Abstract
Background
Adherence to antidepressant treatment is essential for the effective management of patients with major depressive disorder. Adherence to medication is a dynamic decision-making process, and pharmacists play an important role in improving adherence to antidepressant treatment in different settings within the healthcare system. The aim of this study was to assess whether pharmacist interventions based on shared decision making improved adherence and patient-related outcomes.
Methods
This was a randomised controlled study with a 6-month follow-up. Participants were randomly allocated to two groups: 1) intervention group (IG) (usual pharmacy services plus pharmacist interventions based on shared decision making); or 2) control group (CG) (usual pharmacy services). Recruited patients fulfilled the following inclusion criteria: aged 18 to 60 years diagnosed with a major depressive disorder, and no history of psychosis or bipolar disorders. A research assistant blinded to the group allocations collected all data.
Results
Two hundred and thirty-nine patients met the inclusion criteria and were randomised to the IG (n = 119) or CG (n = 120). Nineteen patients dropped out of the study during the follow-up phase. After 6 months, patients in the IG had significantly more favorable medication adherence, treatment satisfaction, general overuse beliefs, and specific concern beliefs. However, the groups did not differ in severitye of depression or health-related quality of life after 6 months.
Conclusions
Our findings emphasise the important role of pharmacists in providing direct patient care in regular pharmacy practice to improve adherence to medications and other patient-reported outcomes.
Trial registration
ISRCTN34879893, Date assigned: 30/12/2014
Background
Medication adherence is a dynamic behaviour that changes over time with changing beliefs and attitudes towards prescribed medications, and it reflects a patient’s effort to optimally manage health, symptoms, and physical function [1]. Adherence to antidepressant treatment is essential for the effective management of major depressive disorder (MDD) [2]. Lack of adherence affects a large proportion of patients, with up to 52 % of patients discontinuing medication after 3 months [3]. Recent data from Saudi Arabia have shown that 53 % of patients have low adherence to antidepressant medication [4].
Adherence to medication is part of a decision-making process. Patients actively make decisions about their medications after weighing their reservations against the perceived benefits [5]. For each medication, patients usually consider their respective beliefs about medication-specific concerns and beliefs about the need for medications, as well as other general beliefs [6]. Patients’ authority and their participation in the decision-making process have increased in the last two decades [7, 8]. Several terms have arisen to describe patient involvement, including ‘informed decision making’ , ‘concordance’ , and ‘evidence-based patient choice’ [9]. Furthermore, progressive concepts have developed, such as shared decision making (SDM). SDM can be defined as ‘a two-way exchange of information, consultation and decision making, where deliberation and decisions are made by both the healthcare professional and the patient’ [9, 10].
A systematic review has shown that there is a positive relationship between patient participation in the decision-making process and outcomes, satisfaction, and improved patient self-esteem, alongside decreased treatment discontinuation rates [11, 12]. Similarly, positive associations have been found between SDM involving depressed patients and patient satisfaction [13]. Additionally, evidence supports the use of decision aids to improve deliberative treatment decisions during the SDM process [14].
Depressed patients have demonstrated an interest in increasing their knowledge about treatment options and participating in treatment decisions [14, 15]. In contrast, psychiatrists have shown poor patient involvement when making treatment decisions [16]. Data from Saudi Arabia showed that the rate of involvement of depressed patients in SDM was 66 %, when measured by observing patient involvement on the decision-making scale (OPTION scale). This increased to 79 % when patients used decision aids [15].
The literature suggests that a patient’s active participation in decision making regarding treatment for depression will result in improved adherence, satisfaction, and improved clinical outcomes. Furthermore, evidence from a systematic review supports the role of pharmacists in providing various interventions to improve medication adherence in antidepressant treatment in different settings [4].
The aim of this study was to evaluate the effectiveness of SDM-based pharmacist intervention for improving adherence and patient outcomes, compared with usual care in patients diagnosed with MDD.
Methods
This study followed the Medical Research Council guidelines for evaluating complex interventions [17]. We conducted a prospective randomised controlled study with a 6-month follow-up, beginning by randomly grouping participants into either: 1) the intervention group (IG; usual pharmacy services plus pharmacist intervention based on SDM); or 2) the control group (CG; usual pharmacy services without SDM-based pharmacist intervention). Patients in the control group received usual care and standard communication regarding their medication when they visited the pharmacy to collect their antidepressants, without any communication aimed specifically at increasing patients’ involvement, such as in SDM. Any inquiries addressed to the pharmacist were answered according to routine practice in the pharmacy.
SDM intervention
During the intervention, pharmacists followed the SDM competency framework, which was designed specifically for depressed patients, to ensure all aspects of SDM were implemented for each patient [18]. Before the SDM session started, the research team distributed a decision aid to patients in the IG. This was developed and validated by Aljumah and colleagues [15, 19] and is specifically designed for Arabic-speaking patients [19]. The intervention focused on enhancing patients’ involvement in decision making by assessing their beliefs and knowledge about antidepressants. The average duration of the first SDM session (baseline) was 15 min, and the second session (final session) lasted 10 min (at 3-month follow-up).
