Abstract
Background
Chorioamnionitis has recently been reported as a risk factor for various neonatal diseases, including cerebral palsy, bronchopulmonary dysplasia, and necrotizing enterocolitis, but its effect on patent ductus arteriosus (PDA) is unclear. We performed a systematic review and meta-analysis to evaluate the effect of chorioamnionitis on PDA.
Methods
We searched PubMed, EMBASE, Cochrane Library, and KoreaMed databases using the terms: “intrauterine infection” or “maternal infection” or “antenatal infection” or “chorioamnionitis” or “placenta inflammation” or “placenta pathology” or “neonatal outcome” or “neonatal morbidity” or “PDA or patent ductus arteriosus” or “ductus arteriosus,” and “prematurity” or “very low birth weight infant.” Studies were included if they were randomized controlled trials, case–control studies, or cohort studies that included information relating to chorioamnionitis and PDA.
Results
Among 1,571 studies, a total of 23 studies (17,708 cases) were included in the meta-analysis to analyze the relationship between chorioamnionitis and PDA, except one study that only included PDA requiring surgical ligation. The association between chorioamnionitis and PDA was statistically significant (odds ratio [OR] 1.43; 95% confidence interval [CI] 1.19, 1.72; P < 0.0001). In subgroup analysis, clinical chorioamnionitis was not associated with PDA (OR 1.28; 95% CI 1.00, 1.64, 1.790; P = 0.05), whereas histologic chorioamnionitis (OR 1.54; 95% CI 1.10, 2.15; P = 0.01) and chorioamnionitis diagnosed from both clinical and histologic findings (OR 1.75; 95% CI 1.07, 2.86; P = 0.03) showed significant associations with PDA. Chorioamnionitis did not increase the risk of PDA requiring surgical ligation (OR 1.23; 95% CI 0.69, 2.17; P = 0.48), and antenatal steroid use reduced the risk of PDA (OR 0.62; 95% CI 0.42, 0.90; P = 0.01) after chorioamnionitis.
Conclusions
The results from this meta-analysis support an association between maternal chorioamnionitis and PDA in offspring.
Introduction
Chorioamnionitis is a risk factor for preterm birth, but the relationship between chorioamnionitis and neonatal morbidity or mortality remains controversial. However, several meta-analyses recently reported chorioamnionitis as a risk factor for various neonatal diseases, such as cerebral palsy [1], retinopathy of prematurity [2], bronchopulmonary dysplasia [3], and necrotizing enterocolitis [4]. Although the routine treatment of patent ductus arteriosus (PDA) is not recommended, persistent shunting through symptomatic PDA could increase the risk of neonatal mortality and morbidity, including chronic lung disease, intraventricular hemorrhage, and necrotizing enterocolitis [5, 6]. In the case of PDA, it has been reported that chorioamnionitis was a risk factor for unresponsiveness to medical therapy with cyclooxygenase inhibitors [7, 8]. The effect of antenatal steroids after chorioamnionitis has been reported [9], but the effect of chorioamnionitis itself on PDA occurrence is unclear. We performed a systematic review and meta-analysis to evaluate the effect of maternal chorioamnionitis on PDA in offspring.
Materials and Methods
Search Strategy and Study Selection
We performed this meta-analysis in accordance with the PRISMA guidelines developed for systematic reviews and meta-analyses (S1 PRISMA Checklist). We searched PubMed, EMBASE, Cochrane Library, and KoreaMed databases using the terms: “intrauterine infection” or “maternal infection” or “antenatal infection” or “chorioamnionitis” or “placenta inflammation” or “placenta pathology” or “neonatal outcome” or “neonatal morbidity” or “PDA” or “patent ductus arteriosus” or “ductus arteriosus,” and “prematurity” or “very low birth weight infant.” Manual searches were also performed on the reference lists of included studies and other electronic databases. No restrictions were applied on language. The last search was performed on September 19, 2014. The titles and abstracts of the articles were initially screened, and the full-text articles were independently reviewed by two reviewers (HW Park and YS Choi) using the selection criteria to determine inclusion in the meta-analysis.
Studies were included for meta-analysis if they met the following criteria: 1) study design: randomized controlled trial, case-control study, or prospectively or retrospectively matched cohort study; 2) patients: preterm infants who were born to a mother for whom information about chorioamnionitis was available; 3) intervention: clinical or histologic chorioamnionitis; and 4) outcomes: PDA (medically treated, surgically treated, or clinically diagnosed). Studies were excluded from the meta-analysis if they were case reports, case series, or single-arm cohort studies.
Data Extraction and Study Quality Assessment
Two authors (HW Park and YS Choi) independently reviewed the full text of all included studies and extracted data with a data extraction form. We extracted the following data: first author, publication year, study design, study location, study period, study population, definition of chorioamnionitis, definition of PDA, and incidence of PDA. We also extracted effect estimates, odds ratios for the case–control studies, or relative risk for the cohort studies, along with the corresponding confidence interval, from each of the studies.
Disagreements were resolved through interpretation by discussion with a third reviewer (KS Kim). Methodological quality was independently assessed by two authors (HW Park and YS Choi) using the Newcastle–Ottawa Scale for all included studies. This scale assesses study quality by evaluating three domains: selection (four items), comparability (one item), and outcome/exposure (three items) for cohort studies and case-control studies, respectively. A study that meets the criteria can be awarded a star, except for the comparability domain (which has a maximum of two stars). The overall score is the total number of stars given, with a maximum of 9. Overall scores of 0–4, 5–7, and 8–9 stars correspond to studies of low, moderate, and high quality. Any disagreements between authors were resolved by discussion.
