Abstract
Introduction
5-Fluoro-2’-deoxycytidine (FdCyd; NSC48006), a fluoropyrimidine nucleoside inhibitor of DNA methylation, is degraded by cytidine deaminase (CD). Pharmacokinetic evaluation was carried out in cynomolgus monkeys in support of an ongoing phase I study of the PO combination of FdCyd and the CD inhibitor tetrahydrouridine (THU; NSC112907).
Methods
Animals were dosed intravenously (IV) or per os (PO). Plasma samples were analyzed by LC-MS/MS for FdCyd, metabolites and THU. Clinical chemistry and hematology were performed at various times after dosing. A pilot pharmacokinetic study was performed in humans to assess FdCyd bioavailability.
Results
After IV FdCyd and THU administration, FdCyd Cmax and AUC increased with dose. FdCyd half-life ranged between 22–56 min, clearance was approximately 15 mL/min/kg. FdCyd PO bioavailability after THU ranged between 9–25% and increased with increasing THU dose. PO bioavailability of THU was less than 5%, but did result in plasma concentrations associated with inhibition of its target CD. Human pilot studies showed comparable bioavailability for FdCyd (10%) and THU (4.1%).
Conclusion
Administration of THU with FdCyd increased the exposure to FdCyd and improved PO FdCyd bioavailability from <1% to 24%. Concentrations of THU and FdCyd achieved after PO administration are associated with CD inhibition and hypomethylation, respectively. The schedule currently studied in phase I studies of PO FdCyd and THU is daily times 3 at the beginning of the first and second weeks of a 28 day cycle.
Keywords: fluorodeoxycytidine, tetrahydouridine, toxicokinetics, toxicity, monkeys, DNA methylation inhibitor
Introduction
5-Fluoro-2’-deoxycytidine (FdCyd, NSC48006) was originally developed as a cytidine deaminase (CDA) activated pro-drug for thymidylate synthase inhibitor 5-fluorouracil. However, by the mid-1980’s, the combination of FdCyd and THU was proposed as an antineoplastic treatment for tumors resistant to 5FU [1–3], suggesting a mechanism of action different from thymidylate synthase inhibition via 5-FU generation. Indeed, it was demonstrated that the administration of FdCyd with 3,4,5,6-tetrahydrouridine (THU, NSC112907) increased the incorporation of FdCyd into DNA and resulted in higher cytotoxicity [4]. Subsequently, FdCyd was shown to have activity as a DNA methyltransferase inhibitor through incorporation into DNA, and formation of tight binding complexes with DNA methyltransferase [5,6]. In this respect, the ratio of FdCyd to FdUrd is an important pharmacological metric because FdCyd incorporation into DNA requires DNA synthesis and cell division, while its CDA generated metabolite FdUrd is a cytotoxic which inhibits DNA replication [7]. The DNA hypomethylating effects of FdCyd in combination with THU have been examined in vitro [8] where the combination of FdCyd and THU was compared with decitabine. Clinically, changes in DNA methyltransferase I and LINE1 methylation as well as upregulation of p16 expression were observed in paracentesis specimens from patients enrolled on an NCI-supported phase I clinical trial of IV FdCyd and THU [8]. The advantage of FdCyd over the FDA approved agents decitabine and azacitidine is the aqueous stability of FdCyd, facilitating both intravenous (IV) dosing, and the pursuit of an oral (PO) dosing regimen [9,10].
FdCyd and THU have been evaluated in phase II clinical studies that use an IV route of administration. However, PO administration of this combination may allow for more prolonged exposure to FdCyd, which is desirable in hypomethylating therapy [11,4]. In addition, it may be more convenient, requiring fewer office visits, which would reduce traveling to and waiting in the clinic. The preclinical studies reported here describe the PO and IV plasma pharmacokinetics of FdCyd, its metabolites, and THU and were designed to provide guidance for first-in-human studies of PO FdCyd and THU.
Materials and Methods
Chemicals and reagents
5-fluoro-2’-deoxycytidine (FdCyd), 5-fluoro-2’-deoxyuridine (FdUrd), 5-fluorouracil (FU), 5-fluorouridine (FUrd), and 3, 4, 5, 6-tetrahydrouridine (THU), and [D4]-tetrahydrouridine (D4-THU) were provided by the Developmental Therapeutics Program, National Cancer Institute (Bethesda, MD). 5-fluorocytosine (FC) and formic acid were obtained from Sigma-Aldrich (St. Louis, MO). [15N2]-5-Fluorouracil was obtained from CDN Isotopes (Pointe-Claire, QC). 5’-deoxy-5-fluorocytidine was generously provided by Roche (Basel, Switzerland). Ethyl acetate and glacial acetic acid were purchased from Fisher Chemicals (Fairlawn, NJ). Acetonitrile and water were purchased from Honeywell Burdick & Jackson (Muskegon, MI). All reagents were of analytical grade. Control plasma from untreated animals was obtained from IIT Research Institute (Chicago, IL) and Lampire Biological Laboratories (Pipersville, PA).