Setting
The study took place between February 2014 and July 2014 in Riyadh, the capital city of Saudi Arabia. This city has a total population of more than 5,000,000 and one psychiatric hospital (Al-Amal Hospital; total of 500 beds) is the main provider of psychiatric care for the entire population.
Patient populations
Recruited patients fulfilled all of the following inclusion criteria
Inclusion criteria
Aged 18 to 60 years;
Newly diagnosed with an MDD, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed (DSM-IV; 1994);
No history of psychosis or bipolar disorders;
No drug or dependency history;
No cognitive impairment that may hinder the assessment.
Patients were excluded from further analysis for the purpose of this study if they met the following criterion:
Exclusion criteria
No response at any level to the antidepressant within 8 weeks of recruitment.
Patients who met the inclusion criteria were briefed about the aims and objectives of the study and invited to participate. Written consent was sought within 24 h of the recruitment visit.
Data collection
Baseline visits took place in the outpatient clinic, and assessments were conducted by independent raters (two trained nurses) who were blinded to patients’ group allocation. This was meant to limit potential observer bias or conscious deception, and reduce the influence of the placebo effect. At baseline, the raters recorded data pertaining to patients’ socio-demographic characteristics, adherence, beliefs, satisfaction with their depression treatment, severity of depression, health-related quality of life, and the quality of patients’ involvement in SDM during clinical interviews. The same measures were administered at the 3- and 6-month follow-up (with the exception of patient involvement in SDM, which was not measured at 6 months). Pharmacists and psychiatrists were not blinded to the patients’ group allocation.
Randomisation
Study participants were individually randomised to one of two parallel groups with an allocation ratio of 1:1 using a computer-generated list. The computer-generated allocation was done by a research assistant with no clinical involvement in the trial. Pharmacists and psychiatrists were not blinded to the patients’ group allocation but the research assistant who collected all data was blinded to group allocation.
Measurements and scales
Assessment of adherence
Direct and indirect measures can be used to evaluate patients’ medication adherence. In this study, we used an indirect method, the Morisky Medication Adherence Scale (MMAS). The MMAS is a well-validated instrument [20–23], with good reliability and an available Arabic version [24]. The MMAS, which is a useful screening technique for antidepressant medications [25], consists of eight items addressing specific medication-taking behaviours and adherence. MMAS scores range from 0 to 8, with higher scores representing better adherence. A score of less than 6 is considered to indicate poor adherence, while a score of 6 or more is considered to indicate good adherence. Written permission to use the questionnaire was obtained from its authors.
Assessment of patients’ beliefs about medicine
Patients’ medication-related beliefs were assessed using the Patients’ Beliefs about Medicine Questionnaire (BMQ; Specific and General versions). This self-report measure has proven validity, reliability, and psychometric capability for both general medical patients and depressed patients [3, 6, 26]. The BMQ-Specific contains two parts: ‘Specific Necessity’ , which evaluates patients’ views about the necessity and importance of their medication; and ‘Specific Concerns’ , which questions patients’ beliefs about the potential harm and adverse effects of their medications. Each part of the questionnaire has a potential score ranging from 5 to 25. The BMQ-General also has two parts: ‘General Harm’ , which measures beliefs that medicines in general are harmful, addictive or poisonous; and ‘General Overuse’ , which measures beliefs that medicines in general are overused by doctors [6]. Again, written permission was obtained from the original author.
Assessment of severity of depression
At each visit, the severity and progression of the disease was assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS). The MADRS is an established scale that is widely used in clinical psychiatry studies to evaluate depression over time. The MADRS focuses more on the psychological symptoms of depression, as opposed to the somatic symptoms [27]. The MADRS comprises 10 items that cover symptoms typical of MDD, with a possible score of 0 to 6 for each item. The total score is a measure of the overall severity of the depression [28, 29]. A score of 30 or more indicates severe depression, while a score of 10 or below indicates remission [27, 28].
Assessment of patients’ involvement in decisions
SDM was assessed quantitatively during the baseline and 3-month visits using the Observing Patient Involvement in Decision-Making scale (OPTION) in the intervention group only. OPTION was developed to evaluate the extent to which patients are involved in the clinical decision-making process [16]. This validated scale consists of 12 rating items, each scored on a five-point Likert scale (0 to 4). A score of ‘0’ indicates that competency is not observed and scores of ‘1’ to ‘4’ represent increasing levels of achievement. An overall OPTION score is obtained by adding the scores for all 12 items together (maximum 48), and then standardising it to obtain a score between 0 and 100 [30]. Trained nurses administered the scale using videotapes of SDM interventions.
