Abstract
Objectives
The purpose of this study was to consecutively capture and quantify nitric oxide (NO) and cGMP, the second messenger of NO, over the skin surface of acupuncture points (acupoints), meridian line without acupoint, and non-meridian control regions of the Pericardium meridian (PC) in humans, and investigate their response to transcutaneous electrical nerve stimulation (TENS).
Design, setting, and main outcome measures
Adhesive biocapture tubes were attached to the skin surface along PC regions and injected with 2-Phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl solution, an NO-scavenging compound, contacting the skin surface for 20 minutes each during 4 consecutive biocapture intervals. TENS (1.0 mA, 6 Hz, 1.0 msec duration) was applied over acupoints PC 8 and PC 3 during the 2nd biocapture for 20 min. Total nitrite and nitrate (NOx-), the stable metabolic products of NO, and cGMP in biocaptured samples were quantified using chemiluminescence and ELISA.
Results
NOx- levels in the 1st biocapture over PC regions are almost two fold higher compared to subsequent biocaptures and are higher over PC acupoints versus non-meridian control region. Following TENS, NOx- concentrations over PC regions were significantly increased, and cGMP is predominantly released from the skin surface of PC acupoints.
Conclusions
TENS induces elevations of NO-cGMP concentrations over local skin region with a high level at acupoints. The enhanced signal molecules improve local circulation, which contributes to beneficial effects of the therapy.
Keywords: Nitric Oxide, cGMP, Transcutaneous Electrical Nerve Stimulation, Biocapture, Acupuncture Meridian
Introduction
Transcutaneous electrical nerve stimulation (TENS) and transcutaneous electrical stimulation of acupuncture points (acupoints, TESA) provide a safe, standardized technique without invasive acupuncture needle insertion and is widely accepted as an alternative therapy for pain relief in the community [1,2]. Several studies have demonstrated that TENS and TESA produce analgesic effects, reduce the need for pain medication, and increase physical functionality [2-4]. A number of studies have demonstrated that localized TENS augments microcirculation and angiogenesis, thus having wound-healing applications in animal and human subjects [2,6-8]. TENS, acupuncture, and acupuncture-like stimulation affects regional blood flow in skin, and produces changes in arterial blood pressure and blood nitric oxide (NO) level [9-11]. Thus, it is suggested that TENS potentially can improve symptoms caused by peripheral vascular diseases [7,8,12]. However, the effects, biochemical changes, and mechanism responsible for the beneficial effects induced by TENS/TESA are not well known.
The Meridian System (Jingluo) described in Traditional Chinese Medicine is an essential pathway system and the core theory of acupuncture, related to TENS and TESA [13-15]. International studies in both humans and animals have shown that acupoints possess characteristics of low electrical resistance (LER) and high electric conductance [14-20]. Several reports have demonstrated that LER is present not only over the skin surface of acupoints, but also over entire meridian lines (about 1.0 mm in width) as described in traditional charts [14,15,20]. Morphological studies have identified that most acupoints are located intimately at the distribution of nerve trunks, blood vessels, sweat glands, and hair follicles [19-21].
It is well-documented that NO is one of the most important messenger molecules, and NO synthase (NOS) expression is exhibited in the skin tissue [22-24]. Figure 1 shows that NO can be produced enzymatically by NOS or non-enzymatically through the nitrate-nitrite-NO pathway, and NO stimulates guanylyl cyclase to generate cyclic guanosine monophosphate (cGMP), a second messenger directing vasodilatation and various biological functions [25,26]. NO has a half-life of a few seconds due to rapid oxidation to both nitrite and nitrate [27,28], and measurements of total nitrite and nitrate (NOx-), stable end products of NO metabolism, are very adequate indicators of the changes in NO activity and its production in the tissue [27-29], as shown in figure 1. Investigators have developed an NO-scavenging compound, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO), which has been used to convert NO into stable measurable nitrite in biological systems [30,31]. Previous studies described a connection between neurotransmitters and organs corresponding to acupuncture points by using an indirect measurement, but requires validation [32]. Our recent studies have developed a painless, non-invasive device to capture and directly quantify NO metabolites using PTIO solution incubated over skin surfaces of acupoints/meridians [33,34]. We have demonstrated, using this biocapture device, that NO levels are increased over acupoints compared to meridian lines without acupoint (MWOP) and non-meridian control regions (NMCR) in humans [23].
Fig. 1. Biological reactivity, metabolism, and key biomolecules in signal transduction pathways of nitric oxide-cGMP system.

Nitric oxide (NO) in the skin tissue is produced enzymatically from L-arginine by nitric oxide synthase and non-enzymatically from bacterial reduction of nitrate through the nitrate-nitrite-NO pathway present in sweat and epidermis. NO stimulates soluble guanylyl cyclase to generate cGMP, which causes vasodilatation and other biological functions. NO rapidly oxidizes into nitrite (NO2-) and nitrate (NO3-), and measurements of these stable metabolites (NOx-, total nitrite plus nitrate) adequately indicate changes in NO activity and production in tissues.
