Abstract
Anthocyanidins were synthesized to study the effect of methoxy substitution on the B ring to their antioxidant property. Comparative FRAP studies show 2′- and 4′-methoxy substituents have higher antioxidant activities, which may be attributed to both resonance and inductive effects.
Graphical abstract:
Anthocyanidins as reducing agents
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-015-1250-x) contains supplementary material, which is available to authorized users.
Keywords: Dyes, Green chemistry, FRAP, One pot synthesis
Background
Anthocyanidins are pigments that are associated with the bright coloration of flowers and fruits. These natural dyes belong to the flavonoid family, with their basic structure comprising of an aromatic ring (A) fused with an heterocyclic ring containing an oxygen (C), which is also bonded to a third aromatic ring (B). These compounds are normally substituted with hydroxy groups, which help stabilize the charge on the flavylium cation. When one of the phenols is substituted with glycosides, the compound is called an anthocyanin.
The natural occurrence of anthocyanins and anthocyanidins warrants their study not only for the evolutionary advantage they confer to plants, but also for their potential applications (Castañeda-Ovando et al. 2009). Besides their utility as colorants for foods and cosmetics (Campanella et al. 2010), they are also explored in materials science (Pina et al. 2012) for example, as photosensitizers for photovoltaics (Calogero et al. 2013; Gokilamani et al. 2013), and as molecular logic gates (Pina et al. 1998). Like many polyphenols, they exhibit biological activities that are beneficial to human health (Pojer et al. 2013) such as in glucose metabolism (Alzaid et al. 2013), protection against cardiovascular disease (Wallace 2011), and mediation of oxidative stress (Zafra-Stone et al. 2007). Their putative roles in human pathologies are of interest, particularly in cancer prevention (Wang and Stoner 2008; Cooke et al. 2005). Despite their biological significance, their pharmacokinetics in humans remains largely unexplored (Kay 2006). Thus, to further the utility of anthocyanins in therapeutics and gain an understanding of their activities as applied to drug design, we synthesized anthocyanidins 1–3 and studied their antioxidant properties.
There are several methods for determining and expressing antioxidant activity, particularly for natural anthocyanins extracted from plants (Thaipong et al. 2006; Huang et al. 2005; Pulido et al. 2000; Sochor et al. 2010). This paper reports the preparation and characterization of three new anthocyanidins with different substitution patterns on the B ring. The antioxidant activities of the synthetic anthocyanidins were studied using a modified ferric reducing activity of plasma (FRAP) assay (Benzie and Strain 1996, 1999).
Results and discussion
Synthesis of the flavylium cation occurs under harsh conditions (Balaban et al. 1969) and preparations of anthocyanidins have been achieved by bubbling the reaction with hydrogen chloride gas (Moncada et al. 2004), treatment with perchloric acid (Sato et al. 1999; Dorofeenko and Olekhnovich 1972), or employment of corrosive Lewis acids such as boron trifluoride etherate (Kuhnert et al. 2001). Recently, milder synthesis using sulfuric acid was reported (Calogero et al. 2013), and described herein is a convenient approach to obtaining anthocyanidins, using less solvent and shorter reaction times. A summary of synthetic methods is listed in Table 1 and the synthesis of flavylium ring has been comprehensively reviewed elsewhere (Iacobucci and Sweeny 1983).
Table 1.