Assessment of health-related quality of life
Quality of life was assessed using the EQ-5D, a generic health-related quality of life (HRQoL) instrument developed by the EuroQoL group. This is a standardised instrument used to measure health outcomes, which provides a simple descriptive profile and single index value for health status that can be used in clinical studies [31]. In this study, we used the Arabic version of the EQ-5D [32]. The first part of the EQ-5D records self-reported problems in one of five domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), according to three levels of severity (no problems, some problems, and extreme problems). The second part records the subject’s self-assessed health on a Visual Analogue Scale (VAS) – a vertical 20 cm line on which the best and worst health states score 100 and 0, respectively [32].
Assessment of patient satisfaction with treatment
Patient treatment satisfaction was measured using the Arabic version of the self-report Treatment Satisfaction Questionnaire for Medication (TSQM 1.4). The TSQM has been shown to be psychometrically robust and to have proven validity and reliability with Arabic patients [33, 34]. It is a 14-item scale consisting of four dimensions, including effectiveness, side effects, convenience, and global satisfaction [35]. The patients were instructed not to respond to the questions in the side effects dimension if they were not suffering from side effects. TSQM 1.4 domain scores range from 0 to 100, with higher scores representing higher satisfaction in that domain [36, 37].
Dropouts
Dropouts were defined as patients who did not attend the follow-up visits at 3 and 6 months, and who could not be assessed again. Patients who decided to stop their treatment without having experienced significant side effects were counted as non-adherents. All analyses were limited to patients who completed all assessments (complete-case analysis).
Sample size and power
We needed to obtain a difference of at least 17 points in the percentage of medication intake at a confidence level of 95 % [38], allowing for a 20 % dropout rate [39] to provide 80 % power, assuming an alpha risk of 0.05 and a beta risk of <0.20. This gave us a target sample size of 195 patients to achieve adequate power.
Data analysis
Descriptive and comparative statistics were analysed using SPSS. Demographic and clinical characteristics of the two study groups were compared at baseline using analysis of variance (continuous variables). ANCOVA analyses were conducted to assess differences in medication adherence, beliefs about depression severity, level of participation in SDM, and satisfaction between treatment groups, controlling for baseline scores. We conducted a mixed ANOVA to analyse changes over time and between groups. We conducted an independent sample t-test to compare overall mean adherence between groups.
Ethical considerations
Approval for the study protocol was received from the Research Ethics Committee of the Ministry of Health in Saudi Arabia received before patients recruitment. Informed written consent was sought from all study participants. All participants were provided with a participant information sheet that included detailed information regarding the nature of the study, to enable informed consent. Participants were informed of their right to drop out of the trial at any stage, without any effects on their treatment or relationship with the treating team. Participants were assured about confidentiality and data protection of gathered personal information, which would only be used by the researchers in an anonymised format.
Results
Two hundred and thirty-nine patients met the inclusion criteria for this study and were randomised to the IG (n = 119) or the CG (n = 120). Of these, 19 dropped out of the study during the follow-up phase (Fig. 1) and were not included in the final analyses. In the group of drop-outs, there was a greater proportion of males and elderly patients compared with the group of patients who completed the study. No other baseline differences existed between the groups.
Fig. 1.
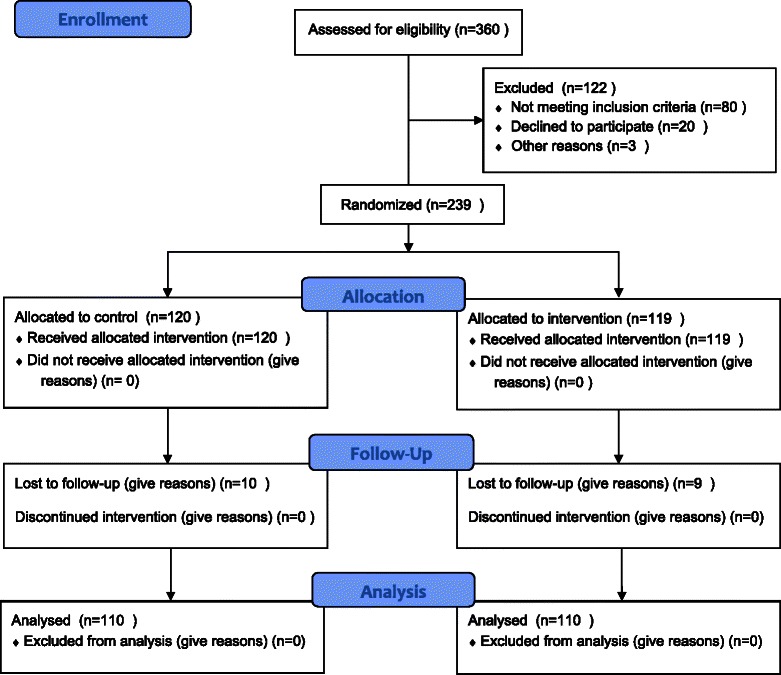
Recruitment flow diagram
Table 1 shows demographic characteristics of the patients, according to group allocation. There were no statistically significant differences between the IG and CG in these variables, including age, education, and number of prescribed antipsychotics. Table 2 shows scores for clinical and patient-related outcome measures at baseline. Patients in the IG and CG reported similarly moderate severe depression, with a mean MADRS score of 22.90 (S.D. 13.27) and 21.89 (S.D. 12.89), respectively. At baseline, there were no significant differences between the groups in terms of beliefs towards antidepressant medications, treatment satisfaction scores or HLQoL.