Animals
Young, sexually mature male and female cynomolgus monkeys were individually housed in stainless steel cages and handled in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and on a protocol approved by the Institutional Animal Care and Use Committee of IIT Research Institute (Chicago, IL). Each animal was uniquely identified by tattoo using number and/or letter combination. All animals were quarantined for a minimum of 42 days prior to the start of the study and received a detailed physical examination by a laboratory veterinarian prior to the initiation of dosing. In addition, all animals had a minimum of two consecutive negative TB tests. Body weights taken one week prior to treatment ranged from 2.5 to 4.0 kg. Room temperature was regulated at 22 ± 1 °C with relative humidity of 50 ± 30%, and the rooms were kept on 12-hour light/dark cycles. Animals were provided with certified, commercial monkey chow twice daily. Diets were supplemented with fresh fruit and/or other primate diet supplements. Water from the public supply was given ad libitum.
Treatments
For PO administration, animals (n=4, 2 per sex/group,) were dosed at 0.1, 1, or 10 mg/kg FdCyd by gavage, 1 h following administration of 500 mg/kg THU by gavage (Table 1). Animals were dosed for four cycles, each consisting of dosing for three consecutive days followed by a 4-day dosing holiday. Dose levels of FdCyd and THU and the dose schedule used in this study were selected on the basis of the results of a previous study, in which severe gastrointestinal toxicity was observed in monkeys receiving daily exposure for seven days to FdCyd at doses higher than 10 mg/kg/day in combination with THU at 150 mg/kg/day PO. Control monkeys (n=8) received two oral gavage doses of water for injection (WFI), spaced 2 h apart. A dosing volume of 5 mL/kg of body weight was used for both THU and FdCyd.
Table 1.
FdCyd and THU dosages studied for pharmacokinetics.
| Route | FdCyd (mg/kg/day) |
THU (mg/kg/day) |
THU timing (relative to FdCyd) |
N (M/F) |
|---|---|---|---|---|
| IV-3h | 4 | 30 | 20% of dose at start | 0/2 |
| IV-3h | 8 | 30 | 20% of dose at start | 0/2 |
| IV-3h | 16 | 30 | 20% of dose at start | 1/1 |
| PO | 0.1 | 0 | - | 2/2 |
| PO | 0.1 | 500 | −1 h | 2/2 |
| PO | 1 | 500 | −1 h | 2/2 |
| PO | 10 | 500 | −1 h | 2/2 |
| PO | 10 | - | - | 2/2 |
| PO | 10 | 150 | −1 h | 2/2 |
To characterize the pharmacokinetics of FdCyd alone, 10 mg/kg FdCyd was administered by gavage. The FdCyd and THU pharmacokinetics of PO FdCyd 1 h after THU was assessed on day 1 in all animals. To assess the bioavailability of FdCyd, animals (n=2 per group) were dosed with 30 mg/kg THU and 4, 8, or 16 mg/kg FdCyd IV. An initial 20% of the daily dose of THU was administered as an IV bolus injection immediately prior to the initiation of the FdCyd dose. The remaining 80% of the THU dose was co-administered with the FdCyd dose as a 3 h infusion. The animals receiving 0.1 mg/kg FdCyd PO were re-used after a 4 day wash-out for the dosing of 0.1 mg/kg FdCyd with 500 mg/kg THU PO, which was justified based on lack of clinical effects and absence of detectable FdCyd concentrations after the first dosing. For clinical pathology determinations, blood was drawn on study days −6, 4, 8, 16, 19, 24, 29, and 43 and hematologic and clinical chemistry parameters were evaluated:
Sample Collection
For pharmacokinetic analysis, heparinized blood samples (3 mL) were collected into tubes preloaded with 30 µL of 100 mg/mL zebularine before, and 15, 30, 60, 120, 240, 360, 480, and 1440 min after PO FdCyd dosing. For the IV group, heparinized blood samples (1 mL) were collected into tubes preloaded with 10 µL of 100 mg/mL zebularine before, and 15, 30, 60, 120, and 150 min after start, and at 15, 30, 60, 120, 240, 360, and 1440 min after end of infusion. Blood samples were centrifuged at 600 × g for 10 min at 4 °C, and the resulting plasma was stored at −80 °C until analysis.
Human Pilot Study
In the first-in-human clinical trial of FdCyd, patients were administered a 3-h infusion of FdCyd concurrent with a fixed dose of THU. At FdCyd doses ≥20 mg/m2/d, peak plasma levels were above those that inhibit DNA methylation in vitro [12]. This trial included an expansion cohort where one of 10 IV dosing occasions was used to study the pharmacokinetics of PO dosed FdCyd 100 mg/m2 (equal to the IV dose) and THU at 1750 mg/m2 (5-fold the IV dose) [11]. The 5-fold higher dose of THU by the PO route was based on earlier preclinical work suggesting approximately 20% bioavailability of THU [13]. Pharmacokinetic samples were obtained both after IV and PO dosing for each patient. Plasma samples were obtained before and 15, 30, 60, 120, 150, 195, 210 min, and 4, 5, 7, 9, and 24 h after the start of the 3 h IV dosing, and before and 15, 30, 60 min, and 2, 4, 6, 9, ad 24 h after PO dosing. Stabilization with zebularine and processing was as described above for animal blood draws.
