=17)
All experimental procedures on rats were approved by the Institutional Animal Care and Use Committee (IACUC) and follow the National Institute of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley male rats (200–300 g, 2–3 months) were anesthetized with an injection of urethane (1.3 g/kg), tracheotomized, and artificially ventilated (70% N2O/30% O2). An intraperitonial line was inserted for administration of ~250 μL of the racemic isomeric mixture of (+/−)MDMA (22±2 mg/kg) or 0.9% saline. A femoral vein was cannulated (PE-10) to administer D-tubocurarine chloride (0.25 mg/kg/h) or TmDOTMA− (0.5 mmol/kg). A femoral artery was cannulated (PE-50) for monitoring of physiological parameters (pCO2, pO2, pH, blood pressure) throughout the experiment. Ventilation parameters were adjusted as needed to maintain normal physiology. The anesthetized rats were prepared with renal ligation as previously described (17,19) to maintain a high concentration of TmDOTMA− in the blood (3–4 mM) and the brain extracellular space (2–3 mM) during the BIRDS experiments (16). For the BIRDS experiments TmDOTMA− was initially infused slowly for the initial 1–2 h followed by the MDMA injection. The majority of the observed TmDOTMA− signal detected in vivo arises mostly from the extracellular space (16,17,19) because the blood represents only 3% of the voxel volume (20). In both magnet (for brain temperature maps with BIRDS) and bench (for simultaneous measurements from cortex and subcortex (i.e., thalamus) of temperature with a thermocuple, neuronal activity with microelectrodes, and tissue perfusion with laser-Doppler) experiments a temperature-controlled and recirculated-water heating pad was used to maintain animal body temperature at physiological levels before MDMA injection. Core body temperature was measured continuously with a rectal temperature probe. No further adjustments in the temperature of the water heating pad were made after MDMA injection, such that the increase in the body temperature represents solely the effects of MDMA.
Study Groups
The magnet (BIRDS) and bench (thermometry and laser-Doppler) experiments were conducted using separate groups of animals with continuous measurements made before (baseline) and after MDMA (BIRDS, n=7; bench, n=4), allowing individual animals to serve as their own controls. Three additional rats in each group (BIRDS, n=3; bench, n=3) were injected only with 0.9% saline to assess potential effects of vehicle on measured parameters. Of all rats injected with MDMA and studied with BIRDS, two rats experienced a large decrease (~50%) in mean arterial blood pressure upon MDMA injection and these animals were excluded from analysis.
In vivo BIRDS (n=10)
CSI data were obtained on a 11.7T horizontal-bore spectrometer using a 1H resonator/surface-coil radio frequency (RF) probe (1.4 cm). A Gaussian-shaped pulse of 200 μs was used for excitation of a 6 mm slice with field-of-view of 2.56×2.56 cm2. The following CSI parameters were used: 16×16 encode steps, recycle time of 11 ms, and 100 averages. A 2D CSI image was acquired in 4 min 40 s of total acquisition time. The 1H spectra were line broadened (150 Hz), phased (zero-order), and baseline corrected (first-order). The temperature (T) depends only on the chemical shift of the -CH3 protons of TmDOTMA− (δCH3)
| [1] |
where δ0 = −103.0 ppm and the coefficients, a0 (34.45±0.01), a1 (1.460±0.003) and a2 (0.0152±0.0009), were calculated from linear least-squares fit of temperature as a function of chemical shift δCH3 (16). The reference temperatures for derivation of calibration coefficients were measured by the thermocouple from a variable temperature controller on a vertical-bore 11.7T system, similar to the magnetic field in which the in vivo experiments were conducted (16). For each voxel, the maximum change in the temperature, ΔTm, was estimated from the difference between the maximum temperature reached after MDMA administration (Tm) and the temperature at the moment of (or prior to) MDMA administration (T0)
| [2] |
The MDMA-induced warming rate (k) immediately after MDMA administration was calculated by fitting the temperature variation over time to a linear function as a first-order approximation to represent the initial rate of heating:
| [3] |
where τ0 represent the time at the moment of MDMA administration. All temperature values from BIRDS were reported in absolute units of °C. The SNR was calculated as signal height / peak-to-peak noise.
In vivo bench experiments (n=7)
Anesthetized rats were mounted on a stereotaxic frame (David Kopf Instruments, Tujunga, CA) placed on a vibration-free table inside a Faraday cage. The scalp and the galea aponeurotica were removed and small burr holes were drilled for insertion of a multisensor probe, which was custom-designed to measure neuronal activity, cerebral blood flow (CBF), and temperature simultaneously. It combined a high impedance (2 MΩ) tungsten microelectrode (FHC, Bowdoinham, ME, USA) that measured extracellular electrical activities of a small neuronal ensemble from the local field potential (LFP) in conjunction with a dual-sensor device for temperature and CBF measurements (250 μm diameter; Oxyflow, Oxford Optronix, UK). The tungsten microelectrode was glued to the side of a needle shaft (30 G; Terumo, Tokyo, Japan), which contained the Oxyflow dual-sensor device. The dual-sensor device was thus isolated from the microelectrode. The dual-sensor probe included a copper-constantan thermocouple probe and a laser-Doppler probe (830 nm) sensitive to red blood cell flow. The thermocouple wire and microelectrodes were grounded separately and the exposed probe tips were physically isolated. These types of multi-modal recordings of LFP with CBF and temperature have been previously used for studies of neurovascular coupling in the cerebral cortex of anesthetized rats (21). The multisensor probe was placed in a stereotaxic holder (Plastic One, Roanoke, VA, USA) and inserted into the cortical and subcortical (i.e., thalamic) regions. Recordings were localized to the middle cortical layers of the forelimb somatosensory cortex (4.4 mm lateral to bregma, 1.0 mm anterior to bregma, 0.9±0.1 mm depth from cortical surface) and ventral posterior lateral nucleus of the thalamus (3 mm lateral to bregma, 3 mm posterior to bregma, 5 mm depth from cortical surface). All simultaneously acquired signals were amplified and filtered before digitization (μ-1401, CED, Cambridge, UK). Electrical and optical signals were digitized using Spike 2 software (CED) at 20 kHz and 50 Hz, respectively. LFP was obtained from low frequency bands using a Butterworth filter (<150 Hz, 24 dB/oct attenuation). All temperature values were used in absolute units of °C, whereas all other measurements (CBF, LFP) were reported relative to their pre-MDMA values. For example, CBF was normalized to the pre-MDMA value and expressed as a fractional change over baseline.