Table 1.
Demographic characteristics of intervention group (n = 110) and control group (n = 110) patients
| Intervention group | Control group | |
|---|---|---|
| No (%) | No (%) | |
| Sex | ||
| Male | 49 (44.5) | 51 (46.4) |
| Female | 61 (55.5) | 59 (53.6) |
| Age | ||
| 18–30 years | 32 (29.1) | 27 (24.5) |
| 31–40 years | 31 (28.2) | 35 (31.8) |
| 41–50 years | 27 (24.5) | 27 (24.5) |
| 51–60 years | 20 (18.2) | 21 (19.1) |
| Education level | ||
| Uneducated | 19 (17.3) | 31 (28.2) |
| Primary | 17 (15.5) | 17 (15.5) |
| Intermediate | 21 (19.1) | 23 (20.9) |
| Secondary | 37 (33.6) | 29 (26.4) |
| College/Bachelor | 16 (14.5) | 10 (9.1) |
| Number of antidepressants prescribed | ||
| One | 86 (78.2) | 95 (86.4) |
| Two | 24 (21.8) | 15 (13.6) |
Table 2.
Clinical characteristics and outcomes in intervention group and control group patients
| Intervention group | Control group | T-value | Sig. | |
|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | |||
| General overuse | 12.42 (2.24) | 12.74 (2.63) | 0.966 | 0.335 |
| General harm | 11.16 (2.50) | 10.88 (2.96) | 0.763 | 0.446 |
| Beneficial effect of pharmaceuticals | 14.69 (1.97) | 14.96 (2.14) | 0.984 | 0.326 |
| Beliefs about medicines | 38.27 (3.92) | 38.58 (4.48) | 0.545 | 0.587 |
| Sensitive | 15.72 (2.66) | 15.55 (3.13) | 0.441 | 0.660 |
| Necessity beliefs | 18.19 (3.68) | 18.30 (4.19) | 0.205 | 0.838 |
| Concern beliefs | 13.07 (3.36) | 13.38 (4.04) | 0.616 | 0.538 |
| Treatment Satisfaction Questionnaire for Medication (TSQM) | 83.20 (11.42) | 82.54 (13.41) | 0.396 | 0.692 |
| Estimated weights for EQ-5D | 0.63 (0.37) | 0.65 (0.38) | 0.414 | 0.680 |
| Montgomery–Åsberg Depression Scale | 22.90 (13.27) | 21.44 (12.89) | 0.830 | 0.408 |
| Medication Adherence Scale | 5.13 (1.80) | 5.19 (2.05) | 0.218 | 0.827 |
T-test for the difference in the characteristics of the study, according to the level of adherence
After 3 months, IG patients had significantly higher scores than CG patients for beliefs about medicine, treatment satisfaction, and medication adherence (Table 3).
Table 3.
Clinical characteristics and outcomes in intervention group and control group patients after 3 months
| Intervention group | Control group | T-value | Sig. | |
|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | |||
| General overuse | 12.17 (2.19) | 12.69 (2.62) | 1.583 | 0.115 |
| General harm | 10.61 (2.37) | 10.77 (2.93) | 0.467 | 0.641 |
| Beneficial effect of pharmaceuticals | 14.91 (1.99) | 15.11 (2.15) | 0.708 | 0.480 |
| Beliefs about medicines | 36.84 (3.77) | 38.43 (4.46) | 2.855 | 0.005a |
| Sensitive | 15.40 (2.61) | 15.47 (3.11) | 0.165 | 0.869 |
| Necessity beliefs | 18.73 (3.78) | 18.46 (4.19) | 0.509 | 0.611 |
| Concern beliefs | 12.42 (3.18) | 13.25 (4.00) | 1.694 | 0.092 |
| Treatment Satisfaction Questionnaire for Medication (TSQM) | 86.68 (11.08) | 82.82 (13.40) | 2.326 | 0.021a |
| Estimated weights for EQ-5D | 0.66 (0.39) | 0.65 (0.38) | 0.076 | 0.939 |
| Montgomery–Åsberg Depression Scale | 21.07 (12.21) | 21.01 (12.63) | 0.036 | 0.971 |
| Medication Adherence Scale | 5.79 (1.89) | 5.04 (1.98) | 2.880 | 0.004a |
aSignificantly different
After 6 months, compared with CG patients, IG patients showed significant differences in adherence to medication, treatment satisfaction, general overuse beliefs, specific concern beliefs, and total general beliefs about medicines (Table 4). Furthermore, severity of depression and HRQoL were not significantly change between IG and CG at the end of six months. Using T-test: to test the difference between the reading at baseline & end of six month among participant in IG, a significantly improved at the end of 6 months in severity of depression 20.65 (11.97) T-value 18.09, and HRQoL 88.71 (10.77) T-value 25.53.
