Abstract
Cleidocranial dysplasia (CCD) is a rare congenital disorder, typically characterized by persistently open skull sutures, aplastic or hypoplastic clavicles, and supernumerary teeth. Mutations in the gene encoding the runt-related transcription factor 2 (RUNX2) protein are responsible for approximately two thirds of CCD patients. We report a 20-year-old CCD patient presenting not only with typical skeletal changes, but also complex dental anomalies. A previously undiagnosed odontoma, 14 supernumerary teeth, a cystic lesion, and previously unreported fused primary teeth were discovered on cone-beam computed tomography (CBCT) scans. Mutation analysis identified the causal c.578G>A (p.R193Q) mutation in the RUNX2 gene. At 20 years of age, the patient had already missed the optimal period for dental intervention. This report describes the complex dental anomalies in a belatedly diagnosed CCD patient, and emphasizes the significance of CBCT assessment for the detection of dental anomalies and the importance of early treatment to achieve good outcomes.
Keywords: Cleidocranial Dysplasia; Cone-Beam Computed Tomography; Odontoma; Tooth, Supernumerary
Introduction
Cleidocranial dysplasia (CCD) is a rare autosomal dominant skeletal disorder. The typical skeletal changes found in CCD patients are short stature, frontal and parietal bossing, open cranial sutures or the delayed closure thereof, the presence of Wormian bones, absent or hypoplastic clavicles, hypoplastic maxilla, hypoplastic iliac wings, and brachydactyly. The associated dental anomalies are the presence of supernumerary teeth, retention of the primary teeth, and delayed eruption and impaction of the permanent dentition. The development of the primary teeth is rarely affected. Patients are generally referred for treatment due to delayed dental development along with the appearance of a short stature, midface dysplasia, and sloping shoulders.1 The worldwide prevalence of this syndrome is approximately 1/1,000,000 individuals. Mutations in the runt-related transcription factor 2 (RUNX2) gene are responsible for approximately two thirds of CCD patients.2 RUNX2 codes for the runt-related transcription factor 2 (RUNX2) protein, which is important in skeletogenesis and dental development.3,4 This report describes the complex dental anomalies that were observed using cone-beam computed tomography (CBCT) imaging in a 20-year-old CCD patient. These novel dental manifestations, the CBCT imaging, and the treatment of CCD are discussed.
Case Report
Written informed consent was obtained from all participants in this study, which was approved by the Ethics Committee of the Zhongshan School of Medicine. The principles outlined in the Declaration of Helsinki were followed.
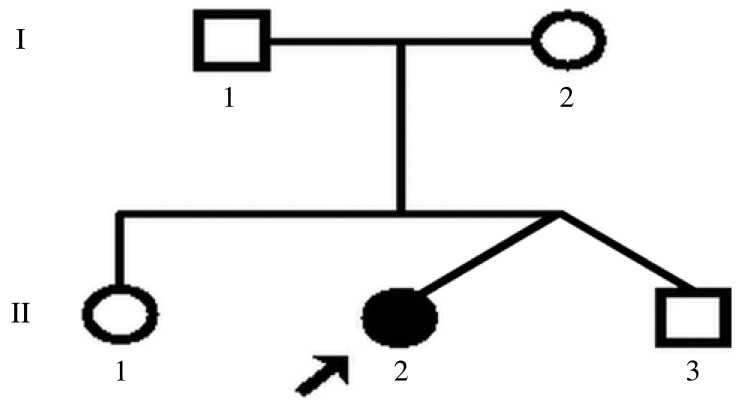
The patient came from a family comprising her dizygotic twin brother, one older sister, her parents, and herself (Fig. 1) from Guangdong Province, China. Phenotypically, this 20-year-old girl was the only affected member of the family. The patient was referred to the Department of Pediatric Dentistry with a complaint of delayed eruption of the permanent teeth.
Fig. 1. Pedigree of the family of our patient. Our patient is indicated with an arrow.
