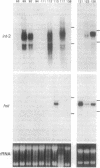
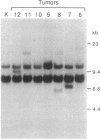
Abstract
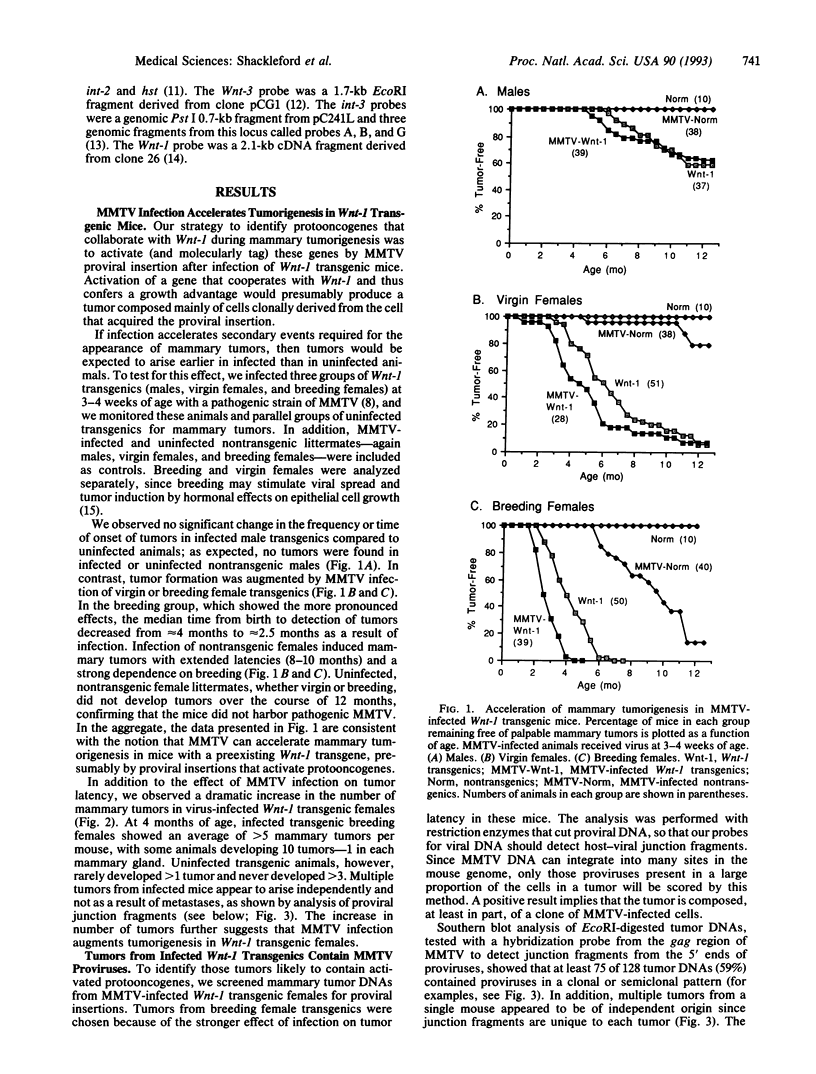
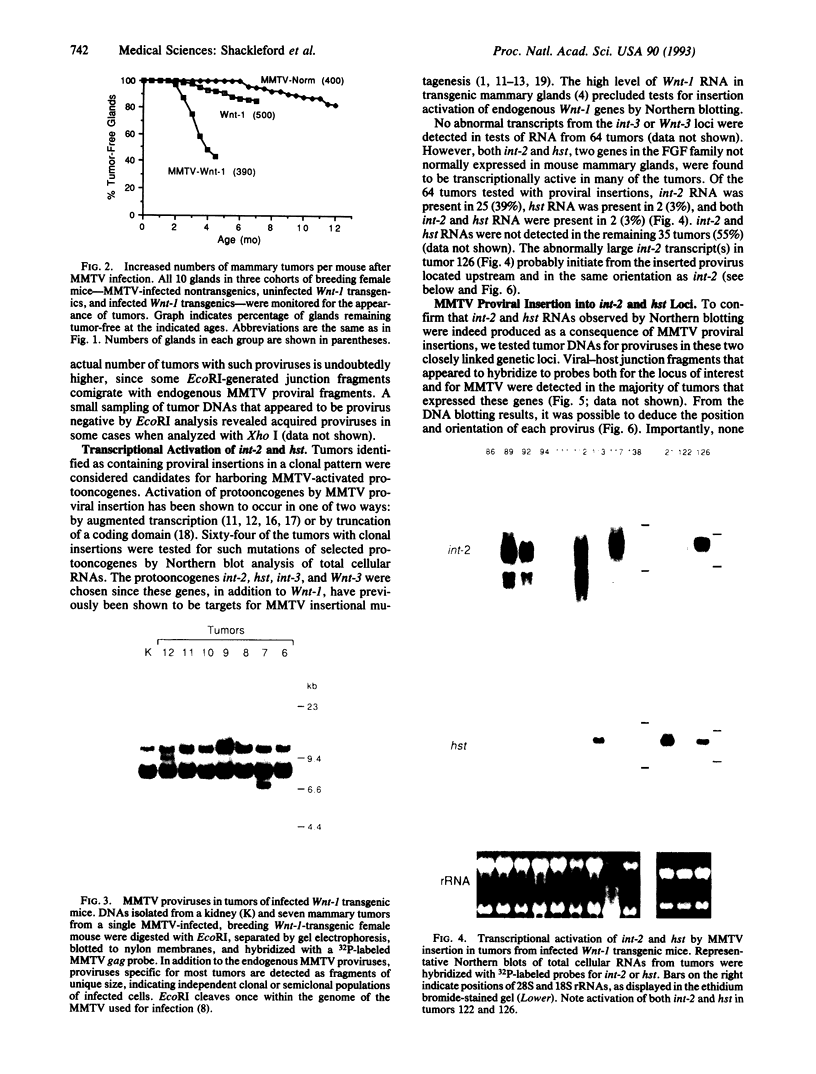
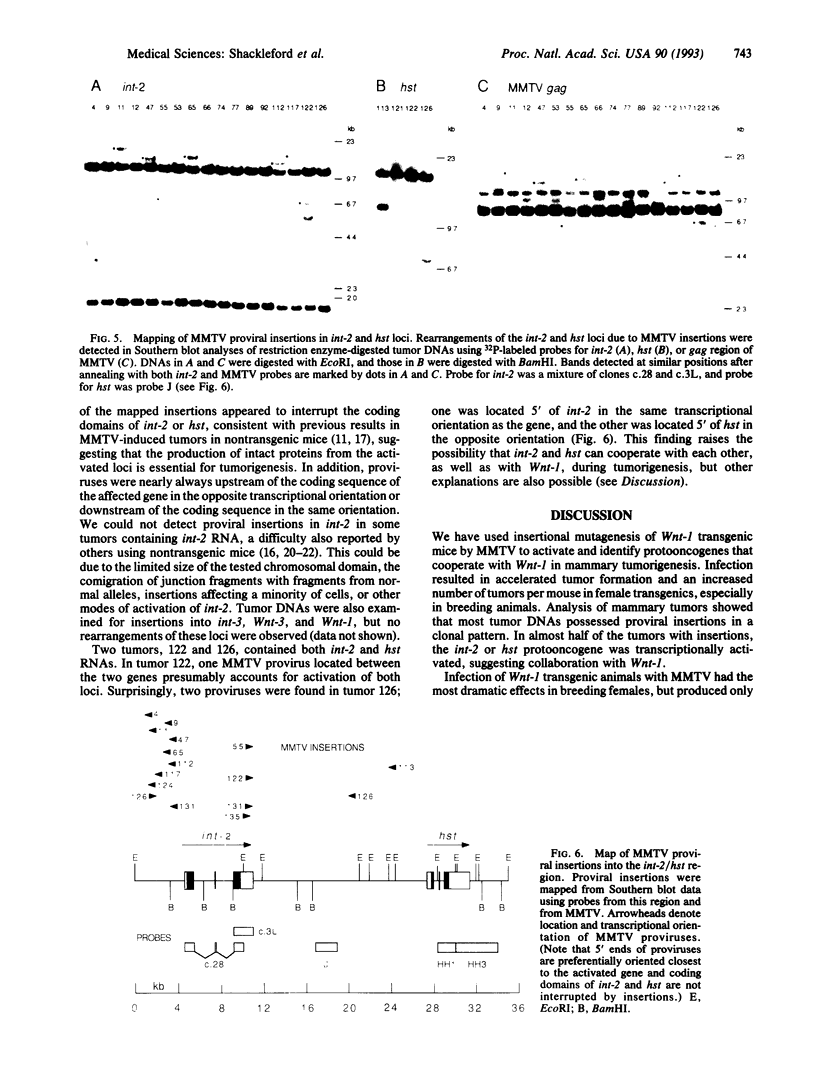
Transgenic mice carrying the Wnt-1 protooncogene modified for expression in mammary epithelial cells exhibit hyperplastic mammary glands and stochastically develop mammary carcinomas, suggesting that additional events are necessary for tumorigenesis. To induce such events and to identify the genes involved, we have infected Wnt-1 transgenic mice with mouse mammary tumor virus (MMTV), intending to insertionally activate, and thereby molecularly tag, cooperating protooncogenes. Infection of breeding female Wnt-1 transgenics decreased the average age at which tumors appeared from approximately 4 months to approximately 2.5 months and increased the average number of primary tumors per mouse from 1-2 to > 5. A smaller effect was observed in virgin females, and infection of transgenic males showed no significant effect on tumor latency. More than half of the tumors from the infected breeding group contained one or more newly acquired MMTV proviruses in a pattern suggesting that most cells in tumors arose from a single infected cell. Analyses of provirus-containing tumors for induced or altered expression of int-2/Fgf-3, hst/Fgf-4, int-3, and Wnt-3 showed activation of int-2 in 39% of tumors, hst in 3%, and both int-2 and hst in 3%. DNA analyses with probes for protooncogenes and MMTV confirmed that the activations resulted from proviral insertions. There was no evidence for proviral insertions at the int-3, Wnt-3, or Wnt-1 loci. These findings provide further evidence that fibroblast growth factors Int-2 and Hst can cooperate with Wnt-1, another secreted factor, in mammary tumorigenesis, and they illustrate the capacity of this system to identify cooperating oncogenes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Wildin R. S., Prendergast T. J., Varmus H. E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986 Sep 26;46(7):1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Olson