This trial was conducted under a City of Hope-sponsored IND with institutional review board approval at each of the participating sites. The protocol design and conduct followed all applicable regulations, guidance, and local policies and was registered on ClinicalTrials.gov: NCT00378807.
Analytical Methods
Plasma samples were analyzed for FdCyd, its metabolites FdUrd, FUrd, FU, FC, and THU by LC-MS/MS. For monkey plasma samples, plasma from untreated cynomolgus monkeys obtained from IIT Research Institute was used for standard curves for both the FdCyd and THU LC-MS/MS assays. Cynomolgus monkey plasma for the quality controls, run in triplicate with each assay, was purchased from Lampire Biological Laboratories (Pipersville, PA). For human samples, human control plasma was obtained from the Central Blood Bank, Pittsburgh, PA. The previously described FdCyd method [7,12] was modified slightly in that [15N2]-5-FU was used as internal standard for FU, FdUrd, and FUrd, while 5’-deoxy-5-fluorocytidine served as internal standard for FdCyd and FC. A 10 µL mix of 10 µg/mL internal standards was added to each 100 µL plasma sample. THU plasma concentrations were quantitated with a previously developed and validated assay [14].
Statistical Analysis
Data were analyzed non-compartmentally using PK Solutions (Montrose, CO). Pharmacokinetic parameters were compared between groups with Minitab (State College, PA) via Kruskall-Wallis tests for overall significance and pairwise comparisons were by Mann-Whitney. PK parameters across different doses were compared after dose-normalization. Significance was set at p≤0.05.
Results
Pharmacokinetics
In cynomolgus monkey plasma, the assays for FdCyd, FdUrd, FU, FC, and THU were linear, accurate, and precise over the same concentration ranges used for human and mouse plasma [7,12]. FUrd could not be quantitated accurately, yet was detectable down to approximately 30 ng/mL; however, no FUrd peaks were detected in any of the samples.