Reported syntheses of anthocyanidins
| Conditions | Yield (%) | References |
|---|---|---|
| Salicylaldehyde, acetophenone, HBF4, HOAc, acetic anhydride, 60 °C, 12 h | 40–58, 23–78 | Katritzky et al. (1998), Gomes et al. (2009) |
| Salicylaldehyde, acetophenone, BF3 etherate, neat | 81 | Kuhnert et al. (2001) |
| Salicylaldehyde, acetophenone, H2SO4, HOAc, overnight | 40–88 | Calogero et al. (2013) |
| Salicylaldehyde, acetophenone, EtOAc, HCl gas, 0 °C, 3 days | 56–75, 55–84 | Mora-Soumille et al. (2013), Mas (2003) |
| Salicylaldehyde, acetophenone, HPF6, HOAc, 2 days | 89 | Kueny-Stotz et al. (2008) |
| Salicylaldehyde, acetophenone, HCl gas, formic acid, 5 h | 56 | Moncada et al. (2004), Michaelidis and Wizinger (1951) |
| Salicylaldehyde, benzaldehyde, ethyl chloroformate, HClO4, 1–12 h | 49–95 | Sato et al. (1999) |
| Salicylideneacetophenone, HBF4OEt2 or HOTf in Et2O | 62–67 | Fichtner et al. (2001) |
| Phenol, arylethynylketone, HPF6, HOAc, r.t. | 82–99 | Kueny-Stotz et al. (2007) |
Scheme 1 shows the condensation of 2,4-dihydroxybenzaldehyde with different acetophenone derivatives using a minimum amount of acetic and sulfuric acid. Heating in a water bath for 30 min facilitated the reaction, which resulted in a dark viscous liquid. The products were purified by trituration with diethyl ether. When performed with minimum exposure to air, fine, brightly colored powders are obtained, which were dried further in a vacuum desiccator. The hygroscopic anthocyanidins were assumed to be bisulfate salts, and the yields were 92–95 %. While the use of concentrated sulfuric acid is still harsh, improvements such as shorter heating time, use of the renewable solvent acetic acid, minimum solvents and adjuvants used during purification, and high yields makes our procedure greener. Characterization by 1H and 13C NMR and HRMS confirms the products, which have nearly similar UV–Vis and IR spectra in the functional group region.
Scheme 1.
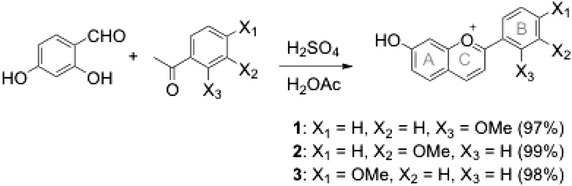
Synthesis of anthocyanidins 1–3
Solutions of 1–3 were prepared by first dissolving in DMF, and subsequent dilution with acetate buffer (pH 3.6). Flavylium salts are in equilibrium with their hydrates in aqueous solutions, with low pH favoring the non-hydrated pyrilium cation (Moncada et al. 2004). Once hydrated, they may undergo ring opening, then tautomerization to the enone, and further isomerization to give trans chalcones. Buffered solutions of 1–3 showed no variation in the UV spectra when kept in the dark, and when kept cold for at least 1 week, hinting on their stability.
A modified FRAP assay was used to study the antioxidant properties of 1–3. Freshly prepared FRAP reagent was admixed with antioxidants at room temperature, which showed rapid development of color characteristic of the formation of the Fe2+ complex. Spectrophotometric measurements were taken 2 min after mixing and all studies were performed in triplicate. The initial color change was fast, however the redox reaction continued for longer than 15 min, similar to what has been observed in polyphenol antioxidants (Pulido et al. 2000). Varying the location of the methoxy substituent on the C ring offers slight differences in the reducing power of the synthesized flavylium salt, with 1 showing the highest antioxidant activity (Fig. 1). This may be attributed to the added stability conferred by conjugation with the B ring substituents (Calogero et al. 2013). It can be reasoned that the higher activity of 1 compared to 3 is due to inductive effects of the proximal 2′ methoxy to the flavylium oxygen, which is absent in the 4′ methoxy (see Additional files 1, 2). The resonance effect is absent for the 3′ methoxy, resulting in least stable derivative (2).
Fig. 1.