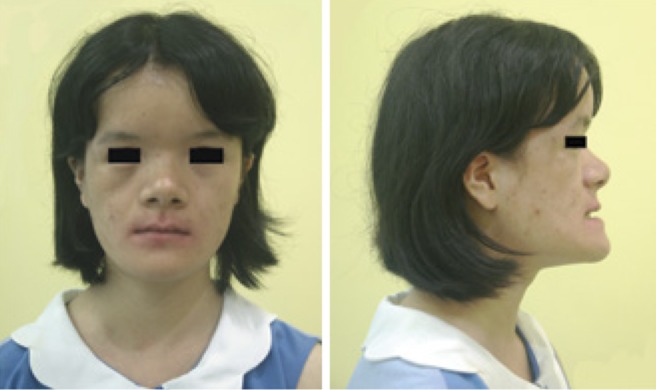
The patient's medical history demonstrated no trauma to the jaws, dentition, or mouth. Upon clinical examination, the 20-year-old girl was found to be of short stature, with a height of 149 cm and a weight of 37.5 kg. No obvious intellectual handicap was found. She had frontal bossing, a hypoplastic midface with hypertelorism, a depressed nasal bridge, a hypoplastic maxilla, and a palpable open frontal fontanelle (Fig. 2). Her drooped shoulders were hypermobile and almost met at the midline.
Fig. 2. A facial photograph shows a brachycephalic head, mild hypertelorism, and midface dysplasia.
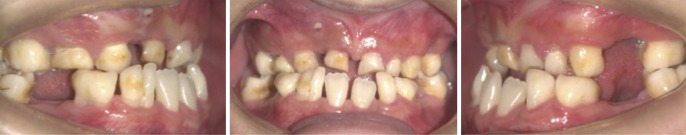
An intraoral examination (Fig. 3) showed mixed dentition, bilateral anterior and posterior crossbite, Angle class III malocclusion, and a high-arched palate. Interestingly, the right mandibular deciduous lateral incisor and canine were fused together, with deep grooves on the labial and lingual surfaces.
Fig. 3. Intraoral photographs show retention of the primary teeth, fusion between the right mandibular deciduous lateral incisor and canine, bilateral anterior and posterior crossbite, and Angle class III malocclusion.
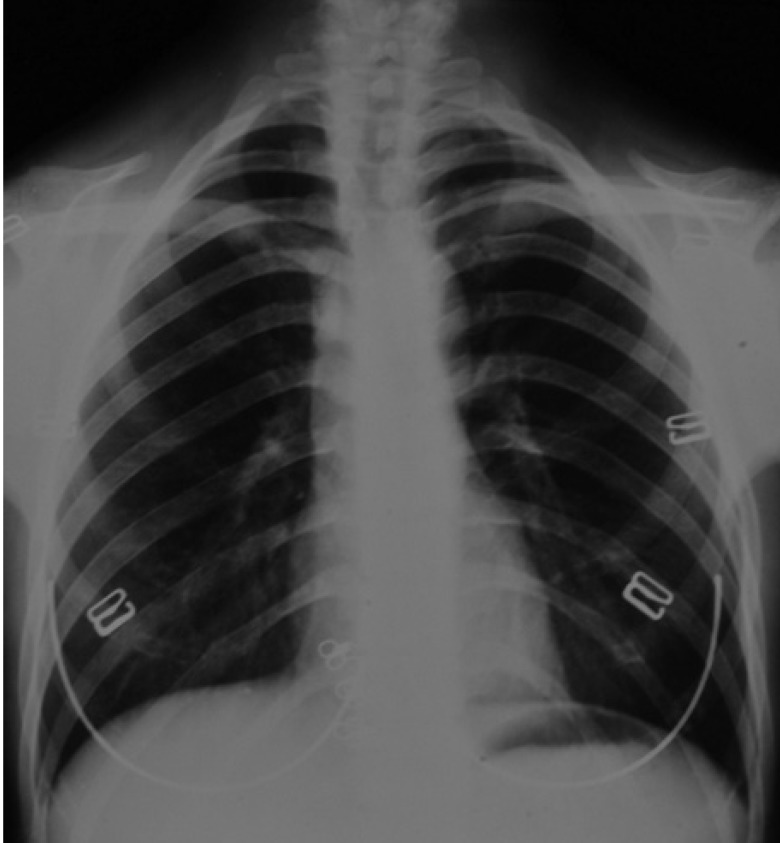
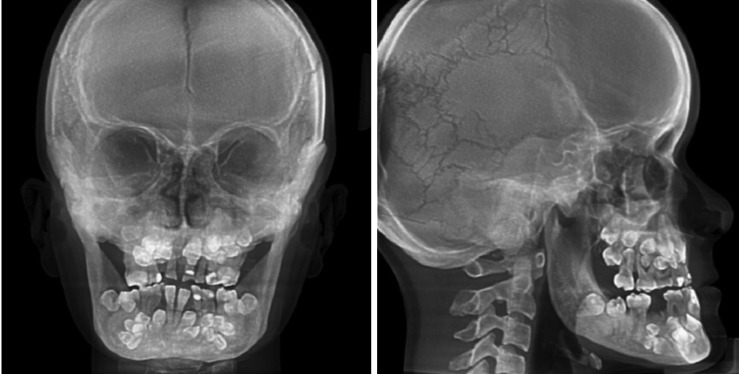
The presence of bilateral hypoplastic clavicles was confirmed with a chest radiograph (Fig. 4). The anteroposterior and lateral cephalogram displayed large fontanelles, unclosed sutures of the skull, numerous Wormian bones in the lambdoidal sutures, and a hypoplastic maxilla (Fig. 5).