Data Synthesis and Statistical Analysis
We conducted a meta-analysis to generate pooled estimates for the association between maternal chorioamnionitis and PDA in offspring using the RevMan 5.3.5 software (Cochrane Library; http://tech.cochrane.org/revman/download) and Comprehensive Meta-Analysis Version 3 (Biostat, Englewood, NJ, USA).
Statistical heterogeneity was assessed using the I2 statistic (percentage of total variation across studies), and I2 >50% was used to detect significant heterogeneity. If there was no heterogeneity, the fixed effects model was used for the meta-analysis. A random effects model was used for analysis if there was substantial statistical heterogeneity. A sensitivity analysis was conducted to evaluate the robustness of the conclusion by removing each study sequentially and to examine the effect of the elimination of each study on the pooled odds ratio (OR) results. A cumulative analysis was performed to detect temporal trends by adding one study at a time according to the year of publication.
Publication bias was assessed by the Begg and Mazumdar rank correlation test, the Egger’s regression test, and inspection of the funnel plot. A funnel plot and the Egger’s regression test were used to detect asymmetry based on the distribution of effect sizes against the standard errors.
Results
Characteristics of the Included Studies
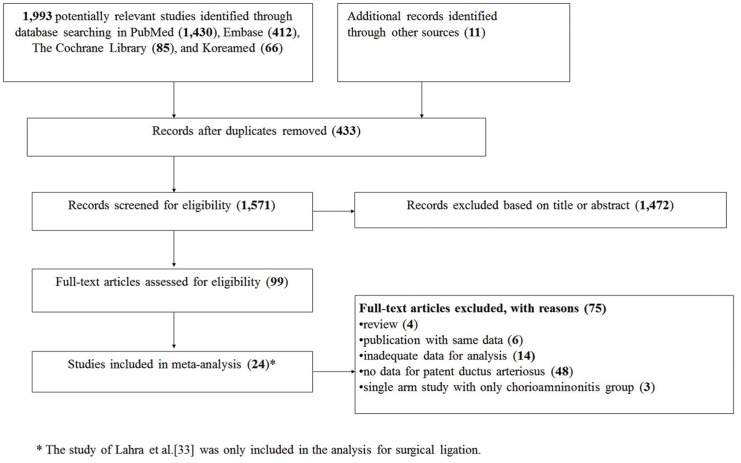
A total of 1,993 potentially relevant studies were identified from database searching, and 1,970 of them were excluded for the reasons listed in Fig 1. After a complete survey of the literature, we included 23 studies (21 cohort and 2 case-control studies) in the systematic review. The 23 eligible studies included in the meta-analysis [10–32] examined a total of 17,708 mothers, of whom 4,681 had chorioamnionitis and 13,027 did not.
Fig 1. Flow diagram of literature selection.
* The study by Lahra et al.[5] was only included in the analysis for surgical ligation.
The quality assessment of the included studies was performed using the Newcastle-Ottawa Scale, and the results are presented in Table 1.
Table 1. Characteristics of studies included in this meta-analysis.
| Study | Location | Study design | Population | Diagnosis, definition of PDA | Quality score* |
|---|---|---|---|---|---|
| Histologic and clinical CA | |||||
| Arayici 2014 | Turkey | Retrospective cohort | GA ≤32 weeks and/or birth weight ≤ 1,500 g | Echocardiography, size >1.5 mm, LA/aorta >1.5 and/or left-to-right shunting, end diastolic reversal of blood flow in aorta | 8 |
| Been 2009 | The Netherlands | Prospective cohort | GA ≤ 32 weeks | Echocardiography | 7 |
| Erdemir 2013 | Turkey | Prospective cohort | GA < 35 weeks | NA | 6 |
| Mu 2008 | Taiwan | Prospective cohort | birth weight ≤ 1,500 g | Echocardiography, and clinical signs of PDA | 7 |
| Ogunyemi 2003 | USA | Retrospective cohort | GA, 24–32 weeks | NA | 7 |
| Seliga-Siwecka 2013 | Poland | Prospective cohort | GA < 32 weeks | Echocardiography | 6 |
| Clinical CA | |||||
| Barrera-Reyes 2011 | Mexico | Prospective cohort | GA < 34 weeks and birth weight < 1,500 g | NA | 8 |
| Botet 2010 | Spain | Case–control | birth weight < 1,500 g | Presence of PDA (including surgical ligation) | 8 |
| Garcia-Munoz 2014 | Spain | Prospective cohort | birth weight < 1,500 g | Echocardiography, requiring treatment (medical or surgical) | 7 |
| Soraisham 2009 | Canada | Prospective cohort | GA < 33 weeks | Clinical signs and requiring treatment (medical or surgical) | 7 |
| Histologic CA | |||||
| Ahn 2012 | Korea | Prospective cohort | GA < 34 weeks | Echocardiography, requiring treatment (medical or surgical) | 8 |
| De Felice 2005 | Italy | Prospective cohort | Birth weight ≤ 1,500 g | NA | 6 |
| Dessardo 2012 | Croatia | Prospective cohort | GA < 32 weeks | Echocardiography, and clinical signs of PDA | 7 |
| Ecevit 2014 | Turkey | Retrospective cohort | GA < 37 weeks | NA | 6 |