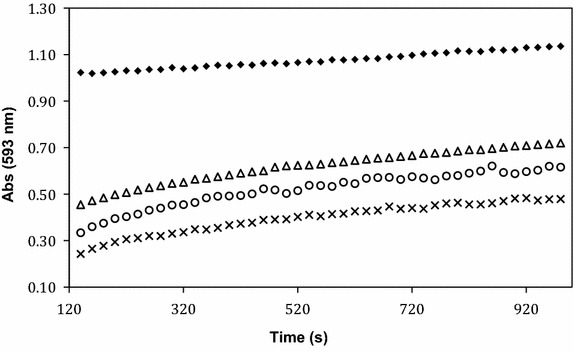
FRAP assay of synthetic anthocyanidin 1 (open triangle), 2 (multiplication sign), and 3 (open circle). Vitamin C (filled diamond) shows higher antioxidant activity under similar conditions. The antioxidants are of the same final concentration (0.15 mM), and the final concentration of Fe3+(TPTZ)2 was 735 mM
The solution chemistry of anthocyanidins is complex (Pina et al. 2012) and analogous anthocyanidins under similar pH exist in equilibrium between the flavylium ion, deporotonated quinoidal base, and as the hydrated hemiketal (Brouillard et al. 1982; Sweeny and Iacobucci 1983). The FRAP assay is non-specific for any antioxidant present under the reaction conditions that could reduce Fe3+, which takes into account the chemistry flavylium ions undergo in solution. Under similar assay conditions, ascorbic acid gives higher FRAP value (2.7) and shows a higher antioxidant activity than anthocyanidins 1–3. FRAP values are normally obtained after 4 min at 37 °C, or 6 min at room temperature. No significant variation of the FRAP value was observed between 4 and 6 min for our experiments, which are 2.2, 2.0, and 2.1 mM for 1, 2, 3, respectively, based on equivalent FeSO4 standard. In comparison, purified anthocyanin extracts from fruit show reducing power one-third that of ascorbic acid, however these comparisons are not straightforward because the reducing power is dose-dependent even for ascorbic acid (Sun et al. 2014).
Conclusion
In conclusion, we demonstrate a greener synthesis of anthocyanidins, which allows facile purification by trituration. This facilitates the study of the effects of various substituents on the different rings to the properties of anthocyanidins. In this case, we show that altering the location of the methoxy substituent on the B ring results in slight variations in the resultant antioxidant activity, as measured by the FRAP assay. The methoxy substituent on the 2′ position of the B ring stabilizes the radical formed in the 7-OH position by conjugation, and by inductive effects due to the proximity of the the methoxy group to the pyrilium oxygen. These results demonstrate the feasibility of tailoring the redox properties of synthetic anthocyanidins.
Experimental
All starting materials and solvents were purchased from commercial sources. NMR analyses were performed using a Bruker 400 MHz Avance, and IR analyses were performed using a Bruker Alpha ATR-IR. High-resolution mass spec were obtained from The City College of New York Mass Spectrometry Facility, and the counter anion was not included in the molecular ion peak calculations.
General procedure for FRAP
Freshly prepared FRAP solution was prepared by mixing acetate buffer at pH 3.6 (10.0 cm3, 20 mM), TPTZ solution (1.0 cm3, 10 mM), and FeCl3 solution (1.0 cm3, 10 mM) in a vial. Stock solutions of the anthocyanidins (35.0 mg) were prepared in DMSO (100 cm3, 1 mM). All solutions were sparged with N2 prior to each experiment. For each experiment, the stock was diluted to 0.5 mM with acetate buffer and equilibrated for 3 min. The experiment was initiated in a new vial containing de-ionized water (900 μL) and TPTZ solution (9.0 cm3). To this was added the diluted anthocyanidins (300 μL), mixed, and immediately transferred to a cuvette. Data capture was started exactly 2 min after the reaction was initiated. The blank was prepared similarly, but adding only buffer instead of the stock anthocyanidin solution. Each experiment was repeated at least three times.
General procedure for anthocyanidins
To a 25-cm3 round bottomed flask was added 2,4-dihydroxybenzaldehyde (414 mg, 3.00 mmol) and the corresponding methoxyacetophenone isomer (0.413 cm3, 3.00 mmol). The mixture was dissolved in acetic acid (1.00 cm3), and sulfuric acid (0.500 cm3) was added. The mixture was equipped with an air condenser and heated in a boiling water bath for 30 min. The solid product was obtained by triturating the oil with diethyl ether (2.0 cm3). Purification was achieved by dissolving the crude in acetic acid and triturating with ether at least three times. The product was vacuum filtered and washed with diethyl ether before drying in a vacuum desiccator.
