Key Points
There is a strong negative association between comorbidities at diagnosis and overall survival.
There is no negative effect of comorbidities on remission rates and progression to advanced phases in CML.
Abstract
We studied the influence of comorbidities on remission rate and overall survival (OS) in patients with chronic myeloid leukemia (CML). Participants of the CML Study IV, a randomized 5-arm trial designed to optimize imatinib therapy, were analyzed for comorbidities at diagnosis using the Charlson Comorbidity Index (CCI); 511 indexed comorbidities were reported in 1519 CML patients. Age was an additional risk factor in 863 patients. Resulting CCI scores were as follows: CCI 2, n = 589; CCI 3 or 4, n = 599; CCI 5 or 6, n = 229; and CCI ≥ 7, n = 102. No differences in cumulative incidences of accelerated phase, blast crisis, or remission rates were observed between patients in the different CCI groups. Higher CCI was significantly associated with lower OS probabilities. The 8-year OS probabilities were 93.6%, 89.4%, 77.6%, and 46.4% for patients with CCI 2, 3 to 4, 5 to 6, and ≥7, respectively. In multivariate analysis, CCI was the most powerful predictor of OS, which was still valid after removal of its age-related components. Comorbidities have no impact on treatment success but do have a negative effect on OS, indicating that survival of patients with CML is determined more by comorbidities than by CML itself. OS may therefore be inappropriate as an outcome measure for specific CML treatments. The trial was registered at www.clinicaltrials.gov as #NCT00055874.
Introduction
Overall survival (OS) in patients with chronic myeloid leukemia (CML) under treatment with imatinib approaches 90% at 5 years and 88% at 8 years.1,2 Since the advent of second- and third-generation tyrosine kinase inhibitors (TKIs), faster and deeper remissions have been reported, including complete cytogenetic remission (CCyR), major molecular remission (MMR), and deep molecular response (MR4, MR4.5).3-6 To date, none of the clinical trials involving these new therapies have shown a convincing advantage in terms of OS, although the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients trial did demonstrate a favorable progression-free survival in CML patients treated with nilotinib.7
A number of different TKIs are now available, giving many treatment options for CML. After failure of first-line TKI, a switch to a second- or third-line therapy is recommended.8 As a result, the influence of a certain TKI therapy on OS has become more difficult to assess.
Comorbidities are known to complicate longitudinal trials in diseases with expected long OS times (eg, in solid cancers such as breast cancer9 and in leukemia and related disorders).10 A tool to measure the influence of relevant comorbid diseases in terms of reduced life expectancies was introduced with the Charlson Comorbidity Index (CCI),11 which considers not only the presence but also the severity of the comorbid condition. The score is well established and validated.12 Comorbidities grouped according to the CCI have been shown to influence OS in patients with myelodysplastic syndrome or chronic lymphocytic leukemia (CLL).10,13,14
The influence of comorbidities on outcome in CML patients has not been studied; comorbidities have only been taken into account when assessing the safety and suitability of different TKIs for CML patients.15-17
We sought to identify the comorbidities in CML patients at diagnosis and to assess the influence of those comorbidities on OS and on remission rates. The Philadelphia chromosome and/or BCR-ABL–positive chronic-phase CML patients from the CML Study IV1 were deemed to be specifically suited for this analysis because the trial was comprehensive with only few exclusion criteria with regard to comorbidities.
Patients and methods
Study design and goals
CML Study IV is a 5-arm randomized study comparing first-line imatinib 400 mg/day vs imatinib 400 mg/day in combination with interferon (IFN)-α vs imatinib 400 mg/day in combination with low-dose cytarabine (AraC) vs imatinib 400 mg/day after IFN failure vs imatinib 800 mg/day. Recruitment was from July 2002 through March 2012. Only low- and intermediate-risk patients were assigned to primary IFN, and during a pilot phase of 3 years, only high-risk patients were given imatinib 800 mg/day. In 2005, recruitment to imatinib+AraC and imatinib after IFN failure was terminated, and imatinib 800 mg/day was commenced as a full study arm. The first primary goal of CML Study IV was to determine the impact of treatment on MMR status at 12 months.1 Other objectives were to assess remission rates and survival probabilities after transplantation.18 A further primary objective was a comparative survival analysis planned 5 years after completion of recruitment.