Table 4.
Clinical characteristics and outcomes in intervention group and control group patients after 6 months
| Intervention group | Control group | T-value | Sig. | |
|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | |||
| General overuse | 11.93 (2.15) | 12.63 (2.61) | 2.197 | 0.029a |
| General harm | 10.08 (2.25) | 10.67 (2.90) | 1.687 | 0.093 |
| Beneficial effect of pharmaceuticals | 15.35 (2.04) | 15.25 (2.15) | 0.356 | 0.722 |
| Beliefs about medicines | 35.36 (3.62) | 38.12 (4.43) | 5.055 | <0.0001a |
| Sensitive | 15.10 (2.56) | 15.39 (3.10) | 0.769 | 0.442 |
| Necessity beliefs | 19.00 (3.83) | 18.50 (4.19) | 0.939 | 0.349 |
| Concern beliefs | 12.05 (3.08) | 13.14 (3.97) | 2.278 | 0.024 |
| Treatment Satisfaction Questionnaire for Medication (TSQM) | 88.71 (10.77) | 82.89 (13.40) | 3.551 | <0.0001a |
| Estimated weights for EQ-5D | 0.68 (0.40) | 0.66 (0.38) | 0.356 | 0.722 |
| Montgomery–Åsberg Depression Scale | 20.65 (11.97) | 20.86 (12.54) | 0.129 | 0.897 |
| Medication Adherence Scale | 5.99 (1.88) | 4.94 (1.94) | 4.059 | <0.0001a |
aSignificantly different
Discussion
In this study, we examined the impact of SDM-based pharmacist interventions on patient adherence to antidepressant medications and related patient-reported outcomes. During the study, we observed changes over time in adherence, treatment satisfaction, and beliefs about antidepressants in the IG but not the CG. After 6 months, IG patients showed statistically significant increases of up to 18 % in adherence to antidepressants and 6 % in treatment satisfaction, and a decrease of 8 % in concern beliefs and general beliefs about medicines.
Our finding of improvements in adherence to antidepressants after pharmacist intervention is consistent with those of other studies (Canales, Bultman, Finley, Brook, 2005 and Rickles et al., 2005 [40, 4]). Furthermore, consistent with previous studies we did not find significant differences in the severity of depression between our groups. Most previous studies that delivered similar interventions as that described in this paper have reported no differences in the severity of depression or depressive symptoms between groups (Capoccia et al., 2004; Bosmans et al., 2007). Only one study has reported significant improvements in depressive symptoms, using the Hamilton Psychiatric Rating to measure severity of depression after pharmacist interventions in acute care psychiatric inpatients [43]. Differences in the patient sample studied (inpatient versus outpatient) may explain these differences.
However, a significant negative correlation (−0.327) between improvements in adherence and severity of depressive symptoms has been reported [44]. In this study, there were improvements in depression severity in that we observed improvements in adherence between baseline and 6 months in IG patients. However, there were no significant differences between the IG and the CG. This lack of a significant difference in depression severity between groups could result from a lack of statistical power, because the sample was calculated to obtain a difference in the main outcome (adherence to antidepressants). This may mean the study was underpowered to detect endpoint changes in secondary outcomes, such as the severity of depression. However, in this study, we used a sensitive scale (MADRS) to detect endpoint changes in depression symptoms in response to the treatment of depression [45].
Both groups reported high treatment satisfaction rates, with IG patients showing significantly greater satisfaction than did CG patients. This finding is consistent with a direct positive correlation between adherence to antidepressants and treatment satisfaction reported elsewhere [46]. It is also similar to findings from another 20 studies reported in a recent literature review [4, 47]. In contrast, both groups reported a moderate health-related quality of life, with no significant differences between groups. HRQoL is influenced by various psychological comorbidities, with depression being one of the most important of these [48, 49]. Furthermore, to assess HRQoL we used the EQ-5D, which is known to be very specific, but which produces information that lacks sensitivity. Therefore, it may not detect small changes, especially as both groups reported moderate scores at baseline.