7-hydroxy-2-(2-methoxyphenyl)chromenylium hydrogen sulfate (1, C16H14O7S)
Rust-colored powder, 0.994 g (95 %). M.p.: 100–107 °C (decomposed); 1H NMR (400 MHz, MeOH-d4) δ = 9.2 (d, 1H, J = 8.7 Hz), δ = 8.7 (d, 2H, J = 8.7 Hz), δ = 8.4 (dd, 1H, J = 8.08, 1.6 Hz), δ = 8.2 (d, 1H, J = 9.0 Hz), δ = 7.8 (td, 1H, J = 7.9, 1.6 Hz), δ = 7.51 (d, 1H, J = 1.6 Hz), δ = 7.48 (dd, 1H, J = 9.0, 2.2 Hz), δ = 7.38 (d, 1H, J = 8.6 Hz), δ = 7.3 (m, 1H), δ 4.1 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 170.3, 170.0. 161.1, 160.3, 154.5, 137.8, 132.8, 131.0, 122.1, 121.7, 119.9, 117.8, 117.0, 113.0, 102.1 55.6 ppm; HRMS (ESI) m/z 253.0897 (M+), calcd for C16H13O3 253.0865.
7-hydroxy-2-(3-methoxyphenyl)chromenylium hydrogensulfate (2, C16H14O7S)
Dark red powder, 0.966 g (92 %). M.p.: 122–155 °C (decomposed); 1H NMR (400 MHz, MeOH-d4) δ = 9.3 (d, 1H, J = 8.5 Hz), δ = 8.5 (d, 1H, J = 8.5 Hz), δ = 8.3 (d, 1H, J = 9.0 Hz), δ = 8.1 (d, 1H, J = 8.2 Hz), δ = 8.0 (s, 1H), δ = 7.64 (m, 1H), δ = 7.62 (d, 1H, J = 1.8 Hz), δ = 7.5 (dd, 1H, J = 9.0, 2.1 Hz), δ = 7.4 (dd, 1H, J = 8.3, 1.9 Hz), δ = 4.0 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 171.9, 170.6, 160.9, 160.2, 155.1, 133.1, 131.0, 130.5, 122.5, 121.8, 121.4, 120.5, 113.4, 113.1, 102.3, 55.0 ppm; HRMS (ESI) m/z 253.0890 (M+)+), calcd for C16H13O3 253.0865.
7-hydroxy-2-(4-methoxyphenyl)chromenylium hydrogensulfate (3, C16H14O7S)
Orange-red powder, 0.990 g (95 %). M.p.: 157–190 °C (decomposed); 1H NMR (400 MHz, MeOH-d4) δ = 9.1 (d, 1H, J = 8.7 Hz), δ = 8.5 (d, 2H, J = 9.1 Hz), δ = 8.4 (d, 1H, J = 8.7 Hz), δ = 8.2 (d, 1H, J = 9.0 Hz), δ = 7.5 (d, 1H, J = 2.0 Hz), δ = 7.4 (dd, 1H, J = 8.9, 2.2 Hz), δ = 7.3 (d, 2H, J = 9.1 Hz), δ = 4.0 (s, 3H); 13C NMR (100 MHz, MeOH-d4) δ 173.7, 170.8, 168.7, 160.1 155.2, 143.2, 133.6, 122.83, 122.76, 120.6, 117.2, 113.7, 103.8, 57.0 ppm; HRMS (ESI) m/z 253.0889 (M+), calcd for C16H13O3 253.0865.
Authors’ contributions
HSB drafted the manuscript and performed synthesis. PC performed synthesis and characterization. AT performed FRAP assays.
Acknowledgements
We are grateful to PSC-CUNY Cycle 41 and the CUNY Office of Vice Chancellor for Research (General Research Initiative 2011) for financial support. We are grateful to Dr. Lijia Yang and the LC-MS Lab at City College, CUNY for mass spectral analyses.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Abbreviation
- FRAP
ferric reducing activity of plasma
Additional files
Additional file 1. Checklist for compound characterization.
Additional file 2. Compound characterization data.
Contributor Information
Homar S. Barcena, Email: homar.barcena@kingsborough.edu
Peishan Chen, Email: pchen@smith.edu.
Abraham Tuachi, Email: avitnyc@gmail.com.