Exclusion criteria
Besides pretreatment with IFN or chemotherapy other than hydroxyurea or anagrelide, the exclusion criteria were defined as follows: (1) second malignancy, if treatment was required and estimated life expectancy was shorter than the median survival of CML; (2) other serious illness; and (3) pregnancy (including lactation period) or other conditions that could prevent compliance with the required protocol.
Treatment
Initial treatment in all study arms, except the arm imatinib after IFN failure, was imatinib 400 mg once daily. If complete hematologic remission was not reached after 2 months or if there was no partial cytogenetic remission after 6 months, a dose increase to 600 or 800 mg/day was recommended.
The full 800 mg/d dose was given after a 6-week run-in period with imatinib 400 mg/d to avoid excessive cytopenia. The dose could be reduced according to tolerability. Further details of the treatment protocol have been published elsewhere.1,2
Definitions and end points
Definitions of CCyR and MMR followed the European LeukemiaNet recommendations.19,20 Risk assignment was done using the European Treatment and Outcome Study for CML (EUTOS) score criteria.21 The starting date for all time-to-event analyses was the date of diagnosis, except for time to adverse drug reactions where beginning of imatinib treatment was the starting date. OS was defined as the time between diagnosis and death of any cause, whether the patient was on TKI treatment or not. All living patients were censored at the time of their last visit. When estimating the cumulative incidences of molecular or cytogenetic remissions, patients were censored when they received a second-generation TKI or allogeneic stem cell transplantation. No patient was taken off the study, except at the patient’s request (n = 4).
Cytogenetic and molecular analyses
Cytogenetic analyses were performed as previously described.2 Follow-up analyses of CCyR included evaluation of ≥20 bone marrow metaphases. Molecular diagnostics for residual BCR-ABL transcripts followed the procedures and definitions of Hughes et al22 and Cross et al23 and were performed in standardized and accredited laboratories with defined conversion factors for equivalence of tests (Mannheim, Basel, Bern, and MLL Munich).2,23
Adverse events
Adverse events were reported at each follow-up visit according to the National Cancer Institute common toxicity criteria version 2.0. Severity grading was from grade 1 to 4. Evaluation focused on probably or definitely treatment-related events (adverse drug reactions [ADRs]) as determined by the investigators. For general safety analyses, patients were only counted if they had received imatinib as their first treatment and as long as they solely received imatinib. For comparisons, patients were only counted as long as they received the randomized treatment (as-treated analysis).24
CCI
The age-adjusted CCI is the most extensively studied comorbidity index11 and has been validated for long-term studies. Initially it was developed to predict the 10-year mortality for a patient who may have a range of comorbid conditions, such as heart disease, AIDS or cancer (a total of 22 conditions). Each condition is assigned a score of 1, 2, 3, or 6, depending on the risk of dying associated with the condition. Scores are summed to provide a total score to predict mortality. The index weighs (1) the severity of comorbidities (eg, 1 point is allocated to myocardial infarction and diabetes, 2 points to nonactive malignancies) and (2) the age of the patient (with 1 point for each decade above 40 years). The CCI at diagnosis was calculated for each randomized patient. Because of the presence of CML, the lowest possible score was 2. For the purpose of analysis, patients were classified in CCI groups 2, 3 to 4, 5 to 6, and ≥7.
The Karnofsky Performance Scale Index (KS) allows patients to be classified according to their functional impairment. The lower the KS, the worse the survival for most serious illnesses. Patients were classified in groups according to their scores: group 1, 50% to 80%; group 2, >80-<100%; group 3, 100%.25
Statistical analysis
The χ2 test was used to assess the association between the KS groups and the CCI. OS probabilities were calculated using Kaplan-Meier curves. For the cumulative incidences of remission and progression to accelerated phase (AP) and blast crisis (BC), death without prior progression was considered as the competing risk.26 Cumulative incidence curves were compared using the Gray test.27 Cox models were estimated for the multivariate analysis. The prognostic significance of a candidate variable was assessed with the Wald test. When comparing BCR-ABL ratios above or below threshold at specific time points, the χ2 test was used. The level of significance was set at .05. All calculations were performed with SAS software (SAS Institute, Cary, NC), except the Gray test, which was performed with R.