In this study, after 3 and 6 months we detected significantly different scores in the IG for beliefs about antidepressants compared with the CG, particularly regarding general beliefs about medicines, general overuse, and specific concern beliefs. This is consistent with results reported elsewhere (Bultman et al., 2002, Rickles et al., 2005; Aikens et al., 2008). Adherence appears to be influenced by specific-concern beliefs and overuse beliefs about antidepressant medications, which is also consistent with findings from other studies [52, 53].
There are some limitations to our study. First, the study was conducted at one site only (an outpatient clinic in a psychiatric hospital) and the intervention was applied by a limited number of hospital pharmacists. This decreases the generalisability of the results to other healthcare settings. Nonetheless, our findings are consistent with studies conducted in other settings. Second, we use subjective methods (self-report scales and questionnaires) to measure patient outcomes, which may be subject to bias. However, we selected all scales carefully, ensuring they were validated with Arabic patients [24, 32, 34, 54]. Furthermore, subjective methods appear reliable and correlate with the clinical state of psychiatric patients in clinical practice [55], and patients reporting low scores are the most likely to be truthful [53]. Finally, there is a chance that researchers who were not blind to intervention group may have biased the results found. However, as those collecting the data were blind to patient group allocation we believe this had a minimal effect on our results.
Despite these limitations, the use of randomised controlled methods is a strength of this study, ensuring equal distribution of demographic and confounding factors across groups (e.g. age, gender, other symptoms, and medical problems) [56]. Furthermore, to the best of our knowledge, this is the first study to investigate pharmacist interventions with patients with depression to enhance adherence to medications using SDM processes.
Further studies are needed to evaluate the cost effectiveness of this intervention and to compare it with usual pharmacy services. New approaches must be designed to target depression symptoms in addition to improving adherence, and to improve our understanding of medication-taking behaviour among depressed patients.
Conclusion
Pharmacist interventions based on SDM delivered to depressed patients showed a significant positive effect on adherence, treatment satisfaction, and patients’ beliefs about antidepressants. When compared with patients provided with usual care, patients given the intervention had better scores on these outcomes. This finding highlights the important role pharmacists could play in providing direct patient care in regular pharmacy practice to improve the adherence to medications and other patient-reported outcomes.
Acknowledgements
We thank the pharmacists at Al-Amal Complex for Mental Health in Riyadh for their support and willingness to recruit patients for this study. We also thank all the patients for their time and participation in the interviews.
Abbreviations
- BMQ
Beliefs about medicine questionnaire
- CG
Control group
- IG
Intervention group
- MDD
Major depressive disorder
- MMAS
Morisky medication adherence scale
- OPTION
Observing patient Involvement in decision
- HRQOL
Health-related quality of life
- SDM
Shared decision making
- TSQM
Treatment satisfaction questionnaire for medication
Footnotes
Competing interests
The authors declare that they have no competing interests (no financial conflicts, no stocks or shares in an organisation that may in any way gain or lose financially from the publication of this manuscript, no patents relating to the content of the manuscript and no non-financial competing interests).
Authors’ contributions
KA contributed to the conception and design of the study, data collection, data analysis, interpretation, and drafting the manuscript, and read, approved the final manuscript. MA contributed to the conception and design of the study, revision of the manuscript and read, approved the final manuscript.
Contributor Information
K. Aljumah, Email: khalafaljumaah@yahoo.com
MA Hassali, Email: alamalpharmacy@yahoo.com.
References
- 1.Remien RH, Hirky AE, Johnson MO, Weinhardt LS, Whittier D, Le GM. Adherence to medication treatment: A qualitative study of facilitators and barriers among a diverse sample of HIV+ men and women in four US cities. AIDS Behav. 2003;7(1):61–72. doi: 10.1023/A:1022513507669. [DOI] [PubMed] [Google Scholar]