Statistics
Values for measured parameters were expressed as mean ± standard deviation (SD). Statistical significance of group differences was assessed by Student’s t test and p-values less than 0.05 were considered significant. Strength of relationship between parameters was assessed by linear correlation analysis using Matlab (Natick, MA).
Results
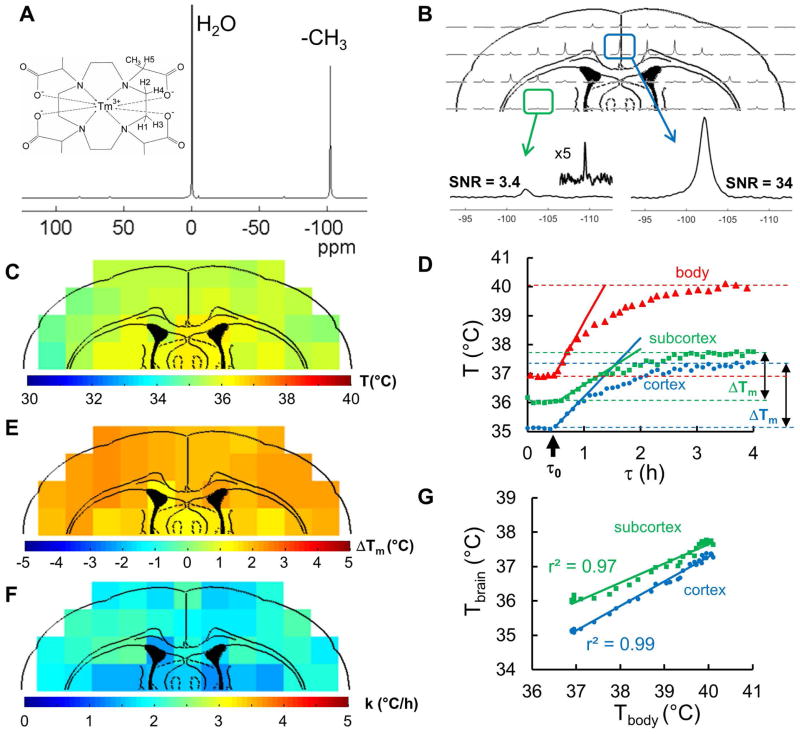
Fig. 1A shows the molecular structure and the 1H MR spectrum of the thermometric biosensor, TmDOTMA−, which possesses four -CH3 groups, each attached to one of the four acetate extensions of the central macrocyclic ring, tetraazacyclododecane. The four methyl protons at ~100 ppm downfield of water are magnetically equivalent, which produce a single resonance with high SNR in the cortex (10:1 to 50:1, Fig.1B, right). However, signals originating in subcortical regions further away from the RF surface coil have lower SNR (<10:1, Fig.1B, left and inset). The high temperature sensitivity (0.7 ppm/°C) of this resonance provides an ideal biosensor when combined with CSI (16,18), permitting localized time-resolved temperature measurements in brain regions before and after MDMA administration (Fig. 1C–D). Fig. 1E–F shows an example of the application of this method in a rat exposed to MDMA, with determination of the main outcome measures: maximum temperature change (ΔTm; Fig. 1E) and warming rate (k; Fig 1F) using equations [1–3]. Good correlations (r2 of 0.99 for cortex and 0.97 for subcortex) were observed between all temperature data points in Fig. 1D from body (Tbody by thermocouple) and brain (Tbrain by BIRDS), before and after MDMA injection.
Figure 1. MDMA-induced temperature changes and dynamics in rat brain measured by TmDOTMA− with BIRDS.
(A) Chemical structure and 1H MR spectrum of TmDOTMA−, which is a paramagnetic complex. A -CH3 group has been inserted into the H6 position of the macrocyclic chelate enabling high SNR for BIRDS. The MR spectrum indicates a prominent hyperfine-shifted 1H signal from the -CH3 group (in relation to water), which is highly temperature sensitive (see eq. 1). (B) Cortical distribution of the 1H signal from the -CH3 group in TmDOTMA− depicted in an in vivo 2D 1H CSI dataset measured by an RF surface coil. Examples of 1H spectra from a cortical voxel (right, SNR=34) and subcortical voxel (left, SNR =3.4) demonstrate that the SNR decreases considerably in the subcortical (deeper) brain regions due to increased distance to the surface coil. However, although different peak heights are observed across voxels, it is the chemical shift of the peak (i.e., not its intensity) that provides the temperature value (see eq. 1). (C) Cortical distribution of temperature derived from the TmDOTMA− signal in B, where prior to MDMA injection subcortical regions (~36 °C) were slightly warmer than cortical areas (~35 °C). (D) Simultaneously measured time courses of body warming (red triangles, measured by a rectal thermocouple) in relation to average cortical (blue circles, by BIRDS, 26 voxels) and subcortical (green squares, by BIRDS, 7 voxels) warming where ΔTm is labeled for BIRDS to show that ΔTm in cortex is slightly larger than ΔTm in subcortex. Although ΔTm in the body (~3 °C) was larger than in the brain (~2 °C), the temperature dynamics were quite similar. Rates of warming in the body (red line), cortex (blue line) and subcortex (green line) immediately after MDMA administration (i.e., black arrow at time τo) were determined from the initial linear rise of temperature over time (see eq. 3). (E) Distribution of maximum temperature change (ΔTm; see eq. 2) induced by MDMA (22±2 mg/kg) - given by difference between maximum temperature reached after (Tm) and before (To) MDMA injection. The difference was larger in the cortex (2.1±0.3 °C) than in subcortical voxels (1.7±0.2 °C). (F) Distribution of warming rates (k; see eq. 3) immediately after MDMA administration. Warming was faster in the cortex (2.1±0.3 °C) than in subcortex (1.5±0.1 °C). (G) Correlation of all temperature data points in D from body (Tbody by thermocouple) and brain (Tbrain by BIRDS), prior to and following MDMA injection, measured independently. Despite the fact that the body was warmer than the brain all throughout, dynamics of Tbrain and Tbody were well correlated in both cortex (r2 = 0.99) and subcortex (r2 = 0.97). All in vivo BIRDS data (at 11.7T) are from the same rat.