The purpose of this study is to quantify the changes in concentrations of NOx- along the skin surface of the acupoints of the Pericardium meridian (PC) compared to their corresponding MWOP and NMCR during consecutive biocaptures in humans. Whether NO-cGMP releases are modified by TENS and whether the effects of TENS-induced NO-cGMP releases are specific to acupoints and/or meridians, are determined by quantification of the molecules along the PC region following the treatments.
Materials and Methods
1. Human Subjects
Twenty six men and women (18- to 55-years-old), recruited at Harbor-UCLA Medical Center, volunteered for the following studies. All participants were healthy subjects who did not have major surgery in the past 12 months nor history of cardiovascular disease. Subjects with dermatological problems, allergic diseases, vascular disorders, infectious diseases, and prescribed medication were excluded from the study. The protocol was approved by the John F. Wolf, MD Human Subjects Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Subjects were given a detailed oral instruction of the study, and their informed consent was obtained. Female participants were not on their menstrual period on the day of study. Experiments were performed in a quiet, air-conditioned room with the temperature maintained at 25-27 °C. Researchers operating the chemiluminescence Nitrogen Oxide Analyzer and analyzing the data were blinded to treatments of the subjects.
2. Identification of Acupoints and Transcutaneous Electrical Nerve Stimulation (TENS)
The region of acupoints, MWOP, and NMCR over the PC meridian of the ventral forearm were studied in each subject as described in Figure 2. Locations were identified by an acupoint/meridian map of the human body [13,15]. These regions were chosen in the experiments for the following reasons: 1) Acupoints/meridians in these regions can be easily and consistently identified on the body surface; and 2) There is enough space to place NO biocapture tubes without touching other meridians [33,34]. TENS was performed using a battery powered acupuncture stimulator to generate a current of 1.0 mA at 6 Hz for a duration of 1.0 msec [35]. The stimulation was applied to the skin surface of PC 8 (cathode) on the hand and PC 3 (anode) on the upper forearm as a pair through a stimulation electrode for 20 min.
Fig. 2. Representation of a Biocapture Device with a Semi-cylindrical Molded Tube Taped to the Skin Surface over Meridians.
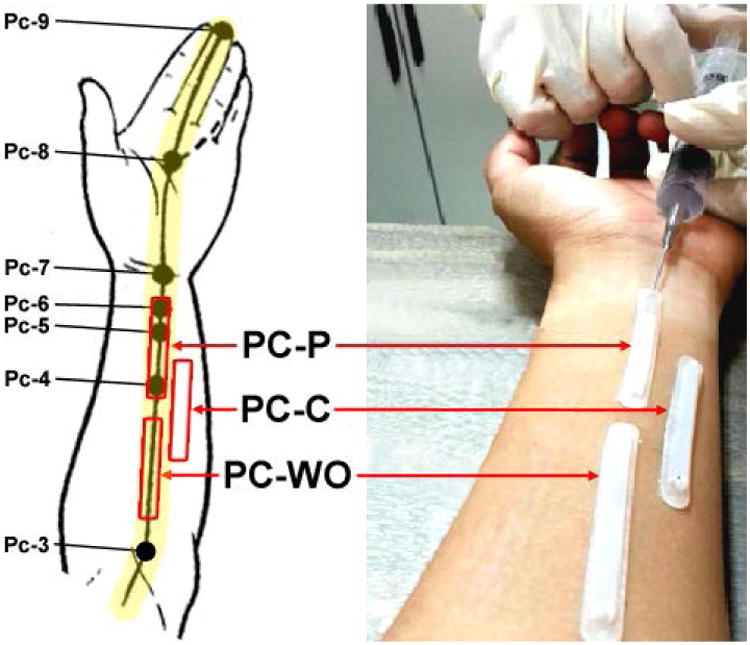
The pericardium meridian (PC or P, bottom left) lines and related acupuncture points are illustrated on the left panel. The region from PC 4 to 6 is defined as acupoint (PC-P, 3 acupoints), the distance between PC 3 to 4 is defined as meridian line without acupoint (PC-WO), and the non-meridian control region is adjacent to the PC meridian (PC-C). NO scavenging solution (100 μM PTIO) was injected into the tubing on the skin surface for 20 min in order to directly absorb NO.