| Elimian 2000 | USA | Retrospective cohort | Birth weight, 500–1,750 g | PDA requiring treatment (medical or surgical) | 7 |
| Hendson 2011 | Canada | Prospective cohort | GA ≤ 32 weeks and birth weight ≤ 1,250 g | Clinical diagnosis or radiologic diagnosis | 6 |
| Liu 2014 | China | Prospective cohort | GA ≤ 34 weeks | Echocardiography, and clinical symptoms of PDA | 7 |
| Perrone 2012 | Italy | Prospective cohort | GA, 23–31 weeks | Echocardiography, size >1.4 mm/kg body weight, LA/aorta >1.4, LA enlargement, shunting, end diastolic reversal of blood flow in descending aorta | 6 |
| Prendergast 2011 | UK | Prospective cohort | GA ≤ 32 weeks | Requiring treatment (medical or surgical) | 5 |
| Rocha 2006 | Portugal | Retrospective cohort | GA ≤ 34 weeks | Echocardiography | 8 |
| Sato 2011 | Japan | Retrospective cohort | GA < 30 weeks | Hemodynamically significant PDA and requiring medical treatment | 7 |
| Schlapbach 2010 | Switzerland | Case–control | GA, 25–32 weeks | NA | 8 |
| Tsiartas 2013 | Czech Republic | Prospective cohort | GA: 24–36 weeks | NA | 7 |
| Lahra 2009 † | Australia | Retrospective cohort | GA < 30 weeks | Echocardiography, requiring surgical treatment | 8 |
*The quality assessment of studies was performed using the Newcastle-Ottawa Scale.
† This study was only included in meta-analysis for the relationship between chorioamnionitis and surgical ligation.
Abbreviation; GA: gestational age at birth, LA: left atrium, PDA: patent ductus arteriosus, CA: chorioamnionitis
Six studies defined chorioamnionitis based on both clinical and histologic findings [15, 18, 19, 27, 28, 30]. In four studies[10–13], chorioamnionitis was diagnosed based on clinical findings, and the remaining 14 studies diagnosed it only on the basis of histologic findings of the placenta (one of these was the study that only included surgical ligation [33]) [14, 16, 17, 20–26, 29, 31–33]. In cases of clinical chorioamnionitis, criteria that were used in most studies were similar: the Gibbs criteria for chorioamnionitis [34], on the basis of maternal fever and two or more of the following additional criteria: maternal tachycardia, fetal tachycardia, uterine tenderness, foul odor of the amniotic fluid, and maternal leukocytosis. There were small variations in diagnostic criteria, such as fever (≥ 38 or 38.3°C), maternal leukocytosis (> 12,000/mm3 or 15,000/mm3 or 18,000/mm3), and fetal tachycardia (heart rate > 160/min or 180/min). In three studies, the elevation of C-reactive protein was included in the diagnostic criteria [11, 19, 20]. Histologic chorioamnionitis was commonly diagnosed based on the infiltration of polymorphonuclear leukocytes in the placental membranes, including the Salfia criteria [35], Blanc criteria [36], and criteria of the Amniotic Fluid Infection Nosology Committee [37].
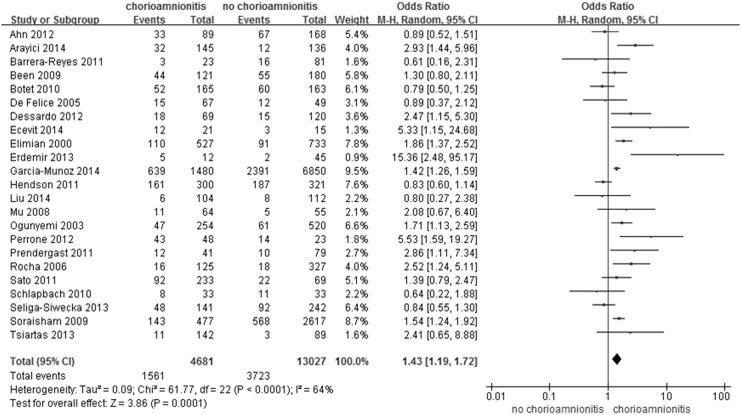
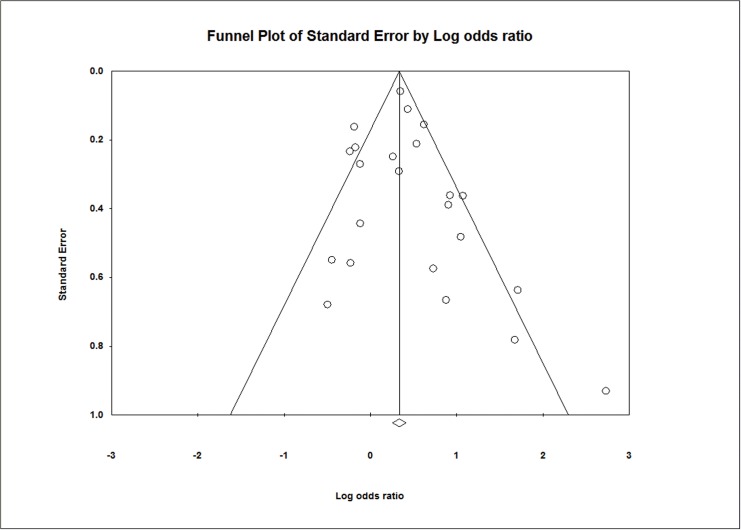
As a primary outcome, PDA was diagnosed in 5,284 of a total of 17,708 (30%) infants, including 1,561 of 4,681 (33.3%) infants of a mother with chorioamnionitis and 3,723 of 13,027 (28.6%) infants of a mother without chorioamnionitis. Overall chorioamnionitis, including that diagnosed based on clinical or histologic findings, was significantly associated with PDA (OR 1.43; 95% CI 1.19, 1.72; P <0.0001; Fig 2). The results of the heterogeneity assessment of overall studies (P < 0.0001; I2 = 64%) indicate the presence of heterogeneity across the studies. Thus, we used a random effects model for meta-analysis. We found no statistical evidence of publication bias in the studies from the results of the Begg-Mazumdar rank correlation test (P = 0.13) and Egger’s regression test (P = 0.48), as well as inspection of the funnel plot (Fig 3). A sensitivity analysis revealed no significant change in the pooled results caused by the sequential exclusion of individual studies (S1 Fig), and the results of a cumulative analysis also provided similar results over time (S2 Fig).