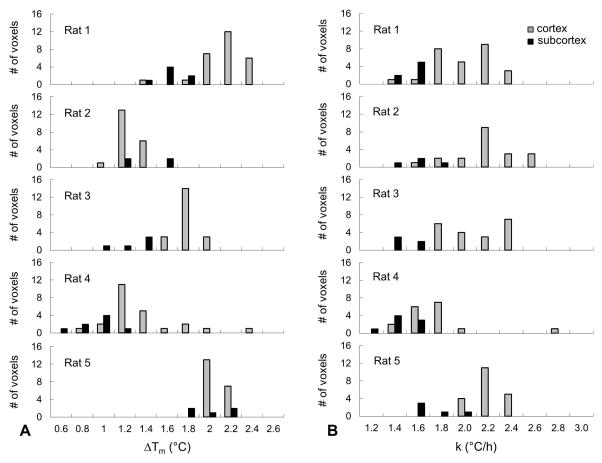
Fig. 2 summarizes BIRDS data in terms of Tm, ΔTm and k calculated from equations 2 and 3. Average baseline temperature was higher in subcortex compared to cortex (36.2±0.3 °C vs. 35.7±0.4 °C; p = 0.056). MDMA injection led to a rise in temperature in both brain regions, eventually reaching similar peak values (cortex, 37.3±0.7 °C; subcortex, 37.5±0.5 °C; p = 0.62). The overall change in temperature was thus greater in cortex than in subcortex (ΔTm: 1.6±0.4 °C vs. 1.3±0.4 °C; p = 0.27). We observed a large between-animal variation in the regional distribution of cortical and subcortical ΔTm, spanning a range of values from 0.6 to 2.4 °C (Fig. 2A). Within individual animals, however, the cortical regional variation was small (SD ≤ 0.34 °C). Warming rates (k) were similar in all rats investigated, 2.0±0.2 °C/h in cortex and 1.5±0.2 °C/h in subcortex, and the values showed a relatively homogenous distribution as observed by their small SD (Fig. 2B). However, two-tail Student’s t test comparison between cortex and subcortex shows that the warming rates were significantly different in these two regions (p = 0.004). The average baseline body temperature was 37.2±0.3 °C, which rose to 39.5±0.7 °C after MDMA injection; thus resulting in an average temperature change ΔTm of 2.3±0.7 °C. The average body warming rate was higher than in the brain, 2.9±0.6 °C/h. Administration of saline through the indwelling catheter showed no effect on brain or body temperature, confirming that the MDMA (and not vehicle or the conditions of animal preparation) was responsible for the observed temperature effects (Supplementary Fig. 1).
Figure 2. Distributions of MDMA-induced maximum temperature change in relation to warming rate in rat brain measured by TmDOTMA− with BIRDS.
(A) Range of maximum temperature change in cortex (gray bars) and subcortex (black bars) induced by MDMA (ΔTm; see Fig. 1 and eq. 2 for how ΔTm was estimated) in different rats. Although ΔTm was slightly different in each rat, the variance in ΔTm was within a narrow range of 0.4–0.8 °C indicating uniform MDMA-induced changes throughout the brain. The average cortical and subcortical ΔTm measured across all rats (n=5) was 1.6±0.4 °C and 1.3±0.4 °C, respectively. (B) Range of cortical warming rate (k) obtained in different rats (see Fig. 1 and eq. 3 for how k was estimated). Calculated values of k were quite similar in all rats, in the range of 1.4–2.6 °C/h. The average k calculated across all rats (n=5) was 2.0±0.3 °C/h in the cortex and 1.5±0.2 °C/h in subcortex.
A strong linear correlation (r2 = 0.96) was observed between the average temperature changes between cerebral cortex and body induced by MDMA (Fig. 3A), although the strength of the correlation between subcortex and body temperature changes was weaker (r2 = 0.46). The change in body temperature consistently exceeded the change in brain temperature (ΔTm,body > ΔTm,brain). In contrast to temperature changes, brain and body warming rates (Fig. 3B) showed a weaker correlation (cortex, r2 = 0.43; subcortex, r2 = 0.47), with the body warming rates spanning a larger range of values (2.1 to 3.5 °C/h) compared to the brain (1.4 to 2.1 °C/h).
Figure 3. Correlation between brain and body measurements of MDMA-induced maximum temperature changes (ΔTm) and warming rates (k).
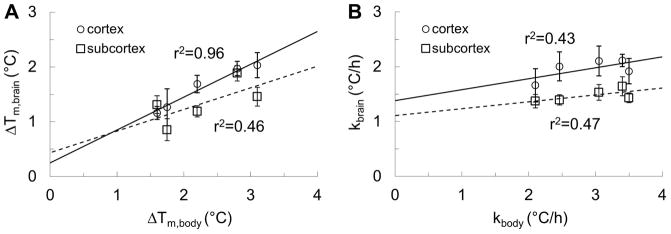
(A) Comparison between the average of maximum temperature changes in the brain (ΔTm,brain) and the body (ΔTm,body) shows a very good correlation (r2 = 0.96; see Fig. 1 and eq. 2 for how ΔTm was estimated) in the cortex (circles), but weak (r2 = 0.46) in the subcortex (squares). Note that ΔTm,body was always larger than ΔTm,brain. The correlated temperatures in the body and the cortex suggest that body warming may be contributing, in part, to cortical warming with MDMA exposure. (B) Comparison between the warming rates in the brain (kbrain) and the body (kbody) shows a poorer correlation in both cortex (circles, r2 = 0.43, see Fig. 1 and eq. 3 for how k was estimated) and subcortex (squares, r2 = 0.47). Since the range of kbody (2–3.5 °C/h) was much larger than the range of kbrain (1.4–2.1 °C/h), the temperature rise in the cortex is more tightly regulated than temperature rise in the body.