FdCyd 4, 8, or 16 mg/kg with 30 mg/kg THU IV in monkeys
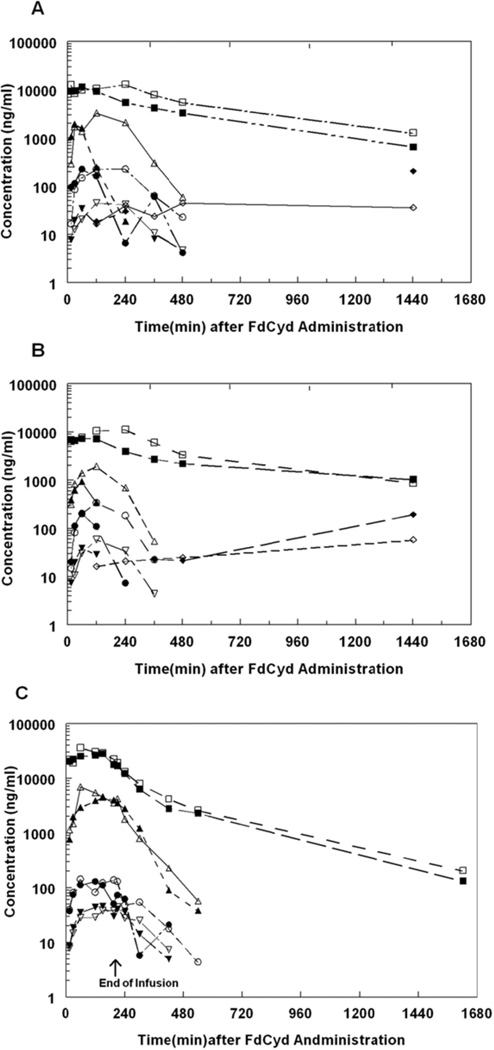
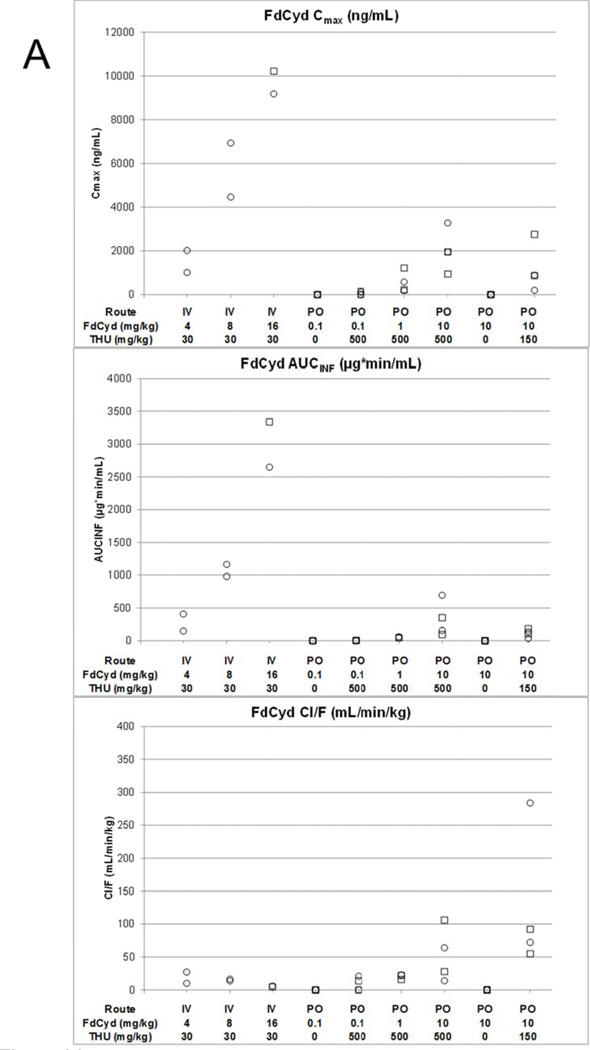
After 4, 8, or 16 mg/kg FdCyd in addition to 30 mg/kg THU IV, mean Cmax of FdCyd were 1.51 (±0.71), 5.69 (±1.74), and 9.70 (±0.74) µg/mL, respectively (Figure 1, Figure 2A, Table 2). In the 16 mg/kg FdCyd animals a sudden increase in plasma concentrations of FdCyd and metabolites was observed at 150 min into the infusion, likely the result of an end-of-infusion push (data not shown), which was omitted for determining Cmax. FdCyd AUC0-inf and Cmax appeared to increase a little more than proportional with dose, but these trends were not statistically significant (p>0.18). No FC was detected in the plasma of any of the monkeys after IV dosing. The FdUrd concentrations were approximately 10–50 fold lower than FdCyd concentrations (Figure 2A vs. Figure 2B).
Figure 1.
Plasma concentration versus time profiles following 500 mg/kg THU and 10 mg/kg FdCyd PO to female (A) and male (B) or 30 mg/kg THU and 8 mg/kg FdCyd IV to female (C) cynomolgus monkeys. For the PO administration, THU was administered as a bolus 1 h prior to bolus FdCyd. For IV administration, the initial 20% of the daily dose of THU was administered as an IV bolus injection immediately prior to the initiation of the FdCyd dose. The remaining 80% of the daily dose of THU was co-administered with the FdCyd dose as a 3 h infusion. Closed and open symbols represent individual monkeys, respectively ▲or △ represents FdCyd; ● or ○ represents FdUrd; ▼ or ▽ represents FU; ♦ or ♢ represents FC; and ■ or □ represents THU.
Figure 2.
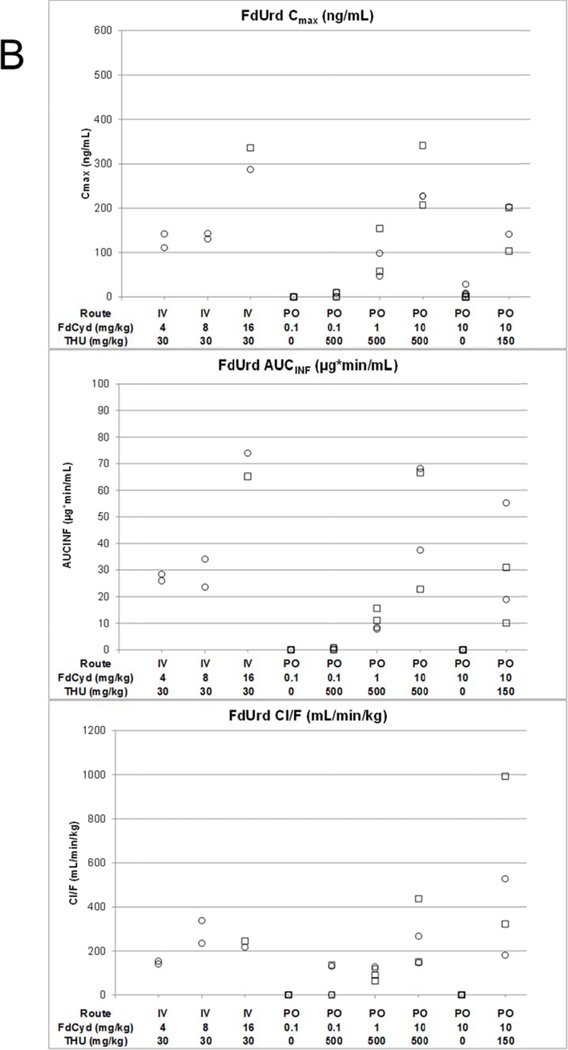
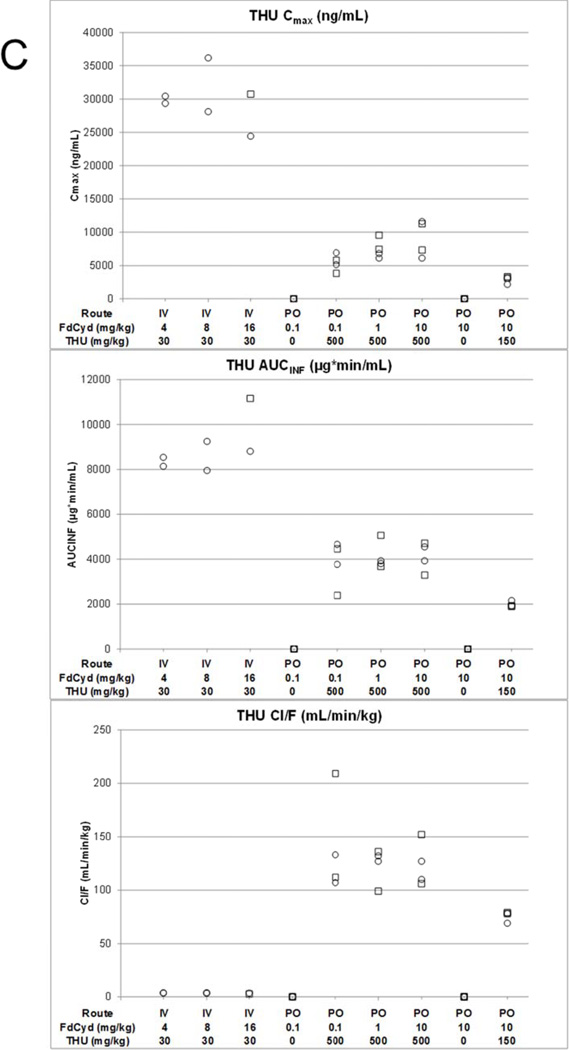
Cmax, AUC and Cl/F of FdCyd (panel A), FdUrd (panel B), and THU (panel C) following IV or PO administration of THU and FdCyd to female (○) and male (□) cynomolgus monkeys.