Fig 2. Meta-analysis for the relationship between maternal chorioamnionitis and neonatal patent ductus arteriosus.
Fig 3. Funnel plot of the publications and meta-analysis evaluating the effect of chorioamnionitis on patent ductus arteriosus.
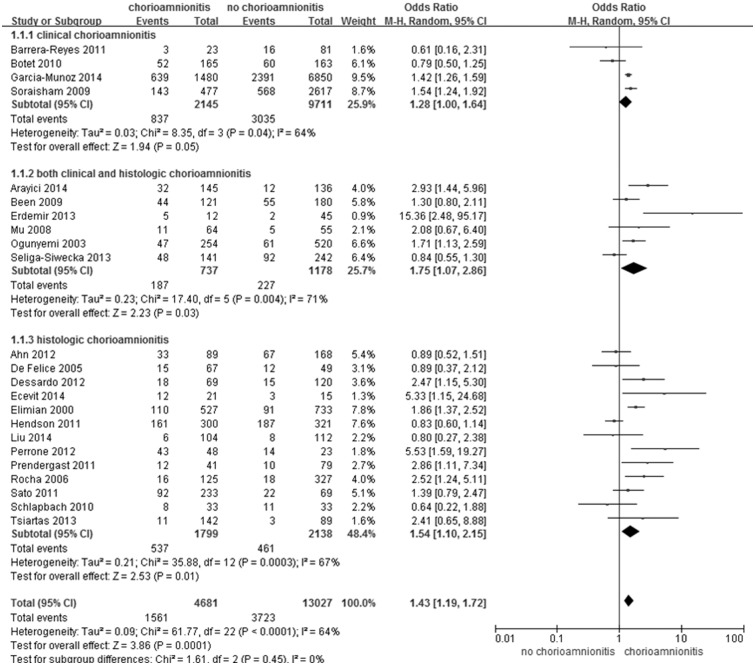
In the subgroup analysis according to type of chorioamnionitis (clinical or histologic or both), the incidences of PDA were 39.0% vs 31.3% in patients with or without clinical chorioamnionitis, 29.8% vs 21.6% in patients with or without histologic chorioamnionitis, and 25.4% vs 19.3% in patients with or without both types of chorioamnionitis. In subgroup analysis according to types of chorioamnionitis, clinical chorioamnionitis was not associated with PDA (OR 1.28; 95% CI 1.00, 1.64, 1.790; P = 0.05; Fig 4). However, both groups (clinical and histologic chorioamnionitis) (OR 1.75; 95% CI 1.07, 2.86; P = 0.03) and histologic chorioamnionitis only (OR 1.54; 95% CI 1.10, 2.15; P = 0.01), including histologic examination in the diagnosis of chorioamnionitis, showed significant associations with PDA (Fig 4).
Fig 4. Subgroup-analysis according to diagnosis of both types of chorioamnionitis for neonatal patent ductus arteriosus.
In the subgroup analysis according to study design (prospective and retrospective, including case–control studies), chorioamnionitis was associated with PDA in both groups (OR 1.346; 95% CI 1.1061, 1.708; P = 0.014 in the prospective studies, and OR 1.440; 95% CI 1.192, 1.739; P < 0.0001 in the retrospective studies; S3 Fig).
The presence of a fetal inflammatory response was not significantly associated with PDA (OR 0.57; 95% CI 0.13, 2.5; P = 0.45).
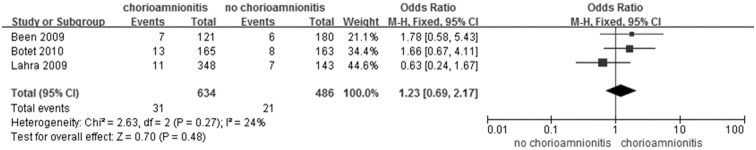
The incidence of PDA requiring surgical ligation did not differ between the chorioamnionitis group and non-chorioamnionitis group (OR 1.23; 95% CI 0.69, 2.17; P = 0.48; Fig 5). We used a fixed effects model because the heterogeneity test showed no evidence of heterogeneity between the included studies (P = 0.27; I2 = 24%). There was no statistical evidence of publication bias; the Begg-Mazumdar rank correlation test (P = 1.0) and Egger’s regression test (P = 0.88) did not suggest the presence of publication bias in this analysis.