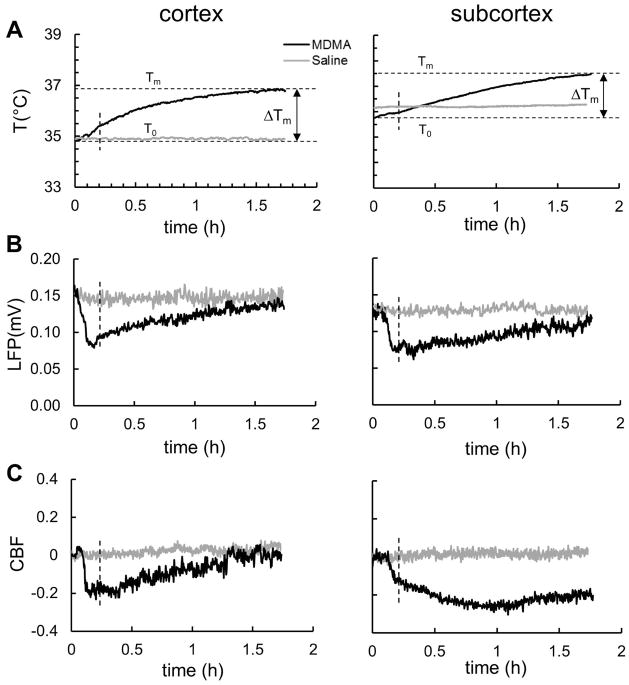
To explore the state of neurovascular coupling in response to MDMA across different brain regions, simultaneous measurements of temperature (thermocouple), LFP (microelectrode), and CBF (laser-Doppler) from both cortex and subcortex (i.e. thalamus) were conducted using a multisensor probe. The average temperature, neuronal activity and blood flow from the cortex and thalamus are shown in Fig. 4. Both the maximal temperature change and the warming rate (Fig. 4A) were higher in the cortex (ΔTm = 2.0 ± 0.6 °C and k = 2.5 ± 1.6 °C/h) than in the thalamus (ΔTm = 1.7 ± 0.4 °C and k = 1.3 ± 0.5 °C/h). The cortical values of ΔTm and k measured by thermocouple did not differ significantly from those determined in cortex by BIRDS (ΔTm = 1.6±0.4 °C; p = 0.27 and k = 2.0±0.2 °C/h; p = 0.50).
Figure 4. Dynamics of temperature (T), local field potential (LFP), and cerebral blood flow (CBF) in cortex and subcortex during MDMA exposure.
MDMA (black; n=4) or saline (gray; n=3) were administered at time 0 and the data shown represent simultaneously recorded average signal. The effect of saline administration was obtained in a separate group of animals. (A) Comparison of T changes (measured by thermocouple) in cortex and subcortex (i.e, thalamus), shows that maximum temperature change ΔTm in the cortex (2.0±0.6 °C) was slightly larger than in the thalamus (1.7±0.4 °C), basal temperature prior to MDMA exposure (To) was slightly higher in the thalamus (35.8±0.6 °C) than in the cortex (34.9±0.8 °C). No significant temperature changes were observed after saline administration in both cortex and thalamus. (B) Comparison of neuronal activity in cortex and thalamus, reflected by LFP (measured by microelectrodes), shows that activity in both regions kept decreasing for ~10 minutes following MDMA injection and after which activity increased at a faster rate in cortex than in thalamus. Saline administration did not significantly alter neuronal activity. (C) Comparison of tissue perfusion in cortex and thalamus, reflected by CBF (measured by laser-Doppler), shows that CBF in both regions decreased fast for 10 minutes of following MDMA injection and after which CBF increased in cortex and not changed significantly in thalamus. No significant CBF changes were induced by saline administration in cortex or thalamus. CBF changes are reflected with respect to the pre-MDMA value and expressed as a fractional change over baseline. See Fig. 5 for details of correlation between each measurement type across both regions (i.e., data points beyond the vertical dashed line were used such that the acute neuronal activity depression immediately following MDMA injection was not included).
MDMA injection led to a rapid initial decline in the LFP (cortex, 255±125 %/h; thalamus, 225±70 %/h) followed by a slower increase in LFP in both regions (cortex, 23±9 %/h; thalamus, 20±2 %/h) (Fig. 4B). The LFP recovery nearly reached pre-MDMA values for both regions. The MDMA-induced CBF dynamics showed early similarities to the LFP patterns but late responses diverged between regions. In both regions there were large initial declines in CBF followed by slower changes in cortex (increase) and thalamus (prolonged decrease) (Fig. 4C). Cortical CBF declined rapidly in the first 10 minutes (110±50 %/h), followed by a slow recovery (31±15 %/h), whereas thalamic CBF decreased more slowly (48±12 %/h) in the first 60 minutes, followed by a much slower increase in CBF (14±11 %/h), which did not recover to pre-MDMA CBF values. For comparison, no significant changes in temperature, neuronal activity or CBF were seen in cortex or subcortex (i.e., thalamus) in response to saline administration (Fig. 4).