Table 2.
Selected FdCyd and THU mean (±SD) pharmacokinetic parameters in monkeys.
| Analyte | FdCyd (mg/kg) |
THU (mg/kg) |
N (IV/PO) |
Cmax (µg/mL) |
Tmax (h) |
T½ (h) |
AUC0-t (mg•min/L) |
AUC0-inf (mg•min/L) |
Cl/F (mL/min/kg) |
Fa (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| FdCyd | 4 | 30 | 2 IV | 1.51 (±0.71) | 2.88 (±0.53) | 0.57 (±0.30) | 274 (±179) | 276 (±182) | 18.6 (±12.2) | - |
| 8 | 30 | 2 IV | 5.69 (±1.74) | 1.75 (±1.07) | 0.87 (±0.12) | 1067 (±127) | 1071 (±128) | 15.1 (±1.8) | - | |
| 16f | 30f | 2 IV | 9.70 (±0.74)f | 2.50 (±0) | 0.56 (±0.20) | 2991 (±491) | 2993 (±491) | 5.42 (±0.89) | - | |
| 10 | 150 | 4 PO | 1.18 (±1.10) | 1.25 (±0.50) | 1.23 (±1.17) | 112 (±62) | 116 (±62) | 126.0 (±106.6) | 8.9b | |
| 10 | 500 | 4 PO | 2.03 (±0.96) | 1.38 (±0.75) | 0.63 (±0.17) | 317 (±274) | 324 (±269) | 53.0 (±41.0) | 25b | |
| THU | 4–16 | 30 | 6 IV | 29.9 (±3.8)c | 2.08 (±0.58) | 3.85 (±0.30) | 8917 (±1160) | 8969 (±1169) | 3.4 (±0.4) | - |
| 10 | 150 | 4 PO | 2.92 (±0.44) | 2.00 (±0.62) | 7.25 (±1.53) | 1105 (±118) | 1976 (±106) | 76.1 (±4.5) | 4.4d | |
| 0.1–10 | 500 | 12 PO | 7.31 (±2.37) | 3.44 (±1.47) | 4.99 (±1.52) | 2666 (±693) | 4017 (±731) | 129.2 (±29.5) | 2.4d |
, calculated by F = (AUCpo/AUCiv)*(Doseiv/Dosepo).
, relative to 4, 8, and 16 mg/kg FdCyd IV; average dose-normalized AUC0-inf = 1300 (±587) mg•min/L/10 mg/kg.
, The THU Cmax values for the two 16 mg/kg FdCyd IV animals were censored as these data indicated an artificial spike possibly due to an increased infusion rate.
, relative to 30 mg/kg THU IV.
, pharmacokinetic data obtained in a separate study cohort
, a sudden increase in plasma concentrations of FdCyd and metabolites was observed at 150 min into the infusion, likely the result of an end-of-infusion push (data not shown), which was omitted for statistics of Cmax.
