Abstract
Idiopathic pulmonary arterial hypertension (IPAH) is a rare and progressive disease of unknown pathogenesis. Vascular remodeling due to excessive proliferation of pulmonary arterial smooth muscle cells (PASMCs) is a critical pathogenic event that leads to early morbidity and mortality. The excessive cell proliferation is closely linked to the augmented Ca2+ signaling in PASMCs. More recently, we have shown by an siRNA knockdown method that the Ca2+-sensing receptor (CaSR) is upregulated in PASMCs from IPAH patients, involved in the enhanced Ca2+ response and subsequent excessive cell proliferation. In this study, we examined whether pharmacological blockade of CaSR attenuated the excessive proliferation of PASMCs from IPAH patients by MTT assay. The proliferation rate of PASMCs from IPAH patients was much higher (~1.5-fold) than that of PASMCs from normal subjects and patients with chronic thromboembolic pulmonary hypertension (CTEPH). Treatment with NPS2143, an antagonist of CaSR or calcilytic, clearly suppressed the cell proliferation in a concentration-dependent manner (IC50 = 2.64 μM) in IPAH-PASMCs, but not in normal and CTEPH PASMCs. Another calcilytic, Calhex 231, which is structurally unrelated to NPS2143, also concentration-dependently inhibited the excessive proliferation of IPAH-PASMCs (IC50 = 1.89 μM). In contrast, R568, an activator of CaSR or calcimimetic, significantly facilitated the proliferation of IPAH-PASMCs (EC50 = 0.33 μM). Similar results were obtained by BrdU incorporation assay. These results reveal that the excessive PASMC proliferation was modulated by pharmacological tools of CaSR, showing us that calcilytics are useful for a novel therapeutic approach for pulmonary arterial hypertension.
Introduction
Pulmonary arterial hypertension (PAH) is caused by functional and structural changes in the pulmonary vasculature. Pulmonary vascular remodeling is triggered by a progressive elevation of pulmonary vascular resistance and pulmonary arterial pressure in patients with PAH. The elevated pulmonary arterial pressure induces extensive changes in heart structure followed by right heart failure, and eventually death. PAH is clinically defined by chronic increases of pulmonary arterial pressure due to various causes and resting mean pulmonary arterial pressure of ≥25 mmHg [1, 2]. The five-year survival rate of PAH after diagnosis is ~57%. In the United States, the mean age of PAH patients was 36.4 years in the 1980s, but it was 53.0 years in 2007, due to improved diagnosis, treatment, and management for PAH [3, 4].
Pulmonary vascular remodeling occurs due to the excessive proliferation of pulmonary arterial smooth muscle cells (PASMCs) [5, 6]. Cell proliferation is closely linked to Ca2+ mobilization and signaling in PASMCs. A major trigger for the PASMC proliferation is elevated cytosolic Ca2+ concentration ([Ca2+]cyt). In PASMCs, [Ca2+]cyt is regulated by the balance of Ca2+ influx through Ca2+-permeable channels in the plasma membrane and Ca2+ release from the intracellular store sites. PASMCs express several Ca2+-permeable channels including voltage-dependent Ca2+ channels, receptor-operated Ca2+ channels, and store-operated Ca2+ channels [7–11]. It has been reported that receptor- and store-operated Ca2+ channels were upregulated in lung tissues and PASMCs from idiopathic pulmonary arterial hypertension (IPAH) patients, compared with PASMCs from normal subjects and normotensive patients, which resulted in enhanced Ca2+ signaling and excessive PASMC proliferation [12, 13]. These channels are also reported to be upregulated in PASMCs during hypoxia [14–18].
In addition to these Ca2+ influx pathways, more recently, we found that the extracellular Ca2+-sensing receptor (CaSR) was expressed at low levels in human PASMCs, and the expression level was upregulated in PASMCs from IPAH patients [19]. CaSR is classified as a member of the G-protein-coupled receptor subfamily C (also known as GPRC2A) [20, 21]. CaSR, which was originally identified from the parathyroid glands, senses the Ca2+ concentration in serum and regulates parathyroid hormone secretion to control serum Ca2+ concentration [22]. It has been reported that CaSR is also expressed in various mammalian tissues including kidney, bone, gastrointestinal tract, skin, brain, and the cardiovascular system [21, 23]. In addition, we previously demonstrated that the upregulation of CaSR enhanced the extracellular Ca2+-induced [Ca2+]cyt increase in IPAH-PASMCs, contributing to enhanced Ca2+ signaling and excessive cell proliferation in IPAH-PASMCs [19].