Whereas good correlations were seen between the dynamics of temperature and LFP in both regions, suggesting that tissue warming is associated with increases in neuronal activity in both regions, this association was higher in cortex than in subcortex (i.e., thalamus) (Fig. 5A). The strong positive correlation between the dynamics of temperature and CBF in the cortex suggests that tissue warming is coupled to tissue perfusion increases in the cortex. In contrast to cortex, the subcortex (i.e., thalamus) displayed a negative and weaker relationship, suggesting that tissue warming is decoupled from tissue perfusion in this region (Fig. 5B). Plotting CBF against LFP for cortex and subcortex (i.e., thalamus) after MDMA injection revealed more clearly the substantial differences in neurovascular coupling between these regions (Fig. 5C). Overall these results suggest that while both regions experience MDMA-induced warming, neurovascular coupling in the subcortex (i.e., thalamus) is more severely affected than in the cortex.
Figure 5. Correlation between temperature (T), local field potential (LFP), and cerebral blood flow (CBF) dynamics in rat cortex and thalamus during MDMA exposure.
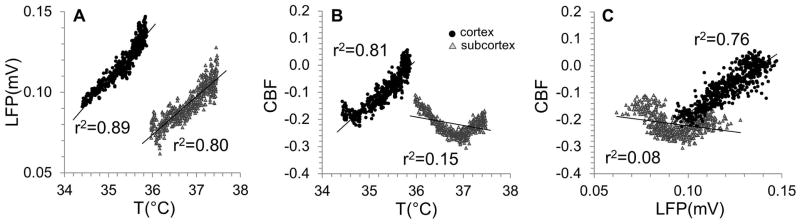
The analysis included multi-modal data from Fig 4 excluding data points prior to the acute neuronal activity depression immediately following MDMA injection (see vertical dashed line in Fig. 4). (A) Linear correlation between dynamics of LFP and T in cortex (black circles) and in thalamus (gray triangles) shows that coupling between temperature and neuronal activity was slightly stronger in cortex than in thalamus (i.e., r2 = 0.89 vs. r2 = 0.80, respectively), suggesting that tissue warming in both regions is associated with increased neuronal activity. (B) Linear correlation between dynamics of CBF and T in cortex (black circles) and in thalamus (gray triangles) shows that the association between tissue perfusion and temperature changes was positive in cortex but negative in thalamus (i.e., r2 = 0.81 vs. r2 = 0.15, respectively), suggesting that tissue warming in cortex is coupled to tissue perfusion increases but decoupled in thalamus. (C) Linear correlation between dynamics of CBF and LFP in cortex (black circles) and in thalamus (gray triangles) shows that coupling between tissue perfusion and neuronal activity was positive in cortex but negative in thalamus (i.e., r2 = 0.76 vs. r2 = 0.08, respectively), suggesting that neurovascular coupling in thalamus is very different than in cortex. CBF was normalized to the pre-MDMA value and expressed as a fractional change over baseline.
Discussion
The high SNR of the biosensor allowed spectroscopic images to be acquired in less than 5 min with BIRDS (Figure 1D), providing sufficient time resolution to capture the rates of rise in both cortical and subcortical temperature after MDMA administration. The baseline cortical and subcortical temperatures before MDMA injection were similar for all rats investigated (SD ≤ 0.4 °C), although the maximum temperature observed following MDMA injection was more variable between rats (SD of 0.7 °C in the cortex and 0.5 °C in subcortex; Fig. 2A), possibly reflecting differences in MDMA absorption through the intraperitoneal route and/or degradation by hepatic metabolism. Interestingly, the temperature gradient between cortical and subcortical regions after MDMA injection was smaller than before the injection, reflecting the lower temperature increase in the subcortex compared to cortex.
Despite differences in peak temperatures between rats administered with MDMA, the distributions of cortical and subcortical temperature changes were relatively homogenous within individual rats (%SD <12%, with exception of one animal (Rat 4) with cortical %SD of 27%; Fig. 2A). The distributions of cortical and subcortical warming rates were also quite homogenous (%SD <13%; with exception of one animal (Rat 4) with cortical %SD of 18%; Fig. 2B), showing little variation across animals, with SD of 0.2 °C/h (or %SD = 10%). Comparing cortical and subcortical temperature changes and warming rates in the same animal we observed that their distributions were more homogenous in subcortex (SDsubcortex < SDcortex), probably due to larger cortical variations of CBF and/or some heat loss to environment.
A consistent observation was that the absolute temperatures and warming rates of the body exceeded that of the brain (Figs. 1D and Fig. 3A and 3B). The average cortical temperature change was highly correlated with body temperature (Fig. 3A, solid line), suggesting that heat transport through blood flow from body-to-brain could contribute, at least partly, to cortical warming. However, subcortical temperature change was not well correlated with body temperature (Fig. 3A, dashed line) suggesting a lower contribution of heat transported from body-to-brain in this region. Moreover, cortical and subcortical warming rates were poorly correlated with body warming rates (Fig. 3B), suggesting that additional factors contributed to cortical warming. These results suggest that the MDMA-induced increase in body temperature was at least partially responsible for cortical hyperthermia, a likely product of body heat generation by MDMA and reduced heat loss by peripheral vasoconstriction. Studies of temperature dynamics in the rat brain suggest that four processes are responsible for local temperature changes: metabolic heat production, heat removal or addition through blood flow, conductive heat loss to adjacent regions and dissipative heat loss to the environment (21,22). Of these, a change in brain metabolism and/or blood flow will have the largest effect on brain temperature, whereas conductive or dissipative heat losses are relatively small and can be neglected to first-order approximation (21). The correlation between the brain and body temperature change (Figs. 1G and 3A) is consistent with these models, and based on the temperature gradient (body > cortex) observed under our experimental conditions, blood flowing from body-to-brain appeared to contribute significantly to cortical warming. Consistent with the model proposed by Zhu et al (22) the more deeply situated thalamus was warmer by ~1°C (i.e., consistent with our findings) than the cortex before MDMA administration, and this temperature gradient was maintained (although smaller) after MDMA injection, despite opposing effects on CBF in subcortex (decreased) and cortex (increased). The opposite CBF responses following MDMA exposure and the induced hyperthermia in subcortex and cortex also correlated with their slower and faster warming rates, respectively.