FdCyd 10 mg/kg with 150 or 500 mg/kg THU PO versus FdCyd 8 mg/kg with 30 mg/kg THU IV in monkeys
Typical plasma concentration versus time profiles of individual monkeys following PO or IV administration of FdCyd and THU are shown in Figure 1. Plasma concentrations of FdCyd or downstream metabolites were not detectable in the plasma of monkeys receiving 10 mg/kg PO FdCyd without THU. Plasma FdCyd concentrations appeared to be slightly higher in monkeys that received FdCyd at 10 mg/kg after 500 mg/kg versus 150 mg/kg THU, however, these plasma FdCyd concentrations were lower than those found in monkeys receiving 8 mg/kg FdCyd IV simultaneously with 30 mg/kg THU IV (Figure 1C). The estimated FdCyd PO bioavailability was 8.9% with 150 mg/kg THU, while an increase of the THU dose to 500 mg/kg increased the FdCyd bioavailability to 25% (Table 2).
FdCyd 0.1, 1, or 10 mg/kg with 500 mg/kg THU PO in monkeys
Animals that received 0.1 mg/kg FdCyd PO as a single agent did not have detectable concentrations of FdCyd in their plasma, nor were any downstream metabolites of FdCyd detected (Figure 2A and B). Of the four animals that received FdCyd at doses of 0.1 mg/kg PO 1 h after 500 mg/kg THU PO, two had detectable plasma concentrations of FdCyd (22.6–117.2 and 34.1–152.2 ng/mL) and three had detectable plasma concentrations of FdUrd out to 60 min. No FU or FC was detected. Animals treated with PO FdCyd at 1 and 10 mg/kg 1 h after PO THU had detectable plasma concentrations of FdCyd, FdUrd, and FU. Low levels of FC were also detected in the plasma of animals receiving 10 mg/kg FdCyd with 500 mg/kg THU, whereas no FC was detected in the plasma of animals receiving 10 mg/kg FdCyd after 150 mg/kg THU. FdCyd Cmax and AUC0-inf increased with dose when FdCyd was administered PO 1 h after 500 mg/kg THU (p =0.03, and p =0.02), although less than proportional. The Tmax for FdCyd also increased with increasing dose with means of 15, 30, and 83 min at 0.1, 1, and 10 mg/kg FdCyd. There was no statistical difference in the plasma terminal half-life between groups receiving 0.1, 1.0, and 10 mg/kg PO FdCyd 1 h after 500 mg/kg PO THU (range 24–162 min). The concentrations of FdUrd and FU were approximately 10-fold lower than those of parent FdCyd.
30 mg/kg THU IV, 150 or 500 mg/kg THU PO with varying doses of FdCyd in monkeys
Within the 500 mg/kg THU dosing group, THU pharmacokinetic parameters appeared independent of FdCyd dose. High THU concentrations were observed at the end of the infusion for the 16 mg/kg FdCyd monkeys and these data were excluded from the calculation of Cmax (see before). The half-life of THU ranged from 203 to 248 min and concentrations of THU remained above 1,000 ng/ml until 6 h after the end of infusion. Individual pharmacokinetic parameters are presented in Figure 2. Mean parameter values are provided in Table 2, with bioavailability estimates of up to 4.4%.
100 mg/m2 FdCyd with 350 mg/m2 THU IV or 100 mg/m2 FdCyd with 1750 mg/m2 THU THU PO in humans
After 100 mg/m2 FdCyd in addition to 350 mg/m2 THU as a 3 h IV infusion, mean Cmax of FdCyd was 4,107 (±1,368) ng/mL, while PO administration of the same dose of FdCyd with a 5-fold dose of THU resulted in a mean Cmax of FdCyd of 623 (±444) ng/mL (Table 3). After IV dosing, the FdCyd to FdUrd ratio for Cmax (average and SD of individual ratios 187±75) and AUC (134±54) were higher than after PO dosing, with FdCyd to FdUrd ratio for Cmax (24±19) and AUC (24±11). FC was detected after PO dosing in 4 out of the 7 patients with an overall maximum concentration observed of 36.9 ng/mL. FdCyd PO bioavailability was estimated at approximately 10%. THU PO bioavailability was estimated to be approximately 4.1%.
Table 3.
Non-compartmental pharmacokinetic parameters of FdCyd and metabolites, and THU after administration of FdCyd and THU to seven humans IV-IV (100 mg/m2–350 mg/m2), PO-PO (100 mg/m2–1750 mg/m2), and PO-IV (100 mg/m2–350 mg/m2) route, respectively.
| FdCyd THU |
IV 100 mg/m2 IV 350 mg/m2 |
PO 100 mg/m2 PO 1750 mg/m2 |
||||
| Dose (mg/m2) |
Analyte | Cmax (ng/mL) |
AUCinf (µg•min/mL) |
Cmax (ng/mL) |
AUCinf (µg•min/mL) |
Fa (%) |
| N=7 | FdCyd | 4107 (1368) | 912 (341) | 623 (444) | 95.9 (62.0) | 10.1 (4.8) |
| FdUrd | 24.1 (8.0) | 7.03 (1.29) | 28.4 (11.5) | 4.04 (1.31) | ||
| FU | 9.72 (6.75) | 6.03 (7.70) | 14.1 (4.1) | 4.47 (5.08) | ||
| THU | 16452 (2154) | 7969 (1925) | 1428 (811) | 1606 (748) | 4.1 (1.5) | |
, calculated by F = (AUCpo/AUCiv)*(Doseiv/Dosepo).