References
- Alzaid F, Cheung H-M, Preedy VR, Sharp PA. Regulation of glucose transporter expression in human intestinal Caco-2 Cells following exposure to an anthocyanin-rich berry extract. PLoS One. 2013;8(11):e78932. doi: 10.1371/journal.pone.0078932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban AT, Schroth W, Fischer G (1969) Pyrylium salts part I. Syntheses. In: Katritzky AR, Boulton AJ (eds) Advances in heterocyclic chemistry, vol 10. Academic Press, pp 241–326. doi:10.1016/S0065-2725(08)60499-7
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Lester P (ed) Methods in enzymology, vol 299. Academic Press, pp 15–27. doi:10.1016/S0076-6879(99)99005-5 [DOI] [PubMed]
- Brouillard R, Iacobucci GA, Sweeny JG. Chemistry of anthocyanin pigments. 9. UV-visible spectrophotometric determination of the acidity constants of apigeninidin and three related 3-deoxyflavylium salts. J Am Chem Soc. 1982;104(26):7585–7590. doi: 10.1021/ja00390a033. [DOI] [Google Scholar]
- Calogero G, Sinopoli A, Citro I, Di Marco G, Petrov V, Diniz AM, Parola AJ, Pina F. Synthetic analogues of anthocyanins as sensitizers for dye-sensitized solar cells. Photochem Photobiol Sci. 2013;12(5):883–894. doi: 10.1039/c3pp25347c. [DOI] [PubMed] [Google Scholar]
- Campanella A, Rustoy E, Baldessari A, Baltanás MA. Lubricants from chemically modified vegetable oils. Bioresour Technol. 2010;101(1):245–254. doi: 10.1016/j.biortech.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Castañeda-Ovando A, Pacheco-Hernández MDL, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chem. 2009;113(4):859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruits and vegetables—does bright colour signal cancer chemopreventive activity? Eur J Cancer. 2005;41(13):1931–1940. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Dorofeenko GN, Olekhnovich LB. Synthesis of pyrylium salts by condensation of benzalacetophenone with aliphatic carbonyl compounds. Chem Heterocycl Compd. 1972;8(7):800–802. doi: 10.1007/BF00475205. [DOI] [Google Scholar]
- Fichtner C, Remennikov G, Mayr H. Kinetics of the reactions of flavylium ions with π-nucleophiles. Eur J Org Chem. 2001;2001(23):4451–4456. doi: 10.1002/1099-0690(200112)2001:23<4451::AID-EJOC4451>3.0.CO;2-F. [DOI] [Google Scholar]
- Gokilamani N, Muthukumarasamy N, Thambidurai M, Ranjitha A, Velauthapillai D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J Sol–Gel Sci Technol. 2013;66(2):212–219. doi: 10.1007/s10971-013-2994-9. [DOI] [Google Scholar]
- Gomes R, Diniz AM, Jesus A, Parola AJ, Pina F. The synthesis and reaction network of 2-styryl-1-benzopyrylium salts: an unexploited class of potential colorants. Dye Pigment. 2009;81(1):69–79. doi: 10.1016/j.dyepig.2008.09.007. [DOI] [Google Scholar]
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Iacobucci GA, Sweeny JG. The chemistry of anthocyanins, anthocyanidins and related flavylium salts. Tetrahedron. 1983;39(19):3005–3038. doi: 10.1016/S0040-4020(01)91542-X. [DOI] [Google Scholar]
- Katritzky AR, Czerney P, Levell JR, Du W. Molecular engineering of benzo[b]pyrylium salts by indirect electrophilic substitution. Eur J Org Chem. 1998;1998(11):2623–2629. doi: 10.1002/(SICI)1099-0690(199811)1998:11<2623::AID-EJOC2623>3.0.CO;2-M. [DOI] [Google Scholar]
- Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr Res Rev. 2006;19(01):137–146. doi: 10.1079/NRR2005116. [DOI] [PubMed] [Google Scholar]
- Kueny-Stotz M, Isorez G, Chassaing S, Brouillard R. Straightforward synthesis of highly hydroxylated phloroglucinol-type 3-deoxyanthocyanidins. Synlett. 2007;2007(7):1067–1070. doi: 10.1055/s-2007-977433. [DOI] [Google Scholar]