Similar MDMA-induced temperature changes of 1.3–1.5 °C in the nucleus accumbens and hippocampus were reported previously by Brown et al (23). Using the time dependence of temperature changes reported in the Brown et al study, we estimated an initial warming rate of ~1.0 °C/h under quiet resting and ~1.2 °C/h under social interaction conditions. These warming rates are approximately half of the warming rates measured in this study (2 °C/h), but this is probably due to the higher MDMA dose used in our study (22 mg/kg) versus that of Brown et al. (9 mg/kg). The MDMA dose in our study is within the range previously investigated in rodents (5 to 40 mg/kg, s.c. or i.p.) (24,25), and is less than half the estimated LD50 dose in rats (26). Generally, the LFP and CBF time courses in both regions behaved similarly with the exception of the immediate effects after MDMA injection (Figs. 4B and 4C). Both of these signals from both these regions showed an initial decrease followed by a much slower rise. While both LFP and CBF seemed to increase to pre-MDMA levels in the cortex, the LFP and CBF stayed well below the pre-MDMA level in the subcortex (i.e., thalamus). Thus, the increased temperature in the cortex triggers a steady blood flow and neuronal activity, while in the thalamus there was a lack of blood flow response in relation to neuronal activity despite temperature increases.
These results suggest that the neurovascular coupling in the cortex is well maintained during cortical warming, whereas there is poor neurovascular coupling in the thalamus during thalamic warming (Fig. 5). The increased cortical blood flow with MDMA was observed also by others using bolus-tracking arterial spin labelling MRI (27) suggesting that some neuroprotection is active in the cortex with MDMA. However lack of thalamic blood flow increase with thalamic hyperthermia suggests that this region may be more susceptible to injury during MDMA exposure. While whole body cooling may be effective for cooling the whole brain, the use of pharyngeal cooling may selectively cool deeper parts of the brain more efficiently (28–32). It is clear that a better description of the mechanisms involved in the MDMA-induced brain hyperthermia is required to separate measurements of intracerebral metabolic heat production, which cannot be achieved directly from temperature measurements due to heat removal/addition by blood flow or conductive and dissipative heat loss to adjacent regions. A quantitative assessment of heat production/removal by each of the mechanisms involved in the MDMA-induced brain warming requires careful separation of each contribution and will be the scope of future studies.
Heat transfer to brain from the body by the flow of warmer blood may not be the only process however that could contribute to cortical MDMA-induced hyperthermia. Mitochondrial UCPs, which dissipate the hydrogen ion gradient across the mitochondrial inner membrane used for ATP production as heat, are expressed in brain (14). UCPs are known to play a role in methamphetamine and MDMA-induced systemic thermogenesis (13,33), primarily UCP3 of skeletal muscle (14), though other UCP isoforms (e.g., UCP2, UCP4, UCP5) are expressed in brain, especially in deep subcortical areas, e.g., thalamus and hypothalamus (15,34,35). However, the finding that the rate of thalamic warming was slower than the cortex and correlated well with the reduction in thalamic CBF, despite increased thalamic activity (LFP), argues against a significant contribution of endogenous metabolism (and UCP-induced thermogenesis) to the observed thalamic temperature change after MDMA.
In the present study, the TmDOTMA− signal was localized mainly in cortex and some subcortical regions (Fig. 1C) due to limited RF coverage by the surface coil, reducing SNR farther away from the cortex, and thus precluding accurate temperature assessment in deeper brain regions with BIRDS. However, the cortical voxels are positioned closer than other (deep) brain structures and therefore the TmDOTMA− signal is sufficient for chemical shift (and thus, temperature) determination. Thus, to investigate the possibility of differential heating profiles in specific brain regions and also potential for variable neurovascular coupling across these regions, we conducted simultaneous cortical and subcortex (i.e., thalamus) recordings of temperature, LFP, and CBF (Fig. 4).
The temperature rise in subcortex lagged that of cortex (Fig. 4A), and both maximum temperature change and warming rate were smaller than in cortex. The different time courses of temperature rise in cortex and subcortex (i.e., thalamus) observed in the current study could be explained by a heat dissipation and conduction model similar to that described by Zhu et al. (22). Their results show a similar trend, where the difference between the brain and the body temperature is negative and larger at the surface of the brain (cortex) becoming smaller in deeper brain regions (thalamus). Thus, the larger temperature rise in the cortex could be due to lower basal temperatures recorded before the MDMA injection in the cortex (34.9±0.8 °C) than in thalamus (35.8±0.6 °C), which was probably a consequence of greater heat loss from cortical regions to the environment.
There are limitations in our study that could affect generalization of our findings. The rats in our study were anesthetized, reducing body and brain blood flow and metabolism, and immobilized so that MDMA-induced heat production did not arise through muscle work. The core temperature increase with MDMA, independent of how it was achieved, did occur under anesthesia and paralysis so this aspect of MDMA action is faithfully reproduced. A more serious potential confounder is the effect of anesthesia on CBF. A reduction in CBF before MDMA administration would reduce the dissipation of heat produced in the brain, as well as reduce MDMA-related heat produced in the periphery from reaching the brain. An imbalance in metabolism over blood flow, i.e., loss of neurovascular coupling, could lead to a rise in brain temperature. However, we and others have shown that neurovascular coupling is maintained under anesthesia, and it was MDMA that disrupted this normally tight coupling in the thalamus but not cortex, and both regions experienced a rise in temperature. Another potential confounder was the use of a heating pad to maintain body temperature during anesthesia, which compensates for body heat loss but not fully for brain (36). For deep pentobarbital anesthesia brain temperature was ~0.6 °C lower than body temperature, but this would be expected to be less as anesthesia depth decreases and brain activity rises. As MDMA led to a temperature increase above the baseline of 37 °C, and saline administration did not alter cortical or thalamic temperature, the maintenance of baseline (normothermic) core temperature prior to MDMA treatment does not alter our conclusions. We note that MDMA exposure in humans generally involves hot ambient environments (i.e., greater than normal room temperature of ~25 °C), and activity levels contributing to elevated body temperature, well beyond the ambient 20 °C temperature of the magnet bore. In awake rats under cool (i.e., 15 °C) ambient temperature conditions MDMA leads to a paradoxical decline in body temperature (37). Our goal in this study was to assess the heat raising effects of MDMA from a uniform baseline of body and brain temperature.