Fig 5. Meta-analysis for the relationship between maternal chorioamnionitis and neonatal patent ductus arteriosus requiring surgical ligation.
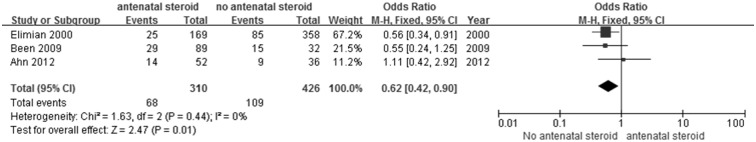
Among patients with chorioamnionitis, administration of antenatal steroid was associated with a decreased risk of PDA compared to the non-use of antenatal steroid (OR 0.62; 95% CI 0.42, 0.90; P = 0.01; Fig 6). Inspection of the funnel plot was not sufficient to rule out publication bias because only three studies were included in this analysis but the Begg–Mazumdar rank correlation test (P = 0.30) and Egger’s regression test (P = 0.50) did not suggest the presence of publication bias. Because there was no evidence of heterogeneity between the included studies (P = 0.44; I2 = 0.0001%), we used a fixed effects model for meta-analysis.
Fig 6. Meta-analysis for the relationship between antenatal steroid and neonatal patent ductus arteriosus after maternal chorioamnionitis.
Discussion
The incidence of PDA has been reported in 55% of extremely low birth weight infants (birth weight < 1000 g), and 60–70% of preterm infants who were born at 28 weeks of gestation or earlier compared to the incidence in term infants (57/100,000 live births) [6]. The patency of ductus arteriosus is regulated by the balance between vasodilating and vasoconstricting factors [38]. Increased sensitivity to vasodilatory material (NO or prostaglandins) and decreased sensitivity to oxygen to constrict the ductus could explain the higher incidence of PDA in preterm than term babies. In the lamb model, the sensitivity of the ductus to nitric oxide (NO; a vasodilator) depends on gestational age; the ductus showed a tendency toward more pronounced NO-mediated relaxation during early gestation (corresponding to approximately 29 weeks in humans) compared with late gestation (corresponding to more than 34 weeks in humans) [39, 40]. Preterm infants also have a persistent response to prostaglandins compared with full term babies because the decrease in the PGE2 receptor has not occurred in preterm infants [38].
In this meta-analysis, chorioamnionitis showed a statistically significant association with PDA (OR 1.43; 95% CI 1.19, 1.72; P <0.0001; Fig 2) when diagnosed by histological examination of the placenta (OR 1.75; 95% CI 1.07, 2.86; P = 0.03 in both groups and OR 1.54; 95% CI 1.10, 2.15; P = 0.01 in histologic chorioamnionitis; Fig 4). The role of infection was also considered in maintaining the patency of ductus arteriosus. In rats, relaxation of the ductus was observed after an injection of lipopolysaccharide via induction the production of inducible nitric oxide synthetase (iNOS) [41]. Infection could induce production of iNOS and cyclooxygenase (COX)-2, resulting in the increased production of vasodilatory prostaglandins as well as constituting NOS and COX-1, which are expressed under normal conditions [39]. Kim et al. [7] demonstrated increased COX-1 expression in the umbilical artery in infants with intrauterine infection compared to those without intrauterine infection. Thus, an infant born to a mother with chorioamnionitis could have a persistently opened ductus arteriosus due to increased NO and vasodilatory prostaglandins. In addition to prenatal infections such as chorioamnionitis, postnatal infections are also associated with PDA. Gonzalez et al. [42] reported increased risks due to failed PDA closure, PDA recurrence, and increased levels of a prostaglandin (6-ketoprostaglandin F1α [6-keto PGF1α]) and tumor necrosis factor alpha in infants with postnatal infections. Ductal vasodilator prostaglandin, 6-keto PGF1α, increased in postnatal infection [42] but not in intrauterine inflammation [7].
Although chorioamnionitis was associated with PDA in the total group with PDA (OR 1.43; 95% CI 1.19, 1.72; P <0.0001), clinical chorioamnionitis did not show an association with PDA (OR 1.28; 95% CI 1.00, 1.64, 1.790; P = 0.05) in subgroup analysis. Clinical chorioamnionitis is diagnosed using only clinical findings. These are often particularly vague, and any diagnosis is thus highly subjective [43]. Pappas et al. [44] reported that 2.7% of cases of clinical chorioamnionitis showed normal findings on histologic examination of the placenta. This group, which was diagnosed on the basis of both clinical and histologic findings (OR 1.75; 95% CI 1.07, 2.86; P = 0.03), showed a slightly higher OR than those with histologic chorioamnionitis only (OR 1.54; 95% CI 1.10, 2.15; P = 0.01).
The presence of chorioamnionitis was significantly associated with unresponsiveness following the use of COX inhibitors [7, 8]; surgical ligation is an indication that the PDA has been unresponsive to medical treatment. In our meta-analysis of populations with or without chorioamnionitis, PDA requiring surgical ligation was not associated with chorioamnionitis (OR 1.23; 95% CI 0.69–2.17; P = 0.48, Fig 5). Various postnatal factors, including infection and the need for fluid management, in addition to chorioamnionitis, may affect PDA outcomes [42, 45]. In addition, the number of studies included in the analysis was small.