- 2.Sawada N, Uchida H, Suzuki T, Watanabe K, Kikuchi T, Handa T, et al. Persistence and compliance to antidepressant treatment in patients with depression: a chart review. BMC Psychiatry. 2009;9(1):38. doi: 10.1186/1471-244X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunot VM, Horne R, Leese MN, Churchill RC. A cohort study of adherence to antidepressants in primary care: the influence of antidepressant concerns and treatment preferences. Primary Care Companion J Clin Psychiatry. 2007;9(2):91. doi: 10.4088/PCC.v09n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Jumah KA, Qureshi NA. Impact of pharmacist interventions on patients’ adherence to antidepressants and patient-reported outcomes: a systematic review. Patient Preference and Adherence. 2011;6:87–100. doi: 10.2147/PPA.S27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson J, Britten N. Patients’ decisions about whether or not to take antihypertensive drugs: qualitative study. BMJ. 2002;325(7369):873. doi: 10.1136/bmj.325.7369.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi: 10.1080/08870449908407311. [DOI] [Google Scholar]
- 7.Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506–11. doi: 10.1007/BF02599549. [DOI] [PubMed] [Google Scholar]
- 8.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clinic Proceedings. Elsevier; 2011;86:4. [DOI] [PMC free article] [PubMed]
- 9.Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competences of involving patients in healthcare choices. Br J Gen Pract. 2000;50(460):892–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray E, Burns J, See TS, Lai R, Nazareth I. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;4. [DOI] [PubMed]
- 12.Drake RE, Cimpean D, Torrey WC. Shared decision making in mental health: prospects for personalized medicine. Dialogues Clin Neurosci. 2009;11(4):455. doi: 10.31887/DCNS.2009.11.4/redrake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson KA, Bastani R, Rubenstein LV, Meredith LS, Ford DE. Effect of mental health care and shared decision making on patient satisfaction in a community sample of patients with depression. Med Care Res Rev. 2007;64(4):416–30. doi: 10.1177/1077558707299479. [DOI] [PubMed] [Google Scholar]
- 14.Orlando M, Meredith LS. Understanding the causal relationship between patient-reported interpersonal and technical quality of care for depression. Med Care. 2002;40(8):696–704. doi: 10.1097/00005650-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Alshammari H, Alaamer K, Albassam M, Aljumah K. The impact of decision aid on depressed patients’ involvement in shared decision making: a Pilot randomized controlled double-blinded study. PHARMACOTHERAPY. 111 River ST, Hoboken 07030–5774, NJ USA: Wiley-Blackwell; 2014. [Google Scholar]
- 16.Elwyn G, Hutchings H, Edwards A, Rapport F, Wensing M, Cheung WY, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision‐making tasks. Health Expect. 2005;8(1):34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons M, Hetrick S, Jorm A. Shared decision-making: benefits, barriers and current opportunities for application. Australas Psychiatry. 2010;18(5):394–7. doi: 10.3109/10398562.2010.499944. [DOI] [PubMed] [Google Scholar]
- 19.Alshammari H, Alaamer K, Albassam M, Aljumah K. Development and validation of patient decision aid for depressed patients. PHARMACOTHERAPY. 111 RIVER ST, HOBOKEN 07030–5774, NJ USA: WILEY-BLACKWELL; 2014. [Google Scholar]
- 20.Morisky DE, Ang A, Krousel‐Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clinical Hypertension. 2008;10(5):348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Krousel-Wood M, Islam T, Webber LS, Re R, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in hypertensive seniors. Am J Manag Care. 2009;15(1):59. [PMC free article] [PubMed] [Google Scholar]
- 23.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64(3):255. doi: 10.1016/j.jclinepi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhalaiqa F, Deane K, Nawafleh A, Clark A, Gray R. Adherence therapy for medication non-compliant patients with hypertension: a randomised controlled trial. J Hum Hypertens. 2011;26(2):117–26. doi: 10.1038/jhh.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George C, Peveler R, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol. 2000;50(2):166–71. doi: 10.1046/j.1365-2125.2000.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mårdby A-C, Åkerlind I, Jörgensen T. Beliefs about medicines and self-reported adherence among pharmacy clients. Patient Educ Couns. 2007;69(1):158–64. doi: 10.1016/j.pec.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Baer L, Blais MA. Handbook of clinical rating scales and assessment in psychiatry and mental health. New York: Humana Press; 2010.
- 28.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Carmody TJ, Trivedi MH, Hughes C, Bernstein IH, Morris DW, et al. A psychometric evaluation of the CDRS and MADRS in assessing depressive symptoms in children. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1204–12. doi: 10.1097/chi.0b013e3180cc2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss MC, Peters TJ. Measuring shared decision making in the consultation: a comparison of the OPTION and Informed Decision Making instruments. Patient Educ Couns. 2008;70(1):79–86. doi: 10.1016/j.pec.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) 1990;16(3):199. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 32.Aburuz S, Bulatova N, Twalbeh M, Gazawi M. The validity and reliability of the Arabic version of the EQ-5D: a study from Jordan. Ann Saudi Med. 2009;29(4):304. doi: 10.4103/0256-4947.55313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweileh WS, Ihbesheh M, Jarar IS, Sawalha AF, Abu Taha AS, Zyoud H, et al. Antipsychotic medication adherence and satisfaction among Palestinian people with schizophrenia. Curr Clin Pharmacol. 2012;7(1):49–55. doi: 10.2174/157488412799218761. [DOI] [PubMed] [Google Scholar]