In summary, we studied acute cerebral hyperthermia in rodents induced by the psychostimulant drug MDMA using a novel magnetic resonance method (BIRDS) employing an exogenous temperature probe, TmDOTMA− to assess temperature distribution and dynamics throughout the cerebral cortex and in some subcortical regions. Single bolus injection of MDMA produced a rapid increase in temperature (2 °C/h) with peak change of 2.4°C at 2 h post-injection. A strong correlation was observed between changes in cortical and body temperature suggesting that the heat produced in the body reached the brain through the circulation, contributing partially to the rise in cortical temperature. Multi-modal assessments of temperature, blood flow and neuronal activity suggest neurovascular coupling differs in thalamus compared to cortex in response to MDMA-induced hyperthermia. Based on the large and rapid change in brain temperature induced by MDMA, future investigations involving repetitive MDMA exposures (38), and measurement of other brain regions where MDMA neurotoxicity is known to manifest, could be highly informative.
Supplementary Material
The time courses of body temperature (red triangles, measured by a rectal thermocouple), cortical temperature (blue circles, by BIRDS) and subcortical temperature (green squares, by BIRDS) show that saline administration through the indwelling catheter (indicated by the black arrow) does not result in an increase of body or brain temperatures.
Acknowledgments
Support: NIH grants P30 NS-52519, R01 MH-067528, R01 CA-140102, R01 EB-011968
We would like to thank Terry Nixon and Peter Brown for engineering help and consultations. Supported by NIH grants P30 NS-52519, R01 MH-067528, R01 CA-140102, R01 EB-011968. MDMA was graciously provided by the Drug Supply Program of NIDA at NIH.
Abbreviations
- BIRDS
biosensor imaging of redundant deviation in shifts
- CBF
cerebral blood flow
- CSI
chemical shift imaging
- DOTMA4−
1,4,7,10-tetramethyl 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate
- LFP
local field potential
- MDMA
(+/−)3,4-methylenedioxymethamphetamine
- RF
radio frequency
- SNR
signal-to-noise ratio
- UCP
uncoupling proteins
Footnotes
Disclosure/Conflict of Interest: Dr. Behar discloses ownership of Pfizer common stock. All authors declare no conflict of interest.
References
- 1.Johnston LD, O’Malley PMJ, JGB . The Monitoring the Future national results on adolescent drug use: overview of key findings, 1999. Rockville, MD: National Institute on Drug Abuse; 2000. [Google Scholar]
- 2.Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96(6):678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- 3.Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992;89(5):1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 2007;189(4):489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- 5.Schindler CW, Thorndike EB, Blough BE, Tella SR, Goldberg SR, Baumann MH. Effects of 3,4-methylenedioxymethamphetamine (MDMA) and its main metabolites on cardiovascular function in conscious rats. British Journal of Pharmacology. 2014;171(1):83–91. doi: 10.1111/bph.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenouda SK. The cardiovascular and cardiac actions of ecstasy and its metabolites. Curr Pharm Biotechnol. 2010;11:470–475. doi: 10.2174/138920110791591526. [DOI] [PubMed] [Google Scholar]
- 7.Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, Mendelson J. Cardiovascular Effects of 3,4-Methylenedioxymethamphetamine: A Double-Blind, Placebo-Controlled Trial. Annals of Internal Medicine. 2000;133(12):969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- 8.Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. The Journal of pharmacology and experimental therapeutics. 1999;290(1):136–145. [PubMed] [Google Scholar]
- 9.Green AR, O’Shea E, Colado MI. A review of the mechanisms involved in the acute MDMA (ecstasy)-induced hyperthermic response. Eur J Pharmacol. 2004;500(1–3):3–13. doi: 10.1016/j.ejphar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Saadat KS, O’Shea E, Colado MI, Elliott JM, Green AR. The role of 5-HT in the impairment of thermoregulation observed in rats administered MDMA (‘ecstasy’) when housed at high ambient temperature. Psychopharmacology (Berl) 2005;179(4):884–890. doi: 10.1007/s00213-004-2106-1. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto Y, Ohkura M, Inoue K, Yamada J. Involvement of serotonergic and dopaminergic mechanisms in hyperthermia induced by a serotonin-releasing drug, p-chloroamphetamine in mice. Eur J Pharmacol. 2001;430(2–3):265–268. doi: 10.1016/s0014-2999(01)01386-3. [DOI] [PubMed] [Google Scholar]
- 12.Rusyniak DE, Sprague JE. Hyperthermic syndromes induced by toxins. Clin Lab Med. 2006;26(1):165–184. ix. doi: 10.1016/j.cll.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Mills EM, Banks ML, Sprague JE, Finkel T. Pharmacology: uncoupling the agony from ecstasy. Nature. 2003;426(6965):403–404. doi: 10.1038/426403a. [DOI] [PubMed] [Google Scholar]
- 14.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci. 2005;6(11):829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 15.Richard D, Clavel S, Huang Q, Sanchis D, Ricquier D. Uncoupling protein 2 in the brain: distribution and function. Biochemical Society transactions. 2001;29(Pt 6):812–817. doi: 10.1042/0300-5127:0290812. [DOI] [PubMed] [Google Scholar]