Ethics
The protocol followed the Declaration of Helsinki and was approved by the local ethics committees. Written informed consent was obtained from all patients before they entered the study.
Results
Patients
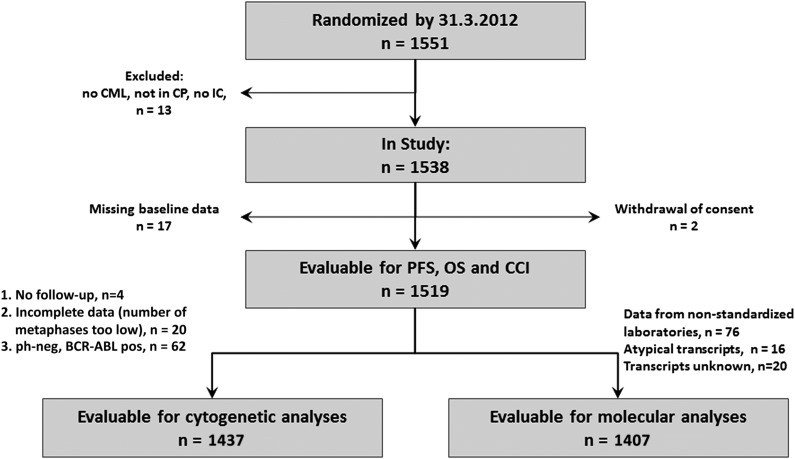
Of 1551 patients, 1519 patients were evaluable (Figure 1). Median follow-up time was 67.5 months (data closing May 24, 2012), and 612 (40.3%) patients had documented comorbidities, 384 (25.3%) with CCI-relevant diseases. In these patients, 511 index comorbidities were reported. The most common CCI relevant comorbidities were diabetes mellitus (n = 106), nonactive cancer (n = 102), chronic pulmonary disease (n = 74), moderate to severe renal insufficiency (n = 47), myocardial infarction (n = 38), cerebrovascular disease (n = 29), congestive heart failure (n = 28), and peripheral vascular disease (n = 28). Comorbidities not considered within the CCI were coronary heart disease/angina pectoris (n = 68), arrhythmia (n = 30), valvular disease (n = 20), arterial hypertension (n = 421), thrombosis/pulmonary embolism (n = 32), thyroid dysfunction (n = 98), acute pulmonary disease, gastrointestinal bleeding, inflammatory bowel disease, neurologic diseases except dementia and hemiplegia, hyperuricemia, benign tumor, anemia, inflammations such as pancreatitis and acute infections, rheumatologic disease, and coagulopathy.
Figure 1.
Flowchart of patient disposition.
In 863 patients, age led to a higher CCI score, resulting in the following CCI groups: (1) CCI 2, 589 patients; (2) CCI 3 or 4, 599 patients; (3) CCI 5 or 6, 229 patients; and (4) CCI ≥ 7, 102 patients.
Patient characteristics according to CCI groups are shown in Table 1. Per definition, age differed in the 4 groups. In concordance with previous reports,28 the leukocyte count was highest in the younger patient group (CCI 2). The distribution of treatment arms was similar within each CCI group.
Table 1.