Fig. 4. A chest radiograph shows bilateral hypoplastic clavicles and a tapered thorax with oblique ribs.
Fig. 5. A posteroanterior cephalogram shows patency of the anterior fontanelle and persistently open skull sutures (left). A lateral cephalogram shows frontal bossing and distinct Wormian bones in the lambdoid region (right).
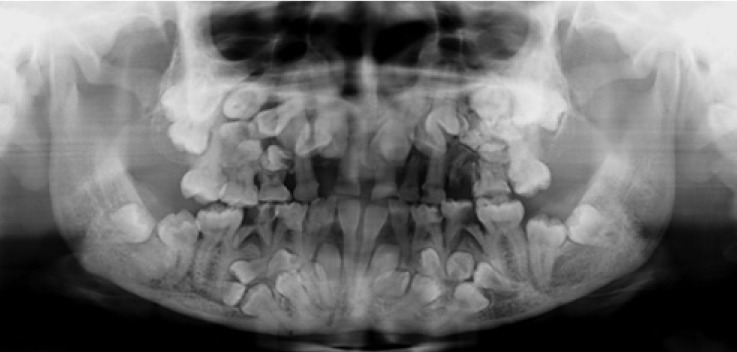
The panoramic radiograph (Fig. 6) revealed retention of the primary teeth, fused teeth, impaction of the permanent successors, multiple supernumerary teeth, and a radiopaque mass in the left maxillary molar region. The primary teeth were retained in the mixed dentition, except for the right maxillary deciduous lateral incisor, the left mandibular deciduous central and lateral incisors, and the right mandibular deciduous central incisor. The retained right mandibular deciduous lateral incisor and canine were fused together. The mandibular central incisors and first molars in all four quadrants were the only erupted permanent teeth in this 20-year-old female patient. The impacted permanent teeth had completed root formation. They were ectopically placed, near the edge of the mandible or close to the maxillary sinus, far from where they are typically placed. Nine supernumerary teeth could be recognized (three in the right maxilla and six in the mandibular premolar regions). The impacted supernumerary teeth were oriented in the buccolingual, mesiodistal, and inverted directions. Some of the supernumeraries were immature with incomplete root development. A radiopaque mass in the left maxillary premolar region appeared to be an odontoma.
Fig. 6. A panoramic radiograph shows retention of the primary dentition, fusion between the right mandibular deciduous lateral incisor and canine, and the impaction of several permanent and supernumerary teeth with varying forms and directions.
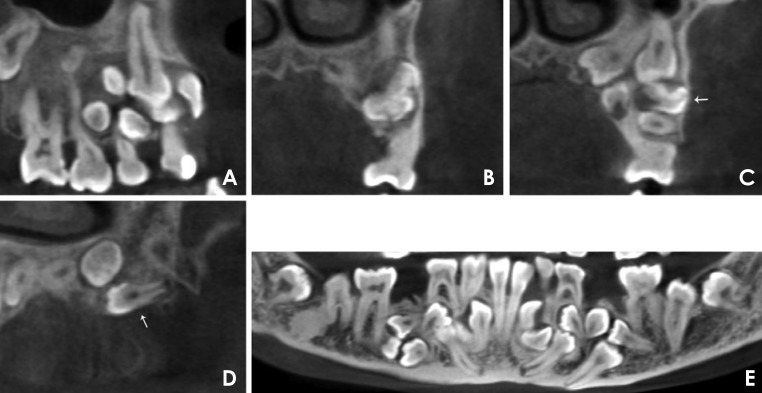
CBCT was performed to obtain more information. The panoramic and sectional CBCT images of both jaws were reconstructed. A 24.8 mm×14.0 mm radiolucency with a well-defined margin was identified in the right maxillary premolar region (Fig. 7A). The crowns of the central and lateral incisors, canine, first and second premolars, and three supernumeraries in the right maxilla were totally or partially involved in the cyst. The radiopaque mass in the left maxilla was found to be an odontoma together with five supernumerary teeth (Figs. 7B-D). The odontoma, measuring 12.8 mm×6.9 mm, showed irregular radiopacity and was surrounded by a radiolucent zone (Fig. 7B). The five supernumerary teeth were oriented in the buccolingual, mesiodistal, and inverted directions (Figs. 7C and D). In the four quadrants of both jaws, a total of 14 supernumerary teeth were found, oriented in different directions. The fusion of the right mandibular deciduous lateral incisor and deciduous canine appeared to be incomplete, sharing part of the pulp chambers with separate root canals. The trabeculation in the mandible was coarse and disordered (Fig. 7E).