3. Instrumentation and NO Biocapture
Acupoints, MWOP, and NMCR were defined as described above, and the biocapture method was described previously in humans [33,34]. A biocapture device, developed by this lab, consists of a molded semi-circular silicone-plastic tube (0.5 × 5 cm), which is adhered to the ventral forearm skin using a custom double-sided adhesive, as shown in Figure 2 [34]. PTIO solution (100 μM) was injected inside the sterilized tube and in direct contact with the surface of the skin for 20 min to absorb NO. After the 20 min incubation, the liquid was collected from the tubes adhered over acupoints, MWOP, and NMCR. Following the first biocapture, the PTIO solution was then replaced and collected at 20 min each for the 2nd, 3rd and 4th biocaptures. The liquid was transferred to a plastic vial and stored in -80 °C. The concentrations of total nitrite plus nitrate (NOx-) and cGMP in the samples were quantified using chemiluminescence and ELISA in a blinded fashion, respectively [33-35].
4. Quantification of NO Metabolites and cyclic GMP
The total NOx- concentration (NO2- and NO3-) was measured in the biocapture solution using an ozone phase chemiluminescence method (Sievers NOA280i, GE Analytical Instruments, Boulder, CO) as described previously [33-35]. Briefly, 5 μl samples were reduced using a Vanadium (III)/HCl solution. The nitrate calibration curve was established using known concentrations of NaNO3 dissolved in the sterile nitrogen-free water. The total amount of NO3- in each collecting sample was calculated by integration of the signal peaks using the nitrate calibration curve. All samples were measured in duplicate. The presence of (NO2-) in our collecting samples was close to the water basal level. The minimum sensitivity level of NO amount is 1.0 picomole.
The concentration of cGMP in the biocapture samples was assayed using a competitive Enzyme-Linked Immunosorbent Assay (Parameter™ Cyclic GMP Assay, R&D Systems, Minneapolis, MN) following manufacturer's protocol [34,35]. For the sample preparation, 40 μl biocapture sample was diluted in 60 μl Calibrator Diluent RD5-5. The optical density of yellow color development in the wells was read using a microplate reader set to 450 nm with a wavelength correction to 570 nm (Molecular Devices Emax, Sunnyvale, CA). The minimal detection level of cGMP is 1.14 pmol/ml.
5. Research Protocols
Volunteers were randomly asked to participate in consecutive biocaptures over PC meridian region either with or without TENS. The NO capturing tubes were taped to the left and right ventral forearm along PC acupoints, MWOP and NMCR, and filled with PTIO solution. In order to measure baseline changes in NOx- concentrations and assess the influence of cumulative NO on the skin surface, four consecutive biocaptures were performed for a period of 80 min over 20 min interval each, respectively.
In the TENS study, the biocapture devices were taped to the skin surface of the PC meridian region on both the left and right forearms, and the 1st biocapture was conducted as described above. TENS was performed over PC 8-PC 3 as a pair on one side of the arm on the skin surface for 20 min; the left or right arm was randomly treated, while the other arm served as self controls in a randomized fashion. Following the 1st biocapture, the PTIO solution was replaced and collected at 20 min during TENS. The liquid was drained from the tubing, and concentrations of NOx- and cGMP were quantified at 20 min each during the stimulation at the stimulated side compared to the control side without the treatment [35].
6. Statistical Analysis
Results were expressed as mean ± standard error of the mean (SEM) of NOx- and cGMP concentrations over the skin surface, measured in the biocapture solution as concentration (μM and nM) and as efflux rate calculated over the surface area (length times width, nmol or pmol/cm2) for 20 min along the skin surface that was in direct contact with the solution, respectively. The significance of differences were determined by three factor-repeated Analysis of Variance (ANOVA), where the three factors are (1) time intervals among the 1st, 2nd, 3rd, and 4th biocaptures; (2) two or three sites between/among PC acupoints, MWOP and NMCR; and (3) with and without TENS. P values less than 0.05 were considered significant.
Results
Baseline NO metabolites over the PC meridian regions during 4 consecutive biocaptures
The baseline NOx- concentrations on the skin surface of PC meridian regions were examined in 13 healthy volunteers. Figure 2 is a representative example of the location of acupoints, MWOP, and non-meridian control along the PC on the forearm. As shown in figure 2 (right panel), a semi-circular plastic tube was taped to the skin surface over acupoints PC 4-6, between PC 3 to 4 defined as MWOP, and adjacent to the defined PC acupoints as NMCR.