Characteristics of patients in the groups defined according to CCI (n = 1519)
| Characteristics | CCI 2 (n = 589) | CCI 3-4 (n = 599) | CCI 5-6 (n = 229) | CCI ≥ 7 (n = 102) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| Median | 39 | 57 | 68 | 72 | ||||
| Range | 16-49 | 18-69 | 31-83 | 53-88 | ||||
| Sex | ||||||||
| Female | 206 | 35 | 258 | 43 | 96 | 42 | 42 | 41 |
| Hemoglobin, g/dL | ||||||||
| Median | 11.7 | 12.6 | 12.7 | 12.3 | ||||
| Range | 4.9-19.1 | 4.7-17.6 | 6.8-17.5 | 6.2-16.2 | ||||
| WBC × 109/L | ||||||||
| Median | 111 | 67 | 46 | 57 | ||||
| Range | 3.8-571 | 2.6-630 | 2.8-582 | 6.3-355 | ||||
| Platelets × 109/L | ||||||||
| Median | 382 | 367 | 401 | 349 | ||||
| Range | 39-2799 | 34-3020 | 70-2337 | 88-1993 | ||||
| EUTOS score | ||||||||
| Low | 493 | 90 | 528 | 90 | 211 | 94 | 91 | 89 |
| High | 93 | 10 | 61 | 10 | 14 | 6 | 11 | 11 |
| Median time from diagnosis to random treatment assignment, days | 14 | 18 | 18 | 20.5 | ||||
| Median observation time, months | 65 | 72 | 66 | 62 | ||||
| Number of patients on | ||||||||
| IM 400 | 145 | 25 | 149 | 25 | 67 | 29 | 35 | 34 |
| IM 400+IFN | 163 | 28 | 166 | 28 | 65 | 28 | 26 | 25 |
| IM 400 + AraC | 59 | 10 | 76 | 13 | 15 | 7 | 8 | 8 |
| IM 400 after IFN | 49 | 8 | 50 | 8 | 21 | 9 | 9 | 9 |
| IM 800 | 173 | 29 | 158 | 26 | 61 | 27 | 24 | 24 |
IM 400, imatinib 400 mg/day; IM800, imatinib 800 mg/day; N, number of patients; WBC, white blood cells.
The distribution of patients to the CCI according to age groups is summarized in Table 2.
Table 2.
Assignment of patients to the CCI according to age
| Age, years | Total (n = 1519) | CCI 2 (n = 1135) | CCI 3-4 (n = 324) | CCI 5-6 (n = 52) | CCI ≥ 7 (n = 8) | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | n | % | n | % | n | % | n | % | |
| <30 | 120 | 114 | 95 | 6 | 5 | 0 | 0 | 0 | 0 |
| 30-49 | 536 | 475 | 88 | 57 | 11 | 4 | 1 | 0 | 0 |
| 50-59 | 340 | 257 | 76 | 76 | 22 | 6 | 2 | 1 | <1 |
| 60-69 | 354 | 230 | 65 | 102 | 29 | 19 | 5 | 3 | 1 |
| 70-79 | 151 | 55 | 36 | 77 | 51 | 15 | 10 | 4 | 3 |
| ≥80 | 18 | 4 | 22 | 6 | 33 | 8 | 45 | 0 | 0 |
We found a positive correlation between the CCI and the KS (P < .001).
Cytogenetic and molecular response according to the CCI
No differences in remission rates were found between patients with CCI 2, 3 to 4, 5 to 6, or ≥7, neither for time to CCyR nor for time to MMR or to MR4.5. Median times to CCyR were 12.9, 12.6, 13.8, and 13.1 months, to MMR were 17.6, 15.8, 15.7, and 19.6 months, and to MR4.5 were 4.5, 4.3, 5.0, and 7.0 years, respectively (Figure 2A-B).
Figure 2.
Cumulative incidence of response according to CCI. (A) Cumulative incidence of CCyR. (B) Cumulative incidence of MMR.
There was no statistically significant difference between the proportions of patients not achieving 10% BCR-ABL (international scale) at 3 or 6 months according to the CCI groups.
Cumulative incidences for AP, BC, and OS
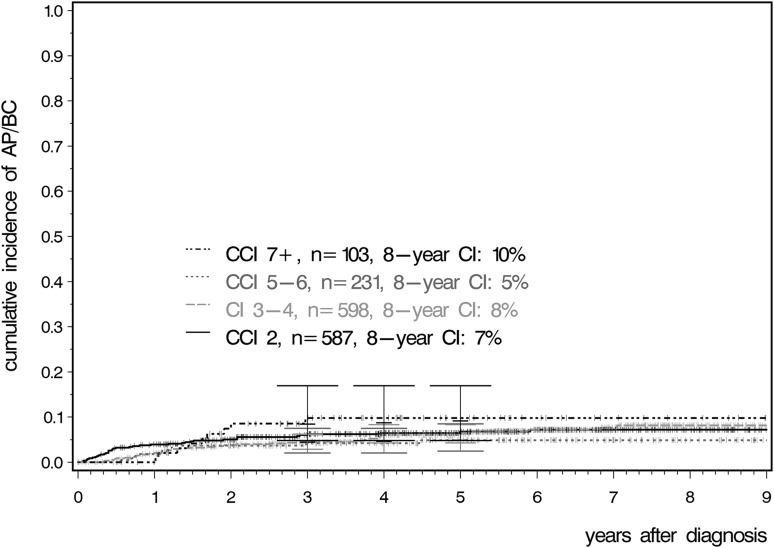
No differences were observed between the CCI groups for the cumulative incidences of AP and BC (Figure 3).