Fig. 7. A. A panoramic reformatted cone-beam computed tomography (CBCT) image shows a 24.8 mm×14.0 mm radiolucency with a well-defined margin in the right premolar region of the maxilla. B. A coronal CBCT image shows an odontoma. The odontoma in the left premolar region of the maxilla presented as a 12.8 mm×6.9 mm irregular radiopacity surrounded by a radiolucent area. C. A coronal CBCT image shows part of the odontoma (arrow) and four supernumerary teeth in the left maxilla. The supernumerary teeth are different in terms of direction (normal, buccolingual, or inverted) and root development. D. A sagittal CBCT image shows a supernumerary tooth (arrow) that grew from the distal to mesial position in the left maxilla. E. A panoramic reformatted CBCT image shows fusion between the right mandibular deciduous lateral incisor and canine, which seems to be incomplete, sharing part of the pulp chamber with separate root canals. The trabeculation in the mandible is coarse and disordered.
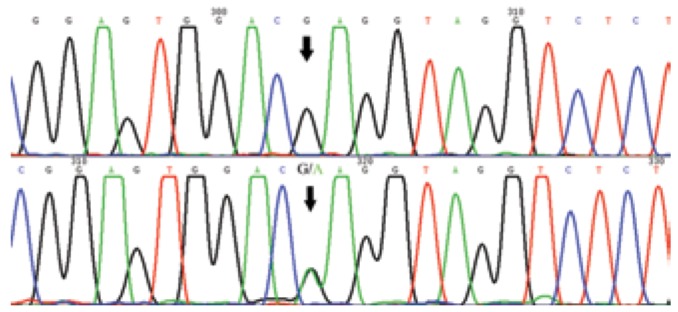
By sequencing the PCR product on both strands, a missense c.578G>A (p.R193Q) mutation in RUNX2 was identified (Fig. 8). A diagnosis of CCD was made based on the clinical and radiographic examinations and mutation analysis.
Fig. 8. Partial sequence of RUNX2. Arrows point to the wild type (top) and the c.578G>A (p.R193Q) mutant type (bottom).
The suggested treatment plan included surgical removal of the odontoma, cystic lesion, and the primary teeth with root resorption and pulpal lesions, followed by prosthetic treatment.
Discussion
Typical characteristics of CCD patients are a short stature, frontal and parietal bossing, open cranial sutures or the delayed closure thereof, the presence of Wormian bones, absent or hypoplastic clavicles, hypoplastic maxilla, supernumerary teeth, and delayed or failed eruption of the permanent teeth. In this case, the patient presented with some remarkable and rare dental anomalies that were found on panoramic and CBCT images, such as a previously undiagnosed odontoma and previously unreported fused primary teeth, the unusual presence of 14 supernumerary teeth, and a cystic lesion.
Supernumerary teeth are one of the most distinct characteristics of CCD patients and show polymorphism. In most previous cases, fewer than 10 supernumerary teeth were present,5,6,7 and the mandibular premolar regions are most frequently involved.8 In this study, panoramic and CBCT images revealed the unusual presence of 14 supernumerary teeth in all four quadrants of both jaws. Unlike other cases, they were oriented in a complex patterns of directions, including the buccolingual, mesiodistal, and inverted directions. The mandibular impacted teeth were located in an extremely low position, which increased the difficulty of extraction.