Preclinical Study Observations
Treatment of male and female monkeys with THU (500 mg/kg) and FdCyd (0.1, 1.0 or 10.0 mg/kg) QDx3 every week, for 3 or 4 repeat 1 week cycles did not result in any mortality or any signs of dose-limiting toxicity. The only test article-related clinical observation was diarrhea (primarily slight to moderate in severity) without any weight loss. This was observed in all monkeys receiving the drug combination at all three dose levels. Hematological changes were limited to decreased leukocyte count and increased reticulocyte count in one female monkey in the high dose group; these changes were reversible within 7 days after completion of dosing.
Discussion
The toxicokinetic studies of FdCyd and THU in monkeys reported here were conducted to guide the NCI-sponsored phase I study of PO FdCyd and THU that is currently being conducted (ClinicalTrials.gov Identifier: NCT01534598). Our data show the tolerability of a QDx3 weekly schedule, which was the starting schedule in an ongoing phase I trial of PO FdCyd and THU in patients with solid tumors (ClinicalTrials.gov Identifier: NCT01534598). To date, the gastro-intestinal toxicity observed in this report has not been observed in the ongoing clinical trial, suggesting that the NHP species used were uniquely sensitive.
PO FdCyd without THU resulted in undetectable FdCyd plasma concentrations, confirming the large first-pass effect of FdCyd and the need for THU co-administration. After PO dosing of FdCyd with 150 or 500 mg/kg THU, FdCyd apparent bioavailability reached 8.9 and 25%, respectively, compared to IV FdCyd with 30 mg/kg THU. These values may be an underestimate of the potential bioavailability of FdCyd as the THU plasma exposure of 150 and 500 mg/kg PO THU was still 4- and 2-fold less, respectively, than the THU plasma exposure after the 30 mg/kg IV THU dose. Furthermore, a 25% PO bioavailability is a reasonable value for a pyrimidine drug and supports the PO route currently pursued in clinical trials as feasible. The 10-fold dose increases from 0.1 to 1 and 10 mg/kg PO FdCyd with 500 mg/kg THU resulted in increasing Tmax and a less than proportional increase in FdCyd exposure, suggesting saturable and more prolonged FdCyd absorption with increasing dose.
THU displayed an oral bioavailability of up to 4.2%, which decreased with increasing dose. Despite the less than proportional 2.1-fold increase in THU plasma exposure, the dose increase of 150 to 500 mg/kg THU did result in effective 3-fold boosting of the FdCyd bioavailability from 8.9 to 25%. Plasma THU exposure may be a determinant for FdCyd clearance, but perhaps less so for the FdCyd first pass effect, which is likely determined by gastrointestinal and hepatic THU concentrations. Based on Freireich et al. [15], 8 mg/kg FdCyd and 30 mg/kg THU in monkeys translates to 96 mg/m2 FdCyd and 360 mg/m2 THU, which approaches the MTD achieved in humans at 100 mg/m2 FdCyd and 350 mg/m2 THU dosed IV [11]. The human pilot data after this IV dose and the PO data after 100 mg/m2 FdCyd and 1750 mg/m2 THU allowed calculation of human bioavailability, and IV and PO exposures (Cmax and AUC) and oral bioavailability showed remarkable agreement between monkeys and humans for both FdCyd and THU.
IV coadministration of FdCyd and THU resulted in favorable plasma exposure ratios of FdCyd to FdUrd, comparable to those observed in clinical studies of this combination [12]. Also after PO coadministration of FdCyd and THU, the FdCyd to FdUrd ratio was approximately 10-fold, suggesting that a pharmacologically desirable ratio is achievable with PO therapy.
We observed 5-FC plasma concentrations after 500, but not after 150 mg/kg THU co-administration with FdCyd, suggesting that only with an abundance of THU to block CD-mediated FdCyd degradation does bacterial pyrimidine nucleoside phosphorylase (EC 2.4.2.2) generate FC in quantities that are detectable in plasma, as observed in murine [7] and the human studies reported here. In man, pyrimidine nucleoside phosphorylase can metabolize most pyrimidine nucleosides except for 4-amino-substituted species like deoxycytidine [16].
The chemical stability of FdCyd confers a significant advantage over both 5-aza-cytidine and decitabine, which are unstable [17]. In addition, our data show the feasibility of achieving meaningful FdCyd concentrations in both monkeys and humans after PO dosing of FdCyd and THU, and the positive effect of higher THU doses on FdCyd bioavailability. Based on our results, a phase I trial of PO FdCyd and THU was initiated in patients with solid tumors (ClinicalTrials.gov Identifier: NCT01534598), and is currently ongoing.