Figure 3.
Cumulative incidence of AP and BC according to CCI.
For the total cohort, causes of death were progression to AP or BC in 64 patients and not related to progression in 95 patients. The causes were different for the groups. In group CCI 2, 16 patients died after progression to AP or BC and 13 patients died without progression; in CCI 3, the numbers were 29 and 23; in CCI 5 to 6, the numbers were 10 and 33; and in CCI >7, the numbers were 9 and 33 patients, respectively.
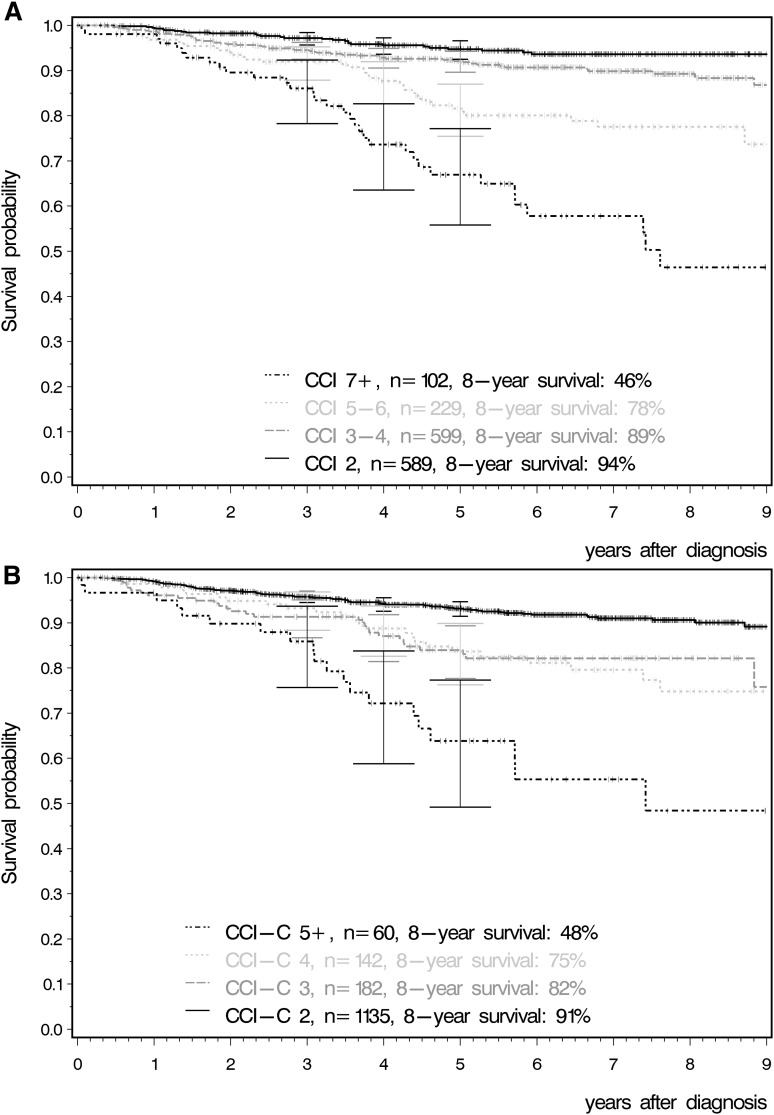
Significant differences were observed for OS (Figure 4A; P < .001). Probabilities of OS at 8 years for patients with CCI 2, 3 to 4, 5 to 6, and ≥7 were 93.6%, (95% confidence interval [CI]: 91.0-95.8%), 89.3% (95% CI: 86.0-92.1%), 77.6% (95% CI: 70.4-84.0%), and 46.4% (95% CI: 31.5-61.7%), respectively.
Figure 4.
OS according to CCI. (A) OS according to CCI considering age. (B) OS according to CCI without considering age.