Odontoma, which is the most common type of odontogenic tumor, is associated with impacted permanent teeth and occasionally with retained primary teeth.9 Odontomas are histopathologically classified as complex and compound. As one of the four subtypes of supernumerary teeth,10 the odontoma in our patient was probably the consequence of the dysfunction of RUNX2. To best of the authors' knowledge, no articles have previously reported odontoma in cases of CCD. The CBCT images showed that the odontoma did not show organized dental structure, which was considered it to be a complex odontoma.
In our case, a cystic lesion was also identified in the right maxilla. Cysts derive from epithelial cells in oral tissues, such as the remnants of odontogenic epithelium in tooth follicles and the epithelial lining of the oral mucosa.11 Adaki et al.12 reported that the incidence of cystic changes in impacted lower third molars was 23.3% (22.1% dentigerous cysts and 1.2% odontogenic keratocysts). If a follicle is thicker than 2.5 mm, a cyst may develop. In our case, we classified the cystic lesion as a dentigerous cyst due to the involvement of the dental crown. However, a definitive diagnosis would have required histopathological confirmation.
The prevalence of fused teeth in the primary dentition is approximately 1%.13 When fusion occurs in the primary dentition, especially in the case of the fusion between the deciduous lateral incisor and canine, 74% of the successional lateral incisors are absent.14 In our case, the fused deciduous lateral incisor and canine were found in the retained primary dentition, although, unusually, the permanent successors were also present. To the best of the authors' knowledge, no articles have been published concerning fusion of the primary teeth in CCD patients. The etiology of fused teeth may include hereditary, genetic, traumatic, or environmental factors. It is clear that haploinsufficiency of RUNX2 is the cause of dental anomalies in CCD. Primary tooth development is rarely affected in CCD patients for the possible reason of insufficiently effective accumulation.4 Therefore, the cause of fused primary teeth in this case has not been established.
CBCT is a non-invasive technique resulting in accurate three-dimensional measurements of anatomic structures. This technique has several advantages and benefits, such as allowing the three-dimensional imaging of craniodental structures, involving easy data transfer compared to panoramic radiographs, and resulting in a lower radiation dose than conventional CT. Although the clinical manifestations and panoramic radiographic characteristics of CCD have been thoroughly described, the characteristics of CCD on CBCT have rarely been discussed in detail. In our case, CBCT confirmed the radiopaque mass to be an odontoma together with five supernumerary teeth, and additionally showed a cystic lesion that was not found on the panoramic radiograph. Therefore, CBCT assessment is advised for the detailed and accurate evaluation of cases with a complex dentofacial presentation.
Increased awareness of this systemic disease among physicians is important because early diagnosis and treatment can improve the clinical outcomes of patients. The manifestations of CCD are often innocuous, with the exception of dental anomalies that cause significant trouble to the patient and require special dental management as early as possible. The management of the dentofacial manifestations of CCD can be challenging. A method combining surgical, orthodontic, and prosthetic treatment is promising, and pediatric dentists play an important role in monitoring the correct eruption and alignment of the permanent teeth.15 Park et al.16 designed a multidisciplinary treatment protocol for the dentofacial manifestations of CCD, involving the following steps: (1) the appropriately timed removal of the retained primary teeth and supernumerary teeth, (2) surgical exposure of the impacted permanent teeth, (3) orthodontic extrusion and alignment, (4) Le Fort I advancement to correct malocclusion, (5) implant placement and prosthetic rehabilitation.
The management of this case was even more challenging. Due to the relatively advanced age of the patient, the impacted permanent teeth had completed root formation and lost their potential to erupt. The complex location and direction of the supernumerary teeth, the impacted permanent teeth, and the limited thickness of the maxilla and mandible made it difficult to carry out surgical extraction of the teeth and remove the odontoma and cystic lesion. An increased risk of jaw fracture, infection, and possible neurological damage was present. Orthodontic treatment, orthognathic surgery, or implantation could have proven challenging for the same reasons. Moreover, comprehensive treatment of this type was a heavy financial burden for the patient. Her anxieties regarding surgery also prevented her from receiving more suitable treatment. After communicating with the patient, a conservative treatment plan was designed. The supernumerary teeth were not extracted, and the odontoma and cystic lesion were not removed. The plan involved prosthetic treatment following the extraction of some primary teeth with root resorption and pulpal lesions, with the aim of restoring the function and aesthetics of the dentition. In addition, maintenance and review would be required. Due to the relatively late diagnosis, the patient missed the best timing for obtaining optimal dental management. Early intervention in CCD patients is very important, and dentists must monitor children with delayed or failed exfoliation of the primary teeth and multiple supernumerary teeth in order to establish an early diagnosis of CCD.