Been et al. [9] previously reported the effects of antenatal steroids on PDA after chorioamnionitis [9]. Our study also showed a decreased risk of PDA with the use of an antenatal steroid in chorioamnionitis (OR 0.62; 95% CI 0.42, 0.90; P = 0.01, Fig 6), as did another study [24] in addition to the results of Been’s study. Although there has been concern about the use of steroids in chorioamnionitis, it could be safe and effective even in women with premature rupture of the membrane based on several studies [9, 46–48]. Intrinsic tone of the ductus is lower in preterm infants who were born earlier than 28 weeks of gestation with increased sensitivity to vasodilators leading to failure of ductus constriction [38, 49][2,3][2,3]. Administration of corticosteroids reduces the sensitivity of the ductus to prostaglandin E2 [38] and inhibits the induction of iNOS and COX-2 in the presence of inflammation [39]. Moreover, the extreme preterm infant commonly has relative adrenal insufficiency because the adrenal cortex cannot produce cortisol sufficiently until 23–30 weeks of gestation [50]. Thus, the use of an antenatal corticosteroid, especially in extreme preterm infants, could help to reduce the occurrence of PDA in the offspring of mothers with chorioamnionitis. The administration of a postnatal corticosteroid also indicated the effect on the decrease in the incidence of PDA [51, 52]. No statistical association was found between the presence of a fetal inflammatory response and PDA (OR 0.57; 95% CI 0.13, 2.5; P = 0.45); however, only three studies [19, 25, 32] were included, and there was substantial heterogeneity among studies (P = 0.02; I2 = 84%).
PDA is a multi-factorial disease that occurs during the neonatal period. We conducted a meta-regression analysis using a random effects model to assess the impact of gestational age on the occurrence of PDA as a covariate. A meta-regression using the length of gestation (both the maximal value and the mean value) as a covariate provides P-values as follows: P = 0.230 for the maximal gestational age (S4 Fig) and P = 0.944 for the mean gestational age (S5 Fig), thus indicating no association of the covariate with the log odds ratio (size of the choroamnionitis effect on PDA).
There are some limitations to be addressed regarding the present study. First, observational study designs are prone to both bias and confounding, which exclusively affect non-randomized studies [4]. However, in the subgroup analysis according to study design (prospective and retrospective, including case–control studies), there was no significant difference in the OR between the studies according to study design (OR 1.346; 95% CI 1.1061, 1.708; P = 0.014 in the prospective studies, and OR 1.440; 95% CI 1.192, 1.739; P < 0.0001 in the retrospective studies; S3 Fig). Moreover, the Newcastle–Ottawa Scale scores indicated moderate to high quality for the methodology used in the included studies. Second, PDA was diagnosed in 30% and 33.3% of infants by virtue of the presence of chorioamnionitis in this study. However, the actual incidences of PDA in the study populations are unclear; 10 studies [10, 12, 15, 16, 20–22, 27, 29, 31] did not state whether PDA was diagnosed echocardiographically.
This systematic review and meta-analysis demonstrated an association between maternal chorioamnionitis and PDA in offspring, especially in histologically diagnosed chorioamnionitis. When routine treatment is not recommended and medical or surgical treatment is associated with potential risks [5], the presence of chorioamnionitis may predict prolonged PDA and a need for early treatment. Chorioamnionitis did not exhibit any association with PDA that required surgical ligation, but the prescription of antenatal steroids reduced the risk of PDA development after chorioamnionitis. Well-designed randomized controlled trials are needed to adjust for other postnatal factors affecting PDA outcomes. It would then be possible to evaluate the effect of chorioamnionitis alone, and thus the need for PDA treatment.
Supporting Information
Abbreviations: CI, confidence interval; CA, chorioamnionitis.
(TIF)
Abbreviations: CI, confidence interval; CA, chorioamnionitis.