Our previous report revealed that excessive cell proliferation due to enhanced CaSR function in IPAH-PASMCs was attenuated by the knockdown of CaSR with siRNA [19]. In this study, we examined whether pharmacological tools for CaSR modulated excessive cell proliferation in IPAH-PASMCs by MTT and BrdU incorporation assays. As pharmacological modulators for CaSR, a synthetic activator of CaSR (calcimimetic), R568, and antagonists of CaSR (calcilytics), NPS2143 and Calhex 231, were used in this investigation. Here, we found that the blockade of CaSR by calcilytics attenuated excessive cell proliferation in IPAH-PASMCs, but did not affect it in PASMCs from normal subjects and patients with chronic thromboembolic pulmonary hypertension (CTEPH).
Materials and Methods
Cell culture
Cell lines of PASMCs (passages 5 to 10) from normal subjects (Lonza, Walkersville, USA), IPAH patients [12], and CTEPH patients [24] were cultured in Medium 199 supplemented with 10% fetal bovine serum, 100 U/ml penicillin plus 100 μg/ml streptomycin (Invitrogen/GIBCO, Grand Island, USA), 50 μg/ml D-valine (Sigma-Aldrich, St. Louis, USA), and 20 μg/ml endothelial cell growth supplement (BD Biosciences, Franklin Lakes, USA) at 37°C.
Cell proliferation assays
The PASMC proliferation was evaluated using Cell Counting Kit-8 (Dojin, Kumamoto, Japan) based on MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay [25] and Cell Proliferation ELISA BrdU Colorimetric Kit (Roche Diagnostics, Mannheim, Germany) based on BrdU (bromodeoxyuridine) incorporation assay. PASMCs were subcultured in 96-well plates (approximately 1×104 cells per well) and incubated at 37°C for 24~72 h. Cellular viability was quantified colorimetrically as the absorbance at 450 nm (A450) for MTT assay or 370 nm for BrdU incorporation assay using Benchmark Plus Microplate Reader and Microplate Manager (ver. 5.2; Bio-Rad Laboratories, Hercules, USA).
Drugs
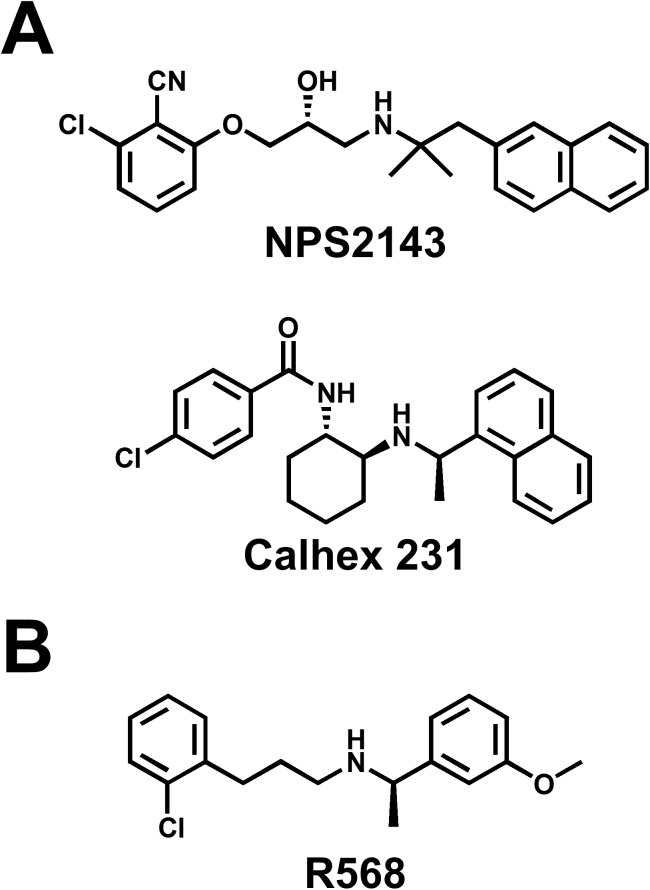
The chemical structures of modulators for CaSR, NPS2143 (2-chloro-6-[(2R)-3-[[1,1-dimethyl-2-(2-naphthalenyl)ethyl]amino-2-hydroxypropoxy]benzonitrile) (Tocris Bioscience, Ellisville, USA), Calhex 231 (4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide) (Sigma-Aldrich), and R568 (2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]benzenepropanamine) (Tocris Bioscience) are shown in Fig 1. These hydrophobic compounds were dissolved in dimethyl sulfoxide at a concentration of 10 mM as a stock solution.