- 16.Coman D, Trubel HK, Hyder F. Brain temperature by Biosensor Imaging of Redundant Deviation in Shifts (BIRDS): comparison between TmDOTP5− and TmDOTMA−. NMR Biomed. 2010;23(3):277–285. doi: 10.1002/nbm.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coman D, Trubel HK, Rycyna RE, Hyder F. Brain temperature and pH measured by (1)H chemical shift imaging of a thulium agent. NMR Biomed. 2009;22(2):229–239. doi: 10.1002/nbm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coman D, de Graaf RA, Rothman DL, Hyder F. In vivo three-dimensional molecular imaging with Biosensor Imaging of Redundant Deviation in Shifts (BIRDS) at high spatiotemporal resolution. NMR Biomed. 2013;26(11):1589–1595. doi: 10.1002/nbm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trubel HK, Maciejewski PK, Farber JH, Hyder F. Brain temperature measured by 1H-NMR in conjunction with a lanthanide complex. J Appl Physiol. 2003;94(4):1641–1649. doi: 10.1152/japplphysiol.00841.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bereczki D, Wei L, Otsuka T, Hans FJ, Acuff V, Patlak C, Fenstermacher J. Hypercapnia slightly raises blood volume and sizably elevates flow velocity in brain microvessels. Am J Physiol. 1993;264(5 Pt 2):H1360–1369. doi: 10.1152/ajpheart.1993.264.5.H1360. [DOI] [PubMed] [Google Scholar]
- 21.Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26(1):68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- 22.Zhu M, Ackerman JJ, Sukstanskii AL, Yablonskiy DA. How the body controls brain temperature: the temperature shielding effect of cerebral blood flow. Journal of applied physiology (Bethesda, Md : 1985) 2006;101(5):1481–1488. doi: 10.1152/japplphysiol.00319.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (‘ecstasy’): modulation by environmental conditions. European Journal of Neuroscience. 2004;20(1):51–58. doi: 10.1111/j.0953-816X.2004.03453.x. [DOI] [PubMed] [Google Scholar]
- 24.Rouine J, Kelly ME, Jennings-Murphy C, Duffy P, Gorman I, Gormley S, Kerskens CM, Harkin A. Investigation of the mechanisms mediating MDMA “Ecstasy”-induced increases in cerebro-cortical perfusion determined by btASL MRI. Psychopharmacology. 2014:1–13. doi: 10.1007/s00213-014-3790-0. [DOI] [PubMed] [Google Scholar]
- 25.Soleimani Asl S, Mousavizedeh K, Pourheydar B, Soleimani M, Rahbar E, Mehdizadeh M. Protective effects of N-acetylcysteine on 3, 4-methylenedioxymethamphetamine-induced neurotoxicity in male Sprague–Dawley rats. Metab Brain Dis. 2013;28(4):677–686. doi: 10.1007/s11011-013-9423-1. [DOI] [PubMed] [Google Scholar]
- 26.Hardman HF. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicology and applied pharmacology. 1973;25(2):299–309. doi: 10.1016/s0041-008x(73)80016-x. [DOI] [PubMed] [Google Scholar]
- 27.Rouine J, Gobbo OL, Campbell M, Gigliucci V, Ogden I, McHugh Smith K, Duffy P, Behan B, Byrne D, Kelly ME, Blau CW, Kerskens CM, Harkin A. MDMA ‘ecstasy’ increases cerebral cortical perfusion determined by bolus-tracking arterial spin labelling (btASL) MRI. Br J Pharmacol. 2013;169(5):974–987. doi: 10.1111/bph.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 29.Trubel H, Herman P, Kampmann C, Novotny E, Hyder F. Selective pharyngeal brain cooling. Biomedizinische Technik Biomedical engineering. 2003;48(11):298–300. doi: 10.1515/bmte.2003.48.11.298. [DOI] [PubMed] [Google Scholar]
- 30.Trubel H, Herman P, Kampmann C, Huth R, Maciejewski PK, Novotny E, Hyder F. A novel approach for selective brain cooling: implications for hypercapnia and seizure activity. Intensive care medicine. 2004;30(9):1829–1833. doi: 10.1007/s00134-004-2350-1. [DOI] [PubMed] [Google Scholar]
- 31.Gunn TR, Wilson NJ, Aftimos S, Gunn AJ. Brain hypothermia and QT interval. Pediatrics. 1999;103(5 Pt 1):1079. doi: 10.1542/peds.103.5.1079. [DOI] [PubMed] [Google Scholar]
- 32.Tooley JR, Eagle RC, Satas S, Thoresen M. Significant head cooling can be achieved while maintaining normothermia in the newborn piglet. Archives of disease in childhood Fetal and neonatal edition. 2005;90(3):F262–266. doi: 10.1136/adc.2003.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague JE, Mallett NM, Rusyniak DE, Mills E. UCP3 and thyroid hormone involvement in methamphetamine-induced hyperthermia. Biochemical pharmacology. 2004;68(7):1339–1343. doi: 10.1016/j.bcp.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Sanchis D, Fleury C, Chomiki N, Goubern M, Huang Q, Neverova M, Gregoire F, Easlick J, Raimbault S, Levi-Meyrueis C, Miroux B, Collins S, Seldin M, Richard D, Warden C, Bouillaud F, Ricquier D. BMCP1, a novel mitochondrial carrier with high expression in the central nervous system of humans and rodents, and respiration uncoupling activity in recombinant yeast. The Journal of biological chemistry. 1998;273(51):34611–34615. doi: 10.1074/jbc.273.51.34611. [DOI] [PubMed] [Google Scholar]
- 35.Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(23):10417–10427. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiology & behavior. 2005;84(4):563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Green AR, O’Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146(2):306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green AR, Sanchez V, O’Shea E, Saadat KS, Elliott JM, Colado MI. Effect of ambient temperature and a prior neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA) on the hyperthermic response of rats to a single or repeated (‘binge’ ingestion) low dose of MDMA. Psychopharmacology (Berl) 2004;173(3–4):264–269. doi: 10.1007/s00213-003-1725-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The time courses of body temperature (red triangles, measured by a rectal thermocouple), cortical temperature (blue circles, by BIRDS) and subcortical temperature (green squares, by BIRDS) show that saline administration through the indwelling catheter (indicated by the black arrow) does not result in an increase of body or brain temperatures.