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Wu YW, Colford JM Jr. (2000) Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 284: 1417–1424. 10.1001/jama.284.11.1417 [DOI] [PubMed] [Google Scholar]
- 2. Mitra S, Aune D, Speer CP, Saugstad OD. (2014) Chorioamnionitis as a risk factor for retinopathy of prematurity: a systematic review and meta-analysis. Neonatology 105: 189–199. 10.1159/000357556 [DOI] [PubMed] [Google Scholar]
- 3. Hartling L, Liang Y, Lacaze-Masmonteil T. (2012) Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 97: F8–F17. 10.1136/adc.2010.210187 [DOI] [PubMed] [Google Scholar]
- 4. Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. (2013) Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr 162: 236–242 e232 10.1016/j.jpeds.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 5. Benitz WE. (2012) Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed 97: F80–82. 10.1136/archdischild-2011-300381 [DOI] [PubMed] [Google Scholar]
- 6. Hamrick SE, Hansmann G. (2010) Patent ductus arteriosus of the preterm infant. Pediatrics 125: 1020–1030. 10.1542/peds.2009-3506 [DOI] [PubMed] [Google Scholar]
- 7. Kim ES, Kim EK, Choi CW, Kim HS, Kim BI, Choi JH, et al. (2010) Intrauterine inflammation as a risk factor for persistent ductus arteriosus patency after cyclooxygenase inhibition in extremely low birth weight infants. J Pediatr 157: 745–750 e741 10.1016/j.jpeds.2010.05.020 [DOI] [PubMed] [Google Scholar]
- 8. Seon H-S, Lee J-B, Kim I-U, Kim S-H, Lee J-H, Kim D-H, et al. (2013) Association with ductus arteriosus closure by ibuprofen and intrauterine inflammation in very low birth weight infants. Korean J Perinatol 24: 158–167. 10.14734/kjp.2013.24.3.158 [DOI] [Google Scholar]
- 9. Been JV, Degraeuwe PL, Kramer BW, Zimmermann LJ. (2011) Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG 118: 113–122. 10.1111/j.1471-0528.2010.02751.x [DOI] [PubMed] [Google Scholar]
- 10. Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. (2009) A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol 200: 372 e371–376. 10.1016/j.ajog.2008.11.034 [DOI] [PubMed] [Google Scholar]
- 11. Botet F, Figueras J, Carbonell-Estrany X, Arca G, The Castrillo Study Group. (2010) Effect of maternal clinical chorioamnionitis on neonatal morbidity in very-low birthweight infants: a case-control study. J Perinat Med 38: 269–273. [DOI] [PubMed] [Google Scholar]
- 12. Barrera-Reyes RH, Ruiz-Macias H, Segura-Cervantes E. (2011) [Neurodevelopment at one year of age [corrected] in preterm newborns with history of maternal chorioamnionitis]. Gynecol Obstet Mex 79: 31–37. [PubMed] [Google Scholar]
- 13. Garcia-Munoz Rodrigo F, Galan Henriquez G, Figueras Aloy J, Garcia-Alix Perez A. (2014) Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology 106: 229–234. 10.1159/000363127 [DOI] [PubMed] [Google Scholar]
- 14. Elimian A, Verma U, Beneck D, Cipriano R, Visintainer P, Tejani N. (2000) Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol 96: 333–336. 10.1016/s0029-7844(00)00928-5 [DOI] [PubMed] [Google Scholar]
- 15. Ogunyemi D, Murillo M, Jackson U, Hunter N, Alperson B. (2003) The relationship between placental histopathology findings and perinatal outcome in preterm infants. J Matern Fetal Neonatal Med 13: 102–109. 10.1080/713605804 [DOI] [PubMed] [Google Scholar]
- 16. De Felice C, Toti P, Parrini S, Del Vecchio A, Bagnoli F, Latini G, et al. (2005) Histologic chorioamnionitis and severity of illness in very low birth weight newborns. Pediatr Crit Care Med 6: 298–302. [DOI] [PubMed] [Google Scholar]
- 17. Rocha G, Proenca E, Quintas C, Rodrigues T, Guimaraes H. (2006) [Chorioamnionitis and neonatal morbidity]. Acta Med Port 19: 207–212. [PubMed] [Google Scholar]
- 18. Mu SC, Lin CH, Chen YL, Ma HJ, Lee JS, Lin MI, et al. (2008) Impact on neonatal outcome and anthropometric growth in very low birth weight infants with histological chorioamnionitis. J Formos Med Assoc 107: 304–310. 10.1016/S0929-6646(08)60091-1 [DOI] [PubMed] [Google Scholar]
- 19. Been JV, Rours IG, Kornelisse RF, Lima Passos V, Kramer BW, Schneider TA, et al. (2009) Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol 201: 587 e581–588. 10.1016/j.ajog.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 20. Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. (2010) Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr 99: 1504–1509. 10.1111/j.1651-2227.2010.01861.x [DOI] [PubMed] [Google Scholar]
- 21. Hendson L, Russell L, Robertson CM, Liang Y, Chen Y, Abdalla A, et al. (2011) Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr 158: 397–402. 10.1016/j.jpeds.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 22. Prendergast M, May C, Broughton S, Pollina E, Milner AD, Rafferty GF, et al. (2011) Chorioamnionitis, lung function and bronchopulmonary dysplasia in prematurely born infants. Arch Dis Child Fetal Neonatal Ed 96: F270–274. 10.1136/adc.2010.189480 [DOI] [PubMed] [Google Scholar]
- 23. Sato M, Nishimaki S, Yokota S, Seki K, Horiguchi H, An H, et al. (2011) Severity of chorioamnionitis and neonatal outcome. J Obstet Gynaecol Res 37: 1313–1319. 10.1111/j.1447-0756.2010.01519.x [DOI] [PubMed] [Google Scholar]
- 24. Ahn HM, Park EA, Cho SJ, Kim YJ, Park HS. (2012) The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks' gestation. Neonatology 102: 259–264. 10.1159/000339577 [DOI] [PubMed] [Google Scholar]
- 25. Dessardo NS, Mustac E, Dessardo S, Banac S, Peter B, Finderle A, et al. (2012) Chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol 29: 133–140. 10.1055/s-0031-1295654 [DOI] [PubMed] [Google Scholar]