Fig 1. Chemical structure of CaSR antagonist (calcilytic) and agonist (calcimimetic).
(A) The chemical structures of calcilytics, or negative allosteric modulators of CaSR, NPS2143 and Calhex 231. (B) The chemical structure of a calcimimetic, or a positive allosteric modulator of CaSR, R568.
Statistical analysis
Pooled data are shown as the mean±S.E. The statistical significance of differences between two groups was determined by Student’s t-test. The statistical significance of differences among groups was determined by Scheffé’s test after one-way analysis of variance (ANOVA). Significant differences are expressed in the figure as *p<0.05 or **p<0.01. The data of the relationship between drug concentrations and cell proliferation were fitted using the following equations: relative value (%) = 100-(100-C)/{1+(Kd/[drug])n) (for inhibition) and Amax/{1+(Kd/[drug])n)+100 (for activation), where Kd is the apparent dissociation constant of drug (IC50 and EC50, respectively), [drug] is the concentration of drug, n is the Hill coefficient, C is the component resistant to drug, and Amax is the maximum value of the response.
Results
Excessive cell proliferation in PASMCs from IPAH patients
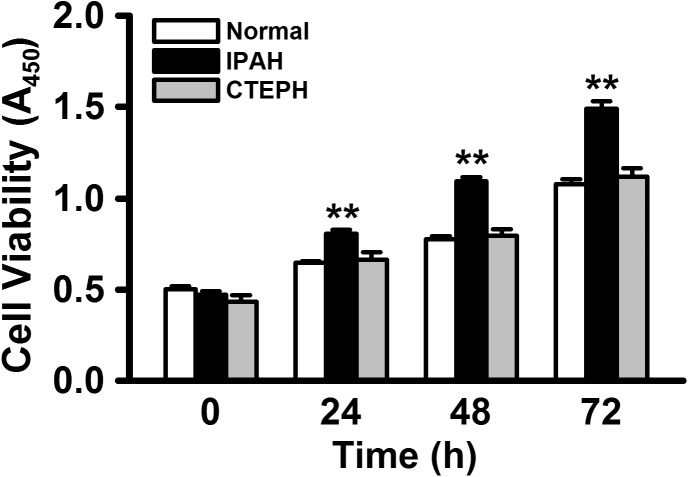
At first, we analyzed the proliferation rates of PASMCs from normal subjects and patients with IPAH and CTEPH by quantitative colorimetric assay based on the MTT test for cellular viability. In normal PASMCs, the cell number was gradually increased until 72 h after subculture (A450 = 0.65±0.01 at 24 h, 0.78±0.01 at 48 h, and 1.08±0.03 at 72 h, n = 8 for each, p<0.01 vs. 0.50±0.02 at 0 h, n = 8; Fig 2). The proliferation rate in IPAH-PASMCs was much higher than that in normal PASMCs (0.81±0.02 at 24 h, 1.09±0.02 at 48 h, and 1.49±0.04 at 72 h, n = 8 for each, p<0.01 vs. 0.47±0.02 at 0 h, n = 8, and p<0.01 vs. normal PASMCs). On the other hand, the proliferation rate in CTEPH-PASMCs was similar to that in normal PASMCs (0.66±0.04 at 24 h, 0.79±0.04 at 48 h, and 1.12±0.05 at 72 h, n = 8 for each, p<0.01 vs. 0.44±0.03 at 0 h, n = 8, and p>0.05 vs. normal PASMCs). This result using a colorimetric cell counting kit was mostly consistent with our previous data using an automated cell counter [19].
Fig 2. Excessive cell proliferation in PASMCs from IPAH patients.
The proliferation rates of PASMCs from normal subjects, IPAH patients, and CTEPH patients were analyzed using quantitative colorimetric assay based on MTT test for cellular viability. When PASMCs were subcultured in 96-well plates, the cell number was made up to approximately 1×104 cells per well. Summarized data show the proliferation rates of PASMCs from normal subjects, IPAH patients, and CTEPH patients. Data were obtained from 8 experiments. **p<0.01 vs. normal PASMCs.