- 34.Sweileh WM, Ihbesheh MS, Jarar IS, Taha ASA, Sawalha AF, Zyoud SH, et al. Self-reported medication adherence and treatment satisfaction in patients with epilepsy. Epilepsy Behav. 2011;21(3):301–5. doi: 10.1016/j.yebeh.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Bharmal M, Payne K, Atkinson MJ, Desrosiers M-P, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2(1):12. doi: 10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8:S9–24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 38.Brook OH, van Hout H, Stalman W, Nieuwenhuyse H, Bakker B, Heerdink E, et al. A pharmacy-based coaching program to improve adherence to antidepressant treatment among primary care patients. Psychiatr Serv. 2005;56(4):487–9. doi: 10.1176/appi.ps.56.4.487. [DOI] [PubMed] [Google Scholar]
- 39.Simon GE, Goldberg SD, Tiemens BG, Ustun TB. Outcomes of recognized and unrecognized depression in an international primary care study. Gen Hosp Psychiatry. 1999;21(2):97–105. doi: 10.1016/S0163-8343(98)00072-3. [DOI] [PubMed] [Google Scholar]
- 40.Rickles NM, Svarstad BL, Statz-Paynter JL, Taylor LV, Kobak KA, 2005b. Pharmacist telemonitoring of antidepressant use: effects on pharmacist-patient collaboration. Journal of the American Pharmacists Association. 45;344-353. [DOI] [PubMed]
- 41.Capoccia KL, Boudreau DM, Blough DK, Ellsworth AJ, Clark DR, Stevens NG, Katon WJ, Sullivan SD. 2004. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. American Journal of Health-System Pharmacy. 61;364-372. [DOI] [PubMed]
- 42.Bosmans JE, Brook OH, Van Hout HP, De Bruijne MC, Nieuwenhuyse H, Bouter LM, Stalman WA, Van Tulder MW. Cost effectiveness of a pharmacy-based coaching programme to improve adherence to antidepressants. Pharmacoeconomics. 2007;25:25–37. doi: 10.2165/00019053-200725010-00004. [DOI] [PubMed] [Google Scholar]
- 43.Canales PL, Dorson PG, Crismon ML. Outcomes assessment of clinical pharmacy services in a psychiatric inpatient setting. Am J Health Syst Pharm. 2001;58(14):1309–16. doi: 10.1093/ajhp/58.14.1309. [DOI] [PubMed] [Google Scholar]
- 44.Al Jumah K, Hassali MA, Al Qhatani D, El Tahir K. Factors associated with adherence to medication among depressed patients from saudi arabia: a cross-sectional study. Neuropsychiatr Dis Treat. 2014;10:2031. doi: 10.2147/NDT.S71697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santen G, Danhof M, Pasqua OD. Sensitivity of the Montgomery Asberg Depression Rating Scale to response and its consequences for the assessment of efficacy. J Psychiatr Res. 2009;43(12):1049–56. doi: 10.1016/j.jpsychires.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Aljumah K, Hassali AA, AlQhatani S. Examining the relationship between adherence and satisfaction with antidepressant treatment. Neuropsychiatr Dis Treat. 2014;10:1433. doi: 10.2147/NDT.S67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbosa CD, Balp M-M, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. doi: 10.2147/PPA.S24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47–56. doi: 10.1007/s00127-009-0041-5. [DOI] [PubMed] [Google Scholar]
- 49.Magán I, Sanz J, García-Vera MP. Psychometric properties of a Spanish version of the Beck Anxiety Inventory (BAI) in general population. Span J Psychol. 2008;11(02):626–40. [PubMed] [Google Scholar]
- 50.Bultman DC, Svarstad BL. 2001. Effects of pharmacist monitoring on patient satisfaction with antidepressant medication therapy. Journal of the American Pharmaceutical Association (Washington, DC: 1996). 42;36-43. [DOI] [PubMed]
- 51.Aikens JE, Nease DE, Klinkman MS. 2008. Explaining patients’ beliefs about the necessity and harmfulness of antidepressants. The Annals of Family Medicine. 6;23-29. [DOI] [PMC free article] [PubMed]
- 52.Rivero-Santana A, Perestelo-Perez L, Pérez-Ramos J, Serrano-Aguilar P, De las Cuevas C. Sociodemographic and clinical predictors of compliance with antidepressants for depressive disorders: systematic review of observational studies. Patient Preference and Adherence. 2013;7:151. doi: 10.2147/PPA.S39382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.AlHewiti A. Adherence to Long-Term Therapies and Beliefs about Medications. Int J Family Med. 2014;2014:8. doi: 10.1155/2014/479596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horne R, Graupner L, Frost S, Weinman J, Wright SM, Hankins M. Medicine in a multi-cultural society: the effect of cultural background on beliefs about medications. Soc Sci Med. 2004;59(6):1307–13. doi: 10.1016/j.socscimed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Velligan D, Wang M, Diamond P, Glahn D, Castillo D, Bendle S, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–92. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 56.Stolberg HO, Norman G, Trop I. Randomized controlled trials. Am J Roentgenol. 2004;183(6):1539–44. doi: 10.2214/ajr.183.6.01831539. [DOI] [PubMed] [Google Scholar]