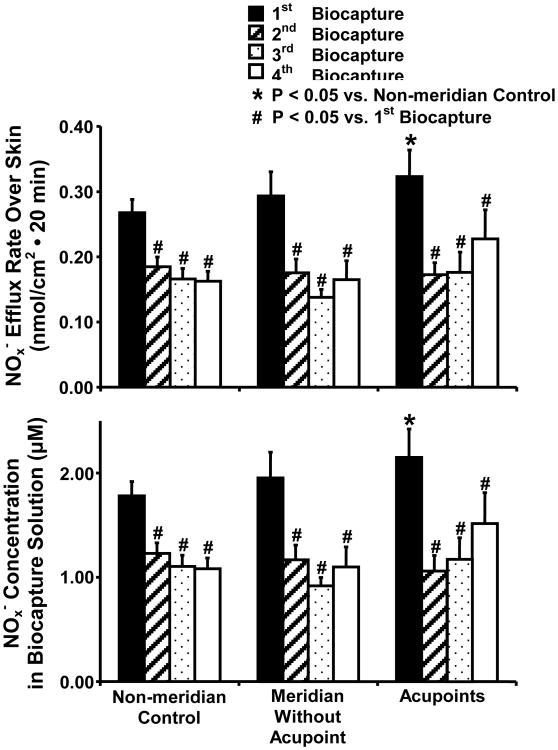
Figure 3 (top panel) shows NOx- concentrations over the skin surface measured in the biocapture solution (μM); and the bottom panel is the NOx- efflux rate calculated over the surface area that is in contact with the biocapture solution over a 20 min duration (nmol/cm2). A two-way ANOVA revealed significant differences in NOx- concentrations between consecutive biocaptures in different time periods in the 8 subjects over PC acupoints, MWOP, and NMCR (F = 23.9, P < 0.001). NOx- concentrations biocaptured during the 1st interval over PC acupoints, MWOP, and NMCR were markedly and consistently higher than those during the 2nd, 3rd, and 4th subsequent biocaptures (P < 0.05). There were no detectable differences among the 2nd, 3rd, and 4th subsequent biocaptures, as shown in figure 3. The baseline NOx- concentrations during the 2nd biocapture over PC acupoints, MWOP, and NMCR were 45.0 ± 5.4 %, 44.7 ± 8.2 %, and 33.7 ± 5.74 % (mean ± SE) less than the 1st biocapture. In the 1st biocapture, baseline NOx- concentration (μM) over PC acupoints was significantly elevated compared to the NMCR (P < 0.05). However, no significant differences were found among NOx- concentrations among the sites of acupoints, MWOP, and NMCR in the subsequent biocaptures, as shown in figure 3.
Fig. 3. Time-response Curves of Biocaptured Nitrite Plus Nitrate (NOx-) from Acupoints, MWOP, and Non-meridian Control along PC Meridian in 13 Healthy Volunteers.
NOx- concentrations over the skin surface were measured in the biocapture solution (μM, top) and NOx- efflux rate was calculated over the surface area in contact with the biocapture solution over a 20 min duration (nmol/cm2, bottom). Skin NOx- was biocaptured at 20 min intervals over 80 min as follows: 0-20 min, 20-40 min, 40-60 min, and 60-80 min. Biocaptured NOx- concentration was increased over PC 4-6 acupoints compared to non-meridian control and was decreased in the 2nd, 3rd, and 4th subsequent biocaptures compared to the 1st biocapture. Each bar represents the mean values and vertical bars represent S.E.M. (n = 8-13). *: P<0.05, compared with non-meridian control; #: P<0.05, compared with 1st biocapture (ANOVA).
Effects of TENS on NO Releases over PC Regions
NOx- releases over the PC meridian regions in response to TENS of PC 8-PC 3 pair were examined in 13 healthy volunteers. Figure 4 shows NOx- concentration measured in the biocapture solution (μM) and efflux rate calculated over the surface area (nmol/cm2) of PC acupoints, MWOP, and NMCR on one side compared to the opposing side (self-control) without treatment. NOx- concentration was significantly increased over PC acupoints and NMCR following TENS compared to the non-stimulation side (P < 0.05), as shown in figure 4.
Fig. 4. Quantification of NO Metabolites over the Pericardium Meridian following TENS.
Concentrations of total nitrite plus nitrate (NOx-) along the PC meridian were collected over acupoints, meridian lines without acupoint (MWOP), and non-meridian control during 20 min of electrical stimulation in healthy volunteers. TENS was performed over PC 8-PC 3 pair on one arm selected at random, while the other arm served as its control. NOx- concentrations in the biocapture solution (μM, top) and efflux rate calculated over the surface area in contact with the biocapture solution over a 20 min duration (nmol/cm2, bottom) were significantly increased over PC acupoints and non-meridian areas by TENS in the stimulated side compared to the side without stimulation. Each bar represents the mean values and vertical bars represent S.E.M. *: p<0.05, compared with untreated side.