Inhibitory effect of NPS2143 on excessive proliferation of PASMCs from IPAH patients
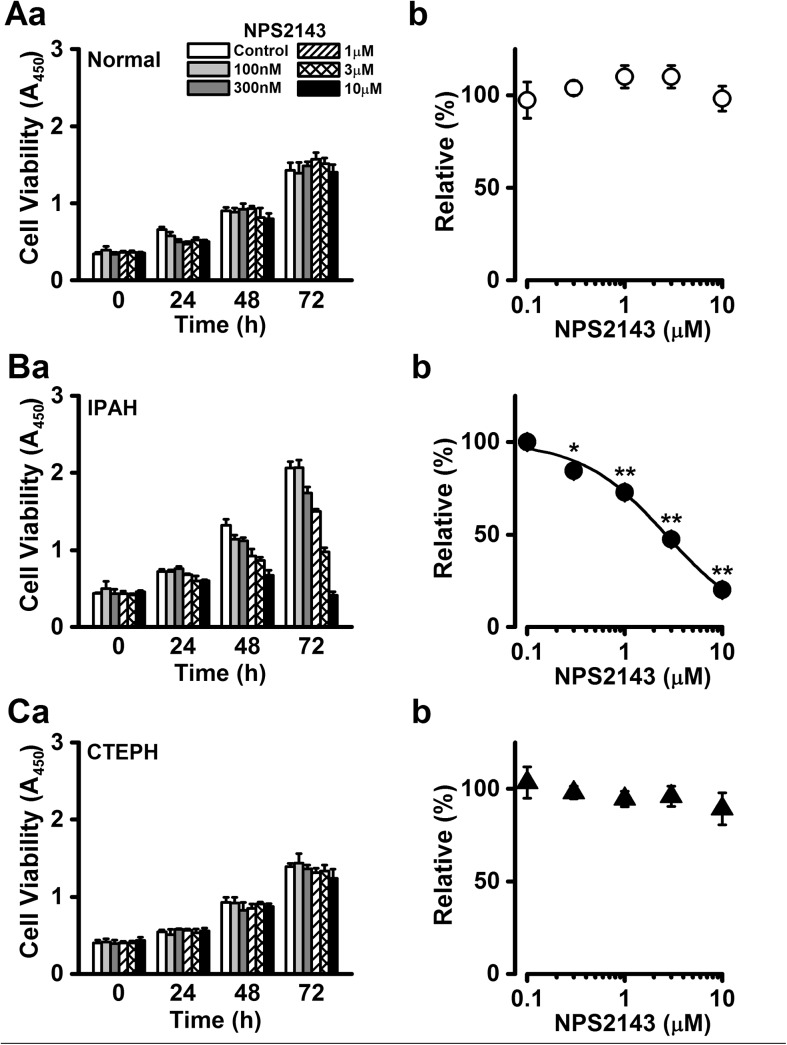
NPS2143 is used as a calcilytic, or negative allosteric modulator of CaSR, and therefore blocks CaSR function. The cell survival of normal PASMCs was not affected by the treatment with NPS2143 regardless of culture time (98±7% at 10 μM and 72 h, n = 5, p>0.05 vs. control (100%), n = 5; Fig 3A). In contrast, the proliferation rate of IPAH-PASMCs was significantly reduced in the presence of 10 μM NPS2143 after 48 h (49% decrease, n = 3, p<0.01 vs. control) and 72 h (80% decrease, n = 10, p<0.01 vs. control) culture (Fig 3Ba). In the next set of experiments, the concentration dependence of the growth inhibition by NPS2143 was analyzed in IPAH-PASMCs. As the inhibitory effect by NPS2143 at 72 h culture was most prominent (Fig 3Ba), we evaluated the dose-response relationship of PASMC proliferation upon 72 h culture. The NPS2143-induced growth inhibition occurred in a concentration-dependent manner (20±2% at 10 μM, n = 10, p<0.01; Fig 3Bb). The IC50 value of NPS2143 was 2.64 μM, and the Hill coefficient was 1.00 (n = 7~13). On the other hand, the proliferation of CTEPH-PASMCs was not influenced by the presence of NPS2143 (89±9% at 10 μM and 72 h, n = 5, p>0.05 vs. control; Fig 3C).
Fig 3. Inhibitory effect of NPS2143 on enhanced proliferation of PASMCs from IPAH patients.