In our case, a belatedly diagnosed CCD patient presented with a previously undiagnosed odontoma, fused primary teeth, the unusual presence of 14 supernumerary teeth, and a cystic lesion. This report described some novel clinical manifestations of CCD and emphasized the importance of CBCT assessment and early dental interventions in the treatment of this disease.
References
- 1.Mundlos S. Cleidocranial dysplasia, clinical and molecular genetics. J Med Genet. 1999;36:177–182. [PMC free article] [PubMed] [Google Scholar]
- 2.Ott CE, Leschik G, Trotier F, Brueton L, Brunner HG, Brussel W, et al. Deletions of the RUNX2 gene are present in about 10% of individuals with cleidocranial dysplasia. Hum Mutat. 2010;31:E1587–E1593. doi: 10.1002/humu.21298. [DOI] [PubMed] [Google Scholar]
- 3.Karsenty G. Update on the transcriptional control of osteoblast differentiation. Bonekey Osteovision. 2007;4:164–170. [Google Scholar]
- 4.Camilleri S, McDonald F. Runx2 and dental development. Eur J Oral Sci. 2006;114:361–373. doi: 10.1111/j.1600-0722.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 5.McNamara CM, O'Riordan BC, Blake M, Sandy JR. Cleidocranial dysplasia: radiological appearances on dental panoramic radiography. Dentomaxillofac Radiol. 1999;28:89–97. doi: 10.1038/sj/dmfr/4600417. [DOI] [PubMed] [Google Scholar]
- 6.Suda N, Hamada T, Hattori M, Torii C, Kosaki K, Moriyama K. Diversity of supernumerary tooth formation in siblings with cleidocranial dysplasia having identical mutation in RUNX2: possible involvement of non-genetic or epigenetic regulation. Orthod Craniofac Res. 2007;10:222–225. doi: 10.1111/j.1601-6343.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Kolokitha OE, Ioannidou I. A 13-year-old Caucasian boy with cleidocranial dysplasia: a case report. BMC Res Notes. 2013;6:6. doi: 10.1186/1756-0500-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang XP, Fan J. Molecular genetics of supernumerary tooth formation. Genesis. 2011;49:261–277. doi: 10.1002/dvg.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Wang JT, Wang YP, Liu BY, Sun A, Chiang CP. Odontoma: a clinicopathologic study of 81 cases. J Formos Med Assoc. 2003;102:876–882. [PubMed] [Google Scholar]
- 10.Rajab LD, Hamdan MA. Supernumerary teeth: review of the literature and a survey of 152 cases. Int J Paediatr Dent. 2002;12:244–254. doi: 10.1046/j.1365-263x.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Saraçoğlu U, Kurt B, Günhan O, Güven O. MIB-1 expression in odontogenic epithelial rests, epithelium of healthy oral mucosa and epithelium of selected odontogenic cysts. An immunohistochemical study. Int J Oral Maxillofac Surg. 2005;34:432–435. doi: 10.1016/j.ijom.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Adaki SR, Yashodadevi BK, Sujatha S, Santana N, Rakesh N, Adaki R. Incidence of cystic changes in impacted lower third molar. Indian J Dent Res. 2013;24:183–187. doi: 10.4103/0970-9290.116674. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumar RS, Arunkumar S, Sadasiva K. Bilateral fusion of mandibular second premolar and supernumerary tooth: a rare case report. J Oral Maxillofac Pathol. 2012;16:128–130. doi: 10.4103/0973-029X.92990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsujino K, Yonezu T, Shintani S. Effects of different combinations of fused primary teeth on eruption of the permanent successors. Pediatr Dent. 2013;35:E64–E67. [PubMed] [Google Scholar]
- 15.Callea M, Bellacchio E, Di Stazio M, Fattori F, Bertini E, Yavuz I, et al. A case of cleidocranial dysplasia with peculiar dental features: pathogenetic role of the RUNX2 mutation and long term follow-up. Oral Health Dent Manag. 2014;13:548–551. [PubMed] [Google Scholar]
- 16.Park TK, Vargervik K, Oberoi S. Orthodontic and surgical management of cleidocranial dysplasia. Korean J Orthod. 2013;43:248–260. doi: 10.4041/kjod.2013.43.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]