Effects of TENS on cGMP Releases over PC Acupoints/Meridians
cGMP releases over the PC meridian regions in response to TENS of PC 8-PC 3 pair were examined in 13 healthy volunteers during the same period of the TENS-induced NO biocapture, which was during the 2nd biocapture after conducting the 1st biocapture. cGMP release over a 20 min interval on skin regions along the PC acupoint and NMCR was measured on one side compared to the opposing side (self-control) without treatment during the stimulation period. Figure 5 shows the cGMP concentrations measured in the biocapture solution (nM) and efflux rate calculated over the surface area that is in contact with the biocapture solution over a 20 min duration (pmol/cm2). cGMP concentrations were significantly increased by TENS over PC acupoints (P < 0.05) but not on NMCR. At the self-control side during the 2nd biocapture, cGMP concentrations at the acupoint are significantly lower compared to those of the NMCR (P < 0.05).
Fig. 5. Quantification of cGMP over the Pericardium Meridian following TENS.
Concentrations of cGMP on acupoints and NMCR were measured along the PC meridian following electrical stimulation of PC 8-PC 3 pair on one arm selected at random, while the other arm served as its control. cGMP concentrations measured in the biocapture solution (nM, top) and efflux rate calculated over the surface area in contact with the biocapture solution over a 20 min duration (pmol/cm2, bottom) were significantly increased over PC acupoints and NMCR by TENS in the stimulated side compared to the side without stimulation. Each bar represents the mean values and vertical bars represent S.E.M. *: p<0.05, compared with non-meridian control; #: p<0.05, compared to untreated side.
Discussion
The purpose of this study was to consecutively biocapture NO over the skin surface of acupoints, MWOP, and NMCR along the PC meridians using a biocapture method in humans. The influences of TENS on NO-cGMP releases were examined by chemical analysis of the samples biocaptured over PC acupoints compared to MWOP and NMCR following the treatments. The major new findings of these studies are that: 1) Baseline NO content was higher over PC acupoints than NMCR during the 1st biocapture, while there were no detectable differences among the 2nd, 3rd, and 4th consecutive biocaptures; 2) NO contents over PC acupoints, MWOP, and NMCR were markedly and consistently reduced during the subsequent consecutive biocaptures compared to the 1st biocapture; 3) TENS produced an increase in NOx-level over PC acupoints and NMCR; and 4) cGMP release was increased over PC acupoints but not on NMCR following TENS. This is the first evidence showing that TENS induces both NO and cGMP releases over PC acupoints. NO releases over the skin surface of the PC meridian region can be consecutively biocaptured and quantified on skin acupoints/meridians. NO contents biocaptured during the 1st interval over PC acupoints and NMCR are almost two fold higher compared to subsequent biocaptures. Baseline NO level is higher at acupoints compared to the NMCR. These findings suggest that both cumulative NO and newly generated NO exist on the skin surface with higher level of cumulative NO over PC acupoints at the physiological level. Newly generated NO-cGMP molecules over acupoints were elevated by TENS, which improve local circulation and/or produce somatosensory signal transduction processes, and contribute to its therapeutic effects.
Our previous studies have demonstrated that NO metabolites are successfully biocaptured over the skin surface in humans using a painless and non-invasive biocapture device, and that concentrations of total nitrite and nitrate (NOx-) are higher over acupoints compared to NMCR in humans [33,34]. The present study shows that the baseline NO level over PC acupoints is only higher than the NMCR during the 1st biocapture but not during the 2nd, 3rd, and 4th consecutive biocaptures. These results are consistent with our previous study reporting that both L-arginine-derived NO synthesis and non-enzymatic reduction of nitrate by bacteria are involved in NO generation on the skin [33]. The present results suggest that cumulative NO mainly contributes to higher levels of NO signal molecules over PC acupoints at the physiological level. This innovative biocapture method is applicable to systematic investigations of both cumulative NO and newly generated NO, and of differences in distribution of NO concentrations over various acupoints and meridian lines during physiological and pathological changes as well as following therapeutic treatments.
The sources and mechanisms of TENS-induced NO-cGMP molecules are not clear. Anatomical studies have identified that the number of nerve fibers/trunks, blood vessels, hair follicles, and sweat glands are enhanced over acupoints compared to their adjacent control areas [19-21]. Our previous studies have demonstrated that NOS protein levels and NO contents are consistently higher in skin regions containing acupoints/meridian lines compared to control tissues in rats [29,36]. EA stimulation induces a significant release of NO following dermal microdialysis in the acupoint but not in the NMCR in humans [35]. L-arginine-derived NO synthesis increases low resistance characteristics of acupoints [37], and norepinephrine turnover rate in acupoints/meridians is facilitated by presence of NO [38]. Consistently, cutaneous vasodilation induced by acupuncture stimulation in the forearms of humans is attenuated by application of NO synthesis inhibitor, which suggest that L-arginine-derived NO synthesis contributes to cutaneous vasodilation induced by acupuncture stimulation [39]. The present results support the possibility that TENS-induced NO generation/release is through L-arginine-derived NO synthesis, since the cumulative NO that mainly comes from non-enzymatic NO generation was already removed by the 1st biocapture. TENS-induced NO-cGMP generation/release predominantly over acupoints agree with previous studies reporting that tissue NO level is high in acupoints, and further suggests that TENS-induced NO-cGMP are mainly from newly-generated molecules. Elevation of NO-cGMP in the acupoint could be achieved through the activation of endothelial and/or neuronal NO synthesis/release system.