Acknowledgements
Funding: Funding for these studies were provided by NCI contracts N01-CM-42202 (IIT Research Institute), N01-CM-52202, N01CM-2011-0015, UM1CA186690 (University of Pittsburgh), and UM1CA186717 (City of Hope). This project used the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30CA047904. The authors would like to thank the Merrill Egorin Writing Group at UPCI for careful review of this manuscript.
Footnotes
Disclosures: None
References
- 1.Mekras JA, Boothman DA, Perez LM, Greer S. Use of 5-fluorodeoxycytidine and tetrahydrouridine to exploit high levels of deoxycytidylate deaminase in tumors to achieve DNA- and target-directed therapies. Cancer research. 1984;44(6):2551–2560. [PubMed] [Google Scholar]
- 2.Boothman DA, Briggle TV, Greer S. Tumor-selective metabolism of 5-fluoro-2'-deoxycytidine coadministered with tetrahydrouridine compared to 5-fluorouracil in mice bearing Lewis lung carcinoma. Cancer research. 1987;47(9):2354–2362. [PubMed] [Google Scholar]
- 3.Boothman DA, Briggle TV, Greer S. Protective, tumor-selective dual pathway activation of 5-fluoro-2'-deoxycytidine provided by tetrahydrouridine in mice bearing mammary adenocarcinoma-755. Cancer research. 1987;47(9):2344–2353. [PubMed] [Google Scholar]
- 4.Kaysen J, Spriggs D, Kufe D. Incorporation of 5-fluorodeoxycytidine and metabolites into nucleic acids of human MCF-7 breast carcinoma cells. Cancer research. 1986;46(9):4534–4538. [PubMed] [Google Scholar]
- 5.Smith SS, Kaplan BE, Sowers LC, Newman EM. Mechanism of human methyl-directed DNA methyltransferase and the fidelity of cytosine methylation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(10):4744–4748. doi: 10.1073/pnas.89.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, Jones PA. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2'-deoxycytidine. Molecular cancer therapeutics. 2005;4(10):1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- 7.Beumer JH, Eiseman JL, Parise RA, Joseph E, Holleran JL, Covey JM, Egorin MJ. Pharmacokinetics, metabolism, and oral bioavailability of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine in mice. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(24):7483–7491. doi: 10.1158/1078-0432.CCR-06-1250. [DOI] [PubMed] [Google Scholar]
- 8.Kinders RJ, Wang L, Kummar S, Khin S, Balasubramanian P, Zhu W, Parchment RE, Newman E, Tomaszewski JE, Doroshow JH. Abstract A106: Investigation of 5-fluorodeoxycytidine with tetrahydrouracil as a demethylation regimen in solid tumors. Molecular cancer therapeutics. 2011;10(Supplement 1):A106–A106. [Google Scholar]
- 9.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 10.Quintas-Cardama A, Santos FP, Garcia-Manero G. Therapy with azanucleosides for myelodysplastic syndromes. Nature reviews Clinical oncology. 2010;7(8):433–444. doi: 10.1038/nrclinonc.2010.87. [DOI] [PubMed] [Google Scholar]
- 11.Newman EM, Morgan RJ, Kummar S, Beumer JH, Blanchard MS, Ruel C, El-Khoueiry AB, Carroll MI, Hou JM, Li C, Lenz HJ, Eiseman JL, Doroshow JH. A phase I, pharmacokinetic, and pharmacodynamic evaluation of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine, administered with tetrahydrouridine. Cancer chemotherapy and pharmacology. 2015;75(3):537–546. doi: 10.1007/s00280-014-2674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beumer JH, Parise RA, Newman EM, Doroshow JH, Synold TW, Lenz HJ, Egorin MJ. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2'-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU) Cancer chemotherapy and pharmacology. 2008;62(2):363–368. doi: 10.1007/s00280-007-0603-8. [DOI] [PubMed] [Google Scholar]
- 13.Beumer JH, Eiseman JL, Parise RA, Florian JA, Jr, Joseph E, D'Argenio DZ, Parker RS, Kay B, Covey JM, Egorin MJ. Plasma pharmacokinetics and oral bioavailability of 3,4,5,6-tetrahydrouridine, a cytidine deaminase inhibitor, in mice. Cancer chemotherapy and pharmacology. 2008;62(3):457–464. doi: 10.1007/s00280-007-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parise RA, Egorin MJ, Eiseman JL, Joseph E, Covey JM, Beumer JH. Quantitative determination of the cytidine deaminase inhibitor tetrahydrouridine (THU) in mouse plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid communications in mass spectrometry : RCM. 2007;21(13):1991–1997. doi: 10.1002/rcm.3054. [DOI] [PubMed] [Google Scholar]
- 15.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer chemotherapy reports Part 1. 1966;50(4):219–244. [PubMed] [Google Scholar]
- 16.De Verdier CH, Potter VR. Alternative pathways of thymine and uracil metabolism in the liver and hepatoma. Journal of the National Cancer Institute. 1960;24:13–29. doi: 10.1093/jnci/24.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22(9):1680–1684. doi: 10.1038/leu.2008.145. [DOI] [PubMed] [Google Scholar]