D. J., Moon R. T. Xwnt-8 modifies the character of mesoderm induced by bFGF in isolated Xenopus ectoderm. EMBO J. 1992 Jan;11(1):33–41. doi: 10.1002/j.1460-2075.1992.tb05024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Shackleford G. M., Brown A. M., Sanders G. S., Varmus H. E. Nucleotide sequence and expression in vitro of cDNA derived from mRNA of int-1, a provirally activated mouse mammary oncogene. Mol Cell Biol. 1985 Dec;5(12):3337–3344. doi: 10.1128/mcb.5.12.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallahan D., Callahan R. Mammary tumorigenesis in feral mice: identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J Virol. 1987 Jan;61(1):66–74. doi: 10.1128/jvi.61.1.66-74.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. A., Jackson D. P., Percy D. H., Morris V. L. Activation of int-1 and int-2 loci in GRf mammary tumors. Virology. 1986 Oct 30;154(2):271–278. doi: 10.1016/0042-6822(86)90453-8. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Alexander W. S., Barri G., Klinken S. P., Adams J. M. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991 May 31;65(5):753–763. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Kwan H., Pecenka V., Tsukamoto A., Parslow T. G., Guzman R., Lin T. P., Muller W. J., Lee F. S., Leder P., Varmus H. E. Transgenes expressing the Wnt-1 and int-2 proto-oncogenes cooperate during mammary carcinogenesis in doubly transgenic mice. Mol Cell Biol. 1992 Jan;12(1):147–154. doi: 10.1128/mcb.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Martin G. R. Four classes of mRNA are expressed from the mouse int-2 gene, a member of the FGF gene family. EMBO J. 1988 Jul;7(7):2035–2041. doi: 10.1002/j.1460-2075.1988.tb03043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A., Robbins J., Campbell G., Buttitta F., Squartini F., Bistocchi M., Callahan R. Host genetic background effect on the frequency of mouse mammary tumor virus-induced rearrangements of the int-1 and int-2 loci in mouse mammary tumors. J Virol. 