TENS stimulation of acupoints and/or painful areas provide a safe, standardized technique and is widely accepted as an alternative therapy for benefiting pain and local circulation in the community [2-4]. Several reports have demonstrated that electrical stimulation causes multiple biological responses in both animals and humans [2,6-8]. These responses can occur locally at or close to the site of the stimulation, or at a distance that is mediated mainly by the neuroendocrine system, and can affect various physiological functions [3-6]. Acupuncture essentially improves local circulation and allows for a flush of analgesic or sensitizing substances, leading to pain relief [40-43]. Previous studies showed that TENS, acupuncture, and acupuncture-like stimulation modifies regional blood flow in skin and changes arterial blood pressure and blood NO level [9-11]. Our results from TENS studies consistently suggest that newly generated NO-cGMP at acupoints is induced by electrical stimulation. Enhanced NO stimulates guanylyl cyclase to generate cGMP, and cGMP induces vasodilatation, which improves local microcirculation and removes pathological sensitizing substances. NO-cGMP may also produce analgesic action, and inhibits releases of inflammatory agents. All of these contribute to biochemical/physiological improvement and beneficial effects of the therapies.
Conclusion
In summary, the results show that baseline NO levels biocaptured during the 1st interval over all PC regions are almost two fold higher compared to the subsequent biocaptures. PC acupoints exude a higher cumulative NO level compared to the NMCR during the 1st biocapture. Newly generated NO release during the 2nd biocapture over PC acupoints and NMCR is induced by TENS. Following TENS, cGMP is predominantly released/generated from the skin surface of PC acupoints. The results suggest that NO-cGMP signal molecules are elevated by TENS with a higher level specifically at acupoints. Improvement of local circulation by enhanced NO-cGMP releases contribute to the beneficial effects of the therapies, and this study demonstrates an innovative biocapture method applicable for monitoring therapeutic treatments.
Acknowledgments
This project was made possible by Gail and Gerald Oppenheimer Family Foundation Grant and NIH Grant (AT002478) from the National Center for Complementary and Alternative Medicine (to SXM). These studies were conducted at the biomedical research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. We are grateful to NIH-supported UCLA Clinical Translational Science Institute (CTSI) at Los Angeles Biomedical Research Institute, and Dr. Peter Christenson, a Biostatistician from CTSI for statistical advice.
Contributor Information
Sheng-Xing Ma, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and Department of Obstetrics and Gynecology, David Geffen School of Medicine at University of California at Los Angeles and Harbor-UCLA Medical Center, Torrance, CA 90502.
Emeran Mayer, Division of Digestive Diseases and Gail and Gerald Oppenheimer Family Center for Neurobiology of Stress, David Geffen School of Medicine at University of California at Los Angeles, Torrance, CA 90502.
Paul Lee, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and Department of Obstetrics and Gynecology, Harbor-UCLA Medical Center, Torrance, CA 90502.
Xi-yan Li, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and Department of Obstetrics and Gynecology, Harbor-UCLA Medical Center, Torrance, CA 90502.
Ellen Z. Gao, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center and Department of Obstetrics and Gynecology, Harbor-UCLA Medical Center, Torrance, CA 90502.