Taking into account the comorbidities only and separating age from the score led to 4 different CCI-C groups: CCI-C 2 (n = 1135), CCI-C 3 (n = 182), CCI-C 4 (n = 142), and CCI-C ≥5 (n = 60). The significant differences for OS were still observed between the groups with increasing CCI counts (Figure 4B; P < .001).
ADRs
No significant differences in the probabilities of ADRs between CCI groups could be detected, neither hematological nor nonhematological (Table 3).
Table 3.
Cumulative incidence of ADRs according to CCI: any ADR grade 1 to 4 and nonhematological ADR grade 1 to 4
| CCI | 3-year cumulative incidence (95% CI) | 5-year cumulative incidence (95% CI) |
|---|---|---|
| Any nonhematological ADR | ||
| 2 | 59.9 (54.4-65.2) | 63.7 (57.9-69.3) |
| 3-4 | 62.8 (57.5-67.8) | 69.4 (63.8-74.7) |
| 5-6 | 52.4 (43.9-60.8) | 58.7 (49.2-67.9) |
| 7+ | 71.9 (58.5-83.6) | 86.4 (69.8-96.9) |
| Any ADR | ||
| 2 | 63.1 (57.8-68.2) | 66.6 (61.0-71.9) |
| 3-4 | 65.3 (60.2-70.2) | 72.7 (67.2-77.9) |
| 5-6 | 55.2 (46.9-63.4) | 60.2 (51.0-69.0) |
| 7+ | 71.9 (58.5-83.6) | 86.4 (69.8-96.9) |
No significant differences were found.
Multivariate analysis
In a multivariate analysis including CCI, KS, and EUTOS Score, the CCI was the most powerful predictive factor for OS (Wald test, P < .001; Table 4). Sex, leukocytes, and hemoglobin level had no significant influence. Hazard ratios for KS >80-<100% and 50% to 80% (each vs 100%) were 1.563 (95% CI: 1.080-2.262; P = .018) and 1.724 (95% CI: 1.071-2.778; P = .025), respectively, and for EUTOS score, high risk vs low risk: 1.793 (95% CI: 1.140-2.821; P = .012). Replacing EUTOS score with the Sokal score gave similar results. Hazard ratios for the CCI group 3 to 4, 5 to 6, and ≥7 (each vs 2) were 1.695 (95% CI: 1.066-2.695; P = .026), 3.231 (95% CI: 1.942-5.376; P < .001), and 6.495 (95% CI: 3.817-11.111; P < .001), respectively. When the CCI was separated into an age-related component and a comorbidity-related component, the comorbidity-related component was still an important predictive factor for OS (Wald test, P = .002).
Table 4.
Results of the multivariate analysis: leukocytes, hemoglobin, and sex without influence
| Variable | Tested category | Reference category | Hazard ratio | Confidence interval | P |
|---|---|---|---|---|---|
| CCI | 3-4 | 2 | 1.695 | 1.066-2.695 | .026 |
| 5-6 | 3.231 | 1.942-5.376 | <.0001 | ||
| ≥7 | 6.495 | 3.817-11.111 | <.0001 | ||
| Karnofsky score | >80- < 100% | 100% | 1.563 | 1.080-2.262 | .018 |
| 50-80% | 1.724 | 1.071-2.778 | .025 | ||
| EUTOS score | High | Low | 1.793 | 1.140-2.821 | .012 |
The hazard ratio including the confidence intervals of tested category vs the reference category for the different variables are listed.