- 26. Perrone S, Toti P, Toti MS, Badii S, Becucci E, Gatti MG, et al. (2012) Perinatal outcome and placental histological characteristics: a single-center study. J Matern Fetal Neonatal Med 25 Suppl 1: 110–113. 10.3109/14767058.2012.664344 [DOI] [PubMed] [Google Scholar]
- 27. Erdemir G, Kultursay N, Calkavur S, Zekioglu O, Koroglu OA, Cakmak B, et al. (2013) Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol 54: 267–274. 10.1016/j.pedneo.2013.03.012 [DOI] [PubMed] [Google Scholar]
- 28. Seliga-Siwecka JP, Kornacka MK. (2013) Neonatal outcome of preterm infants born to mothers with abnormal genital tract colonisation and chorioamnionitis: a cohort study. Early Hum Dev 89: 271–275. 10.1016/j.earlhumdev.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 29. Tsiartas P, Kacerovsky M, Musilova I, Hornychova H, Cobo T, Sävman K, et al. (2013) The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 26: 1332–1336. 10.3109/14767058.2013.784741 [DOI] [PubMed] [Google Scholar]
- 30. Arayici S, Kadioglu Simsek G, Oncel MY, Eras Z, Canpolat FE, Oguz SS, et al. (2014) The effect of histological chorioamnionitis on the short-term outcome of preterm infants ≤32 weeks: a single-center study. J Matern Fetal Neonatal Med 27: 1129–1133. 10.3109/14767058.2013.850668 [DOI] [PubMed] [Google Scholar]
- 31. Ecevit A, Anuk-Ince D, Yapakci E, Kupana-Ayva S, Kurt A, Yanık FF, et al. (2014) Association of respiratory distress syndrome and perinatal hypoxia with histologic chorioamnionitis in preterm infants. Turk J Pediatr 56: 56–61. [PubMed] [Google Scholar]
- 32. Liu Z, Tang Z, Li J, Yang Y. (2014) Effects of placental inflammation on neonatal outcome in preterm infants. Pediatr Neonatol 55: 35–40. 10.1016/j.pedneo.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 33. Lahra MM, Beeby PJ, Jeffery HE. (2009) Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics 123: 1314–1319. 10.1542/peds.2008-0656 [DOI] [PubMed] [Google Scholar]
- 34. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. (1982) Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 145: 1–8. 10.1093/infdis/145.1.1 [DOI] [PubMed] [Google Scholar]
- 35. Salafia CM, Weigl C, Silberman L. (1989) The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol 73: 383–389. 10.1016/0020-7292(89)90252-x [DOI] [PubMed] [Google Scholar]
- 36. Blanc WA. (1981) Pathology of the placenta, membranes, and umbilical cord in bacterial, fungal, and viral infections in man. Monogr Pathol: 67–132. [PubMed] [Google Scholar]
- 37. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, et al. (2003) Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 6: 435–448. 10.1007/s10024-003-7070-y [DOI] [PubMed] [Google Scholar]
- 38. Clyman RI. (2006) Mechanisms regulating the ductus arteriosus. Neonatology 89: 330–335. 10.1159/000092870 [DOI] [PubMed] [Google Scholar]
- 39. Yeung MY. (2006) Hypotension, persistent ductus arteriosus and the underlying adrenal insufficiency in low gestation newborns. World J Pediatr 1: 8–13. [Google Scholar]
- 40. Clyman RI, Waleh N, Black SM, Riemer RK, Mauray F, Chen YQ. (1998) Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation, oxygen tension, and vasa vasorum. Pediatr Res 43: 633–644. 10.1203/00006450-199805000-00012 [DOI] [PubMed] [Google Scholar]
- 41. Bustamante SA, Pang Y, Romero S, Pierce MR, Voelker CA, Thompson JH, et al. (1996) Inducible nitric oxide synthase and the regulation of central vessel caliber in the fetal rat. Circulation 94: 1948–1953. 10.1161/01.cir.94.8.1948 [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E, et al. (1996) Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 128: 470–478. 10.1016/s0022-3476(96)70356-6 [DOI] [PubMed] [Google Scholar]
- 43. Tita AT, Andrews WW. (2010) Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 37: 339–354. 10.1016/j.clp.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, et al. (2014) Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr 168: 137–147. 10.1001/jamapediatrics.2013.4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, et al. (2008) Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 28: 123–128. 10.1038/sj.jp.7211895 [DOI] [PubMed] [Google Scholar]
- 46. Lievense S, Kramer BW, Nijhuis JG, Zimmermann LJ, Been JV. (2010) Antenatal Steroids in Chorioamnionitis: Friend or Foe? a Nationwide Survey Among Perinatologists. Pediatr Res 68: 615–615. [Google Scholar]
- 47. Roberts D, Dalziel S. (2006) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev: CD004454 [DOI] [PubMed] [Google Scholar]
- 48. Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. (2006) The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 195: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 49. Clyman RI. (2000) Ibuprofen and patent ductus arteriosus. N Engl J Med 343: 728–730. 10.1056/nejm200009073431009 [DOI] [PubMed] [Google Scholar]
- 50. Chung HR. (2014) Adrenal and thyroid function in the fetus and preterm infant Korean J Pediatr 57: 425–433. 10.3345/kjp.2014.57.10.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halliday HL, Ehrenkranz RA, Doyle LW. (2014) Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev: CD001146 10.1002/14651858.CD001146 [DOI] [PubMed] [Google Scholar]
- 52. Halliday HL, Ehrenkranz RA. (2001) Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev: CD001146 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: CI, confidence interval; CA, chorioamnionitis.
(TIF)
Abbreviations: CI, confidence interval; CA, chorioamnionitis.
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.