References
- 1.Novey DW. Clinician's Complete reference to Complementary & Alternative Medicine. St. Louis, MO: 2000. [Google Scholar]
- 2.Blum K, Ho CK, Chen AL, Fulton M, Fulton B, Westcott WL, Reinl G, Braverman ER, Dinubile N, Chen TJ. The H-Wave((R)) Device Induces NO Dependent Augmented Microcirculation and Angiogenesis, Providing Both Analgesia and Tissue Healing in Sports Injuries. Physician and Sportsmedicine. 2008;36:103–114. doi: 10.3810/psm.2008.12.18. [DOI] [PubMed] [Google Scholar]
- 3.Yuan CS, Attele AS, Dey L, Lynch JP, Guan X. Transcutaneous electrical acupoint stimulation potentiates analgesic effect of morphine. The Journal of Clinical Pharmacology. 2002;42:899–903. doi: 10.1177/009127002401102812. [DOI] [PubMed] [Google Scholar]
- 4.Liu SS, Gerancher JC, Bainton BG, Kopacz DJ, Carpenter RL. The effects of electrical stimulation at different frequencies on perception and pain in human volunteers: epidural versus intravenous administration of fentanyl. Anesthesia & Analgesia. 1996;82:98–102. doi: 10.1097/00000539-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Resende MA, Sabino GG, Cândido CR, Pereira LS, Francischi JN. Local transcutaneous electrical stimulation (TENS) effects in experimental inflammatory edema and pain. European Journal of Pharmacology. 2004;504:217–222. doi: 10.1016/j.ejphar.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 6.Taşkan I, Ozyazgan I, Tercan M, Kardaş HY, Balkanli S, Saraymen R, Zorlu U, Ozügül Y. A comparative study of the effect of ultrasound and electrostimulation on wound healing in rats. Plastic and Reconstructive Surgery. 1997;100:966–972. doi: 10.1097/00006534-199709001-00020. [DOI] [PubMed] [Google Scholar]
- 7.Clover AJ, McCarthy MJ, Hodgkinson K, Bell PR, Brindle NP. Noninvasive augmentation of microvessel number in patients with peripheral vascular disease. Journal of Vascular Surgery. 2003;38:1309–1312. doi: 10.1016/s0741-5214(03)00895-4. [DOI] [PubMed] [Google Scholar]
- 8.Morris KA, McGee MF, Jasper JJ, Bogie KM. Evaluation of electrical stimulation for ischemic wound therapy: a feasibility study using the lapine wound model. Archives of Dermatological Research. 2009;301:323–327. doi: 10.1007/s00403-008-0918-2. [DOI] [PubMed] [Google Scholar]
- 9.Cramp AF, Noble JG, Lowe AS, Walsh DM. Transcutaneous electrical nerve stimulation (TENS): the effect of electrode placement upon cutaneous blood flow and skin temperature. Acupuncture & Electro-Therapeutics Research, The International Journal. 2001;1-2:25–37. doi: 10.3727/036012901816356036. [DOI] [PubMed] [Google Scholar]
- 10.Francis RP, Johnson MI. The characteristics of acupuncture-like transcutaneous electrical nerve stimulation (acupuncture-like TENS): a literature review. Acupuncture & Electro-Therapeutics Research, The International Journal. 2011;3-4:231–258. doi: 10.3727/036012911803634139. [DOI] [PubMed] [Google Scholar]
- 11.Severcan C, Cevik C, Acar HV, Sivri AB, Mit SS, Geçioğlu E, Paşaoğlu OT, Gündüztepe Y. The effects of acupuncture on the levels of blood pressure and nitric oxide in hypertensive patients. Acupuncture & Electro-Therapeutics Research, The International Journal. 2012;4:263–275. doi: 10.3727/036012912x13831831256320. [DOI] [PubMed] [Google Scholar]
- 12.Hudlicka O, Brown MD. Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. Journal of Vascular Research. 2009;46:504–512. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- 13.Beijing College of Traditional Chinese Medicine, Shanghai College of Traditional Chinese Medicine, Nanjing College of Traditional Chinese Medicine, The Acupuncture Institute of the Academy of Traditional Chinese Medicine. Essentials of Chinese Acupuncture. Beijing, China: 1980. pp. 301–310. [Google Scholar]
- 14.Zhu ZX. The advances and prospect in physiological and biophysical approaches of acupuncture meridian system. Acupuncture Research. 1988;13:81–89. [PubMed] [Google Scholar]
- 15.Zhu ZX, Hao JK. Acupuncture Meridian Biophysics-Scientific Verification of the First Great Invention of China. Beijing, China: 1989. pp. 150–230. [Google Scholar]
- 16.Voll R. The phenomenon of medical testing in electroacupuncture according to Voll. American Journal of Acupuncture. 1980;8:92–104. [Google Scholar]
- 17.Fraden J. Active acupuncture point impedance and potential measurements. American Journal of Acupuncture. 1979;7:137–144. [Google Scholar]
- 18.Sullivan SG, Eggleston DW, Martinoff JT, Kroening RJ. Evoked electrical conductivity on the lung acupuncture points in healthy individuals and confirmed lung cancer patients. American Journal of Acupuncture. 1985;13:261–266. [Google Scholar]
- 19.Luciani RJ. Direct observation and photography of electroconductive points on human skin. American Journal of Acupuncture. 1978;6:311–317. [Google Scholar]
- 20.Wang ZT, Wu SL, Cao YC, Zhu ZX, Xu RM. Morphological study on the low impedance line along channels. Acupuncture Research. 1987;12:82–85. [PubMed] [Google Scholar]