Discussion
The most important finding of this analysis is the strong negative association between comorbidities at diagnosis and overall survival. Imatinib-treated CML patients in this analysis die from their comorbidities rather than from CML. The data show that patients with multiple comorbidities derived significant benefit from treatment with imatinib as there is no negative effect of comorbidities on remission rates and progression to advanced phases, but comorbidities do have an impact on OS. In fact, comorbid patients’ chances of achieving MMR and CCyR were similar to their more healthy counterparts without comorbidities. Our findings are in line with results in other hematological and oncological diseases with long-term outcomes (eg, in CLL or breast cancer). In a recent analysis of CLL patients, comorbidities were an independent prognostic parameter for progression-free survival and OS. In contrast to our CML population, in CLL patients, the major cause of death remained the disease itself, even in patients with >2 comorbidities.10 When separating the CCI into an age-related and a comorbidity-related component, the effect was still relevant without the age component. It turned out that age is not as much of a factor in remission rates as has been believed previously. This is in line with reports on the development of the EUTOS score. This score was designed to predict CCyR at 18 months, and age had no influence on either the univariate or multivariate analysis.21 In contrast, in a new evaluation of the EUTOS population taking long-term survival as an end point, age played an important role.29,30 Within the last decade, CML has changed from a disease that was almost always fatal to a chronic condition maintained by regular drug therapy. The registration studies for TKIs had more stringent exclusion criteria than CML Study IV from which our cohort was taken and that most probably represented a more untypical CML population.3,4
There are a few limitations of this analysis. Although the recruitment to CML Study IV had less exclusion criteria than other trials, patients with limited survival expectancies because of comorbidities should not be randomized. Besides, clinical trial patients are usually younger and fitter.31 Furthermore, we cannot completely exclude underreporting of comorbidities. Therefore, the distribution of the CCI here is probably not fully representative of the distribution of the CCI in CML patients in routine care.
Because TKIs have reduced CML-related mortality so effectively, we also conclude that a sole unadjusted analysis of OS is no longer appropriate for assessing the efficacy of a new specific treatment of CML. An appropriate measurement seems to be progression-free survival, as this was, at least in our cohort, not influenced by comorbidities. There is a need for a consensus definition of surrogate end points in CML studies. We suggest that in future CML studies, the analysis of OS should be stratified according to comorbidities: at least as part of an additional sensitivity analysis. For a valid comparative analysis and interpretation of OS between trials with CML patients, adjustment for comorbidity needs to be discussed.
Acknowledgments
The contributions of S. Dean, E. Matzat, R. Pleil-Lösch, I. Stalljann, G. Bartsch, U. Kossak, B. Müller, A. Elett, C. Sodan-Boyer, and U. Böhm are acknowledged. The authors thank the German Chronic Myeloid Leukemia Study Group for its participation in this study.
This work was supported by the Deutsche Krebshilfe (Grant 106642), Novartis (Nürnberg, Germany), Kompetenznetz für Akute und Chronische Leukämien (Bundesministerium für Bildung und Forschung 01GI0270), Deutsche José-Carreras Leukämiestiftung (Deutsche Jose-Carreras-Stiftung H09/01f, H06/04v, H03/01, R05/23), European LeukemiaNet (LSHC-CT-2004-503216), Roche (Grenzach-Wyhlen, Germany), and Essex Pharma (München, Germany).
Footnotes
Presented in part at the Annual Meeting of the American Society of Hematology, New Orleans, LA, December 8, 2013.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.S., M.-P.K. and M.C.M. had the primary responsibility for the publication; S.S., M.-P.K., R.H., M.L., U.P., L.K., B.H., A.F., D.K., W.E.B., M.B., P.S., M.d.W., M.W., F.Z., H.F.H., M.H., J. Heymanns, I.S.-W., N.S., M.J.E., W.G., A. Bartholomäus, A.P., E.O.L., D.H., S.W.K., A. Burchert, W.-K.H., J. Hasford, A.H., M.P., and M.C.M. contributed to the design of the study, to the statistical analysis, and to the interpretation of the results; and all authors checked and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.S. received honoraria from Pfizer, Novartis, and Bristol Myers Squibb and received research funding by Bristol Myers Squibb and Novartis; R.H. received research funding by Bristol Myers Squibb and Novartis; S.W.K. declares honoraria and research funding by Novartis; A.H. acted as a consultant for and received honoraria and research funding from Novartis, Bristol Myers Squibb, Pfizer, and ARIAD and received research funding by Novartis, Bristol Myers Squibb, and Pfizer; and M.C.M. received honoraria from ARIAD, Bristol Myers Squibb, and Novartis and received research funding by Bristol Myers Squibb and Novartis. All other authors declare no competing financial interests.
A complete list of the members of the Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung and the German CML Study Group appears in the online data supplement.
Correspondence: Susanne Saußele, III Medizinische Klinik, Medizinische Fakultät Mannheim, Universität Heidelberg, Pettenkoferstr. 22, 68169 Mannheim, Germany; e-mail: susanne.saussele@medma.uni-heidelberg.de.