- Kueny-Stotz M, Chassaing S, Brouillard R, Nielsen M, Goeldner M. Flavylium salts as in vitro precursors of potent ligands to brain GABA-A receptors. Bioorg Med Chem Lett. 2008;18(17):4864–4867. doi: 10.1016/j.bmcl.2008.07.107. [DOI] [PubMed] [Google Scholar]
- Kuhnert N, Clifford MN, Radenac A-G. Boron trifluoride–etherate mediated synthesis of 3-desoxyanthocyanidins including a total synthesis of tricetanidin from black tea. Tetrahedron Lett. 2001;42(52):9261–9263. doi: 10.1016/S0040-4039(01)01975-X. [DOI] [Google Scholar]
- Mas T. A new and convenient one-stepSynthesis of the natural 3-deoxyanthocyanidins apigeninidinand luteolinidin chlorides from 2,4,6-triacetoxybenzaldehyde. Synthesis. 2003;2003(12):1878–1880. doi: 10.1055/s-2003-40982. [DOI] [Google Scholar]
- Michaelidis C, Wizinger R. Beitrag zur Kenntnis der 2-Aryl-benzopyryliumsalze. Helvetica Chimica Acta. 1951;34(6):1761–1770. doi: 10.1002/hlca.19510340608. [DOI] [Google Scholar]
- Moncada MC, Pina F, Roque A, Parola AJ, Maestri M, Balzani V. Tuning the photochromic properties of a flavylium compound by pH. Eur J Org Chem. 2004;2004(2):304–312. doi: 10.1002/ejoc.200300508. [DOI] [Google Scholar]
- Mora-Soumille N, Al Bittar S, Rosa M, Dangles O. Analogs of anthocyanins with a 3′,4′-dihydroxy substitution: synthesis and investigation of their acid–base, hydration, metal binding and hydrogen-donating properties in aqueous solution. Dye Pigment. 2013;96(1):7–15. doi: 10.1016/j.dyepig.2012.07.006. [DOI] [Google Scholar]
- Pina F, Roque A, Melo MJ, Maestri M, Belladelli L, Balzani V. Multistate/multifunctional molecular-level systems: light and pH switching between the various forms of a synthetic flavylium salt. Chem Eur J. 1998;4(7):1184–1191. doi: 10.1002/(SICI)1521-3765(19980710)4:7<1184::AID-CHEM1184>3.0.CO;2-6. [DOI] [Google Scholar]
- Pina F, Melo MJ, Laia CAT, Parola AJ, Lima JC. Chemistry and applications of flavylium compounds: a handful of colours. Chem Soc Rev. 2012;41(2):869–908. doi: 10.1039/C1CS15126F. [DOI] [PubMed] [Google Scholar]
- Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12(5):483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48(8):3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Sato S, Watanabe T, Kumagai H, Kitamura N, Matsuba S, Kumazawa T, Onodera J-I, Suzuki M. Convenient synthesis of 1,6,7,8-substituted 2-(3′,4′-substituted-phenyl)-4-quinolones via a 4-ethoxyflavylium salt. J Heterocycl Chem. 1999;36(5):1189–1193. doi: 10.1002/jhet.5570360513. [DOI] [Google Scholar]
- Sochor J, Ryvolova M, Krystofova O, Salas P, Hubalek J, Adam V, Trnkova L, Havel L, Beklova M, Zehnalek J, Provaznik I, Kizek R. Fully automated spectrometric protocols for determination of antioxidant activity: advantages and disadvantages. Molecules. 2010;15(12):8618–8640. doi: 10.3390/molecules15128618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L-L, Gao W, Zhang M-M, Li C, Wang A-G, Su Y-L, Ji T-F. Composition and antioxidant activity of the anthocyanins of the fruit of Berberis heteropoda Schrenk. Molecules. 2014;19(11):19078. doi: 10.3390/molecules191119078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny JG, Iacobucci GA. Effect of substitution on the stability of 3-deoxyanthocyanidins in aqueous solutions. J Agric Food Chem. 1983;31(3):531–533. doi: 10.1021/jf00117a017. [DOI] [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19(6–7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Wallace TC. Anthocyanins in cardiovascular disease. Adv Nutr: Int Rev J. 2011;2(1):1–7. doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-S, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51(6):675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]