- 21.Chan SH. What is being stimulated in acupuncture: evaluation of the existence of a specific substrate. Neuroscience & Biobehavioral Reviews. 1984;8:25–33. doi: 10.1016/0149-7634(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. European Journal of Clinical Investigation. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 23.Salter M, Knowles RG, Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. Federation of European Biochemical Societies Letters. 1991;291:145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- 24.Dippel E, Mayer B, Schönfelder G, Czarnetzki BM, Paus R. Distribution of constitutive nitric oxide synthase immunoreactivity and NADPH-diaphorase activity in murine telogen and anagen skin. Journal of Investigative Dermatology. 1994;103:112–115. doi: 10.1111/1523-1747.ep12391865. [DOI] [PubMed] [Google Scholar]
- 25.Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochimica et Biophysica Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 26.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends in Biochemical Sciences. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 27.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annual Review of Pharmacology and Toxicology. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 29.Ma SX. Enhanced nitric oxide concentrations and expression of nitric oxide synthase in acupuncture points/meridians. The Journal of Alternative and Complementary Medicine. 2003;9:207–215. doi: 10.1089/10755530360623329. [DOI] [PubMed] [Google Scholar]
- 30.Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida M, Akaike T, Wada Y, Sato K, Ikeda K, Ueda S, Maeda H. Therapeutic effects of imidazolineoxyl N-oxide against endotoxin shock through its direct nitric oxide-scavenging activity. Biochemical and Biophysical Research Communications. 1994;202:923–930. doi: 10.1006/bbrc.1994.2018. [DOI] [PubMed] [Google Scholar]
- 32.Omura Y. Connections found between each meridian (heart, stomach, triple burner, etc.) & organ representation area of corresponding internal organs in each side of the cerebral cortex; release of common neurotransmitters and hormones unique to each meridian and corresponding acupuncture point & internal organ after acupuncture, electrical stimulation, mechanical stimulation (including shiatsu), soft laser stimulation or Qi Gong. Acupuncture & Electro-Therapeutics Research, The International Journal. 1989;14:155–186. doi: 10.3727/036012989816358470. [DOI] [PubMed] [Google Scholar]
- 33.Ma SX, Li XY, Sakurai T, Pandjaitan M. Evidence of enhanced non-enzymatic generation of nitric oxide on the skin surface of acupuncture points: An innovative approach in humans. Nitric Oxide. 2007;17:60–68. doi: 10.1016/j.niox.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Ma SX, Li XY, Smith BT, Jou NT. Changes in nitric oxide, cGMP, and nitrotyrosine concentrations over skin along the meridians in obese subjects. Obesity. 2011;19:1560–1567. doi: 10.1038/oby.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jou NT, Ma SX. Responses of nitric oxide-cGMP release in acupuncture point to electroacupuncture in human skin in vivo using dermal microdialysis. Microcirculation. 2009;16:434–443. doi: 10.1080/10739680902915012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham TS, Chen ML, Ma SX. TRPV1 expression in acupuncture points: response to electroacupuncture stimulation. Journal of Chemical Neuroanatomy. 2011;41:129–136. doi: 10.1016/j.jchemneu.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JX, Ma SX. Effects of nitric oxide and noradrenergic function on skin electric resistance of acupoints and meridians. The Journal of Alternative and Complementary Medicine. 2005;11:423–431. doi: 10.1089/acm.2005.11.423. [DOI] [PubMed] [Google Scholar]
- 38.Chen JX, Ibe BO, Ma SX. Nitric oxide modulation of norepinephrine production in acupuncture points. Life Science. 2006;79:2157–2164. doi: 10.1016/j.lfs.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Kimura K, Takeuchi H, Yuri K, Wakayama I. Effects of nitric oxide synthase inhibition on cutaneous vasodilation in response to acupuncture stimulation in humans. Acupuncture in Medicine. 2013;31:74–80. doi: 10.1136/acupmed-2012-010177. [DOI] [PubMed] [Google Scholar]
- 40.Sandberg M, Lundeberg T, Lindberg LG, Gerdle B. Effects of acupuncture on skin and muscle blood flow in healthy subjects. European Journal of Applied Physiology. 2003;90:114–119. doi: 10.1007/s00421-003-0825-3. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Kim HK, Park YI, Sohn IC, Choi DO, Kim MS, Park BR. Moxibustion at ST36 alleviates pain in complete Freund's adjuvant-induced arthritic rats. The American Journal of Chinese Medicine. 2006;34:57–67. doi: 10.1142/S0192415X06003631. [DOI] [PubMed] [Google Scholar]
- 42.Sandberg M, Lindberg LG, Gerdle B. Peripheral effects of needle stimulation (acupuncture) on skin and muscle blood flow in fibromyalgia. European Journal of Pain. 2004;8:163–171. doi: 10.1016/S1090-3801(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 43.Sandberg M, Larsson B, Lindberg LG, Gerdle B. Different patterns of blood flow response in the trapezius muscle following needle stimulation (acupuncture) between healthy subjects and patients with fibromyalgia and work-related trapezius myalgia. European Journal of Pain. 2005;9:497–510. doi: 10.1016/j.ejpain.2004.11.002. [DOI] [PubMed] [Google Scholar]