References
- 1.Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-α in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29(12):1634–1642. doi: 10.1200/JCO.2010.32.0598. [DOI] [PubMed] [Google Scholar]
- 2.Hehlmann R, Müller MC, Lauseker M, et al. Deep Molecular Response (MR4.5) is reached by the majority of imatinib-treated patients, predicts survival, and is achieved faster by optimized high-dose imatinib - results from the randomized CML-Study IV. J Clin Oncol. 2014;32(5):415–423. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 3.Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2014;123(4):494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ording AG, Garne JP, Nyström PM, Frøslev T, Sørensen HT, Lash TL. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis—a Danish nationwide matched cohort study. PLoS ONE. 2013;8(10):e76013. doi: 10.1371/journal.pone.0076013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goede V, Cramer P, Busch R, et al. German CLL Study Group. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica. 2014;99(6):1095–1100. doi: 10.3324/haematol.2013.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res. 2009;33(12):1594–1598. doi: 10.1016/j.leukres.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breccia M, Federico V, Loglisci G, Salaroli A, Serrao A, Alimena G. Evaluation of overall survival according to myelodysplastic syndrome-specific comorbidity index in a large series of myelodysplastic syndromes. Haematologica. 2011;96(10):e41–e42. doi: 10.3324/haematol.2011.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gugliotta G, Castagnetti F, Fogli M, Cavo M, Baccarani M, Rosti G. Impact of comorbidities on the treatment of chronic myeloid leukemia with tyrosine-kinase inhibitors. Expert Rev Hematol. 2013;6(5):563–574. doi: 10.1586/17474086.2013.837279. [DOI] [PubMed] [Google Scholar]
- 16.Breccia M, Latagliata R, Stagno F, et al. Charlson comorbidity index and adult comorbidity evaluation-27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second-line dasatinib. Haematologica. 2011;96(10):1457–1461. doi: 10.3324/haematol.2011.041251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousselot P, Cony-Makhoul P, Nicolini F, et al. French Intergroup For Chronic Myelogenous Leukemia (Fi-LMC) Long-term safety and efficacy of imatinib mesylate (Gleevec®) in elderly patients with chronic phase chronic myelogenous leukemia: results of the AFR04 study. Am J Hematol. 2013;88(1):1–4. doi: 10.1002/ajh.23330. [DOI] [PubMed] [Google Scholar]
- 18.Saussele S, Lauseker M, Gratwohl A, et al. German CML Study Group. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 19.Baccarani M, Saglio G, Goldman J, et al. European LeukemiaNet. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Cortes J, Pane F, et al. European LeukemiaNet. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 22.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–2175. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 24.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV [published online ahead of print February 13, 2015]. Leukemia. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 25.Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45(8):2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Pfirrmann M, Hochhaus A, Lauseker M, Saussele S, Hehlmann R, Hasford J. Recommendations to meet statistical challenges arising from endpoints beyond overall survival in clinical trials on chronic myeloid leukemia. Leukemia. 2011;25(9):1433–1438. doi: 10.1038/leu.2011.116. [DOI] [PubMed] [Google Scholar]
- 27.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 28.Kalmanti L, Saussele S, Lauseker M, et al. German Chronic Myeloid Leukemia Study Group; Schweizerische Arbeitsgemeinschaft für Klinische Krebsforschung (SAKK) Younger patients with chronic myeloid leukemia do well in spite of poor prognostic indicators: results from the randomized CML study IV. Ann Hematol. 2014;93(1):71–80. doi: 10.1007/s00277-013-1937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfirrmann M, Saussele S, Baccarani M, et al. Long-term survival outcome and prognosis in 2277 patients with chronic myeloid leukemia allocated to first-line imatinib treatment - new results from the EUTOS in-study registry. Haematologica. 2013;98(s1):298.
- 30.Pfirrmann M, Saussele S, Baccarani M, et al. Survival and prognosis in patients with first-line imatinib treatment under particular consideration of death due to chronic myeloid leukemia. Blood. 2014;124(21):153. [Google Scholar]
- 31.Rohrbacher M, Berger U, Hochhaus A, et al. Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia. 2009;23(3):602–604. doi: 10.1038/leu.2008.245. [DOI] [PubMed] [Google Scholar]