1991 Aug;65(8):4550–4554. doi: 10.1128/jvi.65.8.4550-4554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester J., Wagenaar E., Sluyser M., Nusse R. Activation of int-1 and int-2 mammary oncogenes in hormone-dependent and -independent mammary tumors of GR mice. J Virol. 1987 Apr;61(4):1073–1078. doi: 10.1128/jvi.61.4.1073-1078.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Placzek M., Dickson C. The mouse homolog of the hst/k-FGF gene is adjacent to int-2 and is activated by proviral insertion in some virally induced mammary tumors. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5678–5682. doi: 10.1073/pnas.86.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Activation of cellular gene by mouse mammary tumour virus may occur early in mammary tumour development. Nature. 1984 May 17;309(5965):273–275. doi: 10.1038/309273a0. [DOI] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., van Deemter L., Wagenaar E., Sonnenberg A., Nusse R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987 Jan;6(1):127–131. doi: 10.1002/j.1460-2075.1987.tb04729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Blondel B. J., Gallahan D., Callahan R. Mouse mammary tumor gene int-3: a member of the notch gene family transforms mammary epithelial cells. J Virol. 1992 Apr;66(4):2594–2599. doi: 10.1128/jvi.66.4.2594-2599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Nusse R. Amplification and proviral activation of several Wnt genes during progression and clonal variation of mouse mammary tumors. Oncogene. 1992 Mar;7(3):487–492. [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9655–9659. doi: 10.1073/pnas.85.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto A. S., Grosschedl R., Guzman R. C., Parslow T., Varmus H. E. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988 Nov 18;55(4):619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Krimpenfort P., Domen J., Saris C., Radaszkiewicz T., Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989 Feb 24;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Scheijen B., Wientjens E., van der Gulden H., Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991 May 31;65(5):737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]