The effects of NPS2143, which is widely used as a calcilytic or negative allosteric regulator of CaSR, on cell proliferation were examined in normal, IPAH, and CTEPH PASMCs. Summarized data (a) show the effect of NPS2143 on cell viability at the concentration range between 100 nM and 10 μM in normal (A), IPAH (B), and CTEPH (C) PASMCs. Dose-response curve (b) of cell proliferation at 72 h for NPS2143 in normal (A), IPAH (B), and CTEPH (C) PASMCs. The absorbance at several concentrations of NPS2143 was normalized by the value in the absence of NPS2143 (as 100%). The IC50 value of NPS2143 for the proliferation of IPAH-PASMCs was 2.64 μM and the Hill coefficient was 1.00. Data were obtained from 5~13 experiments. *p<0.05 or **p<0.01 vs. control (100%).
Attenuation of excessive cell proliferation by Calhex 231 in PASMCs from IPAH patients
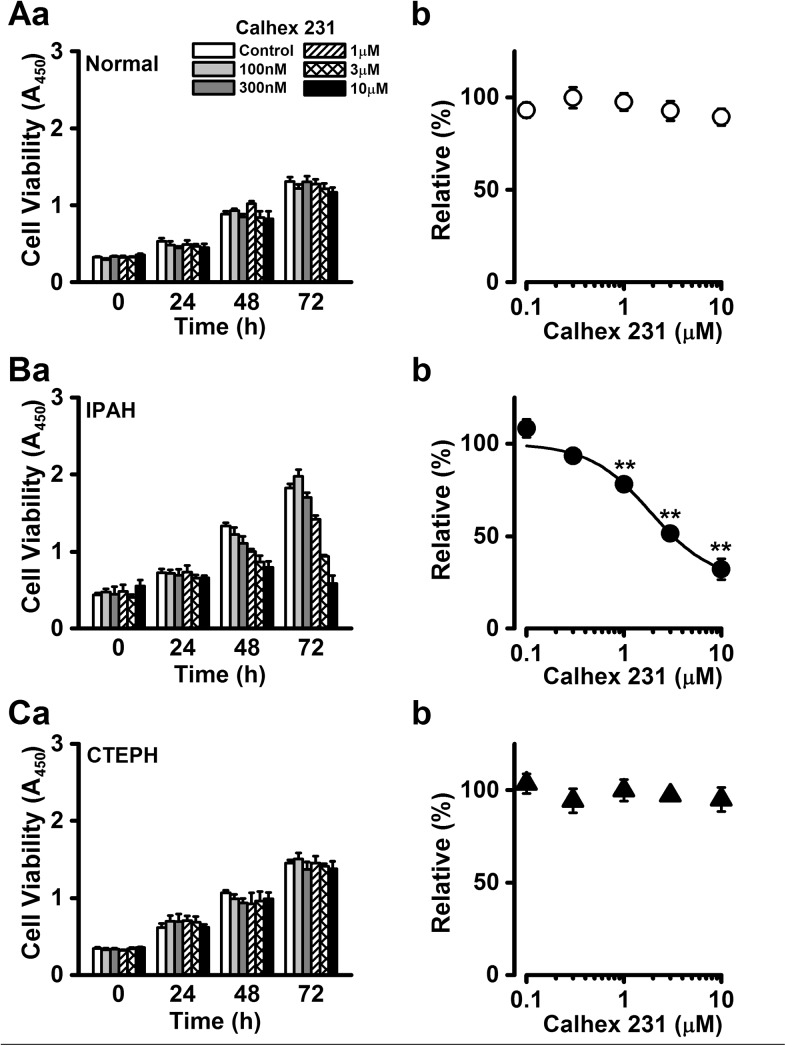
To confirm that the inhibitory effect of NPS2143 on excessive cell proliferation was mediated by CaSR antagonism in IPAH-PASMCs, the effect of Calhex 231, which is another calcilytic structurally unrelated to NPS2143, on cell proliferation was examined in normal, IPAH, and CTEPH PASMCs. The cell survival of normal PASMCs was not affected by the presence of Calhex 231 (89±4% at 10 μM and 72 h, n = 8, p>0.05 vs. control; Fig 4A). When IPAH-PASMCs were incubated with medium containing various concentrations of Calhex 231 for 72 h, Calhex 231 dramatically inhibited cell growth in a concentration-dependent manner (32±6% at 10 μM, n = 6, p<0.01 vs. control; Fig 4B) with an IC50 value of 1.89 μM and a Hill coefficient of 1.39 (n = 6). In CTEPH-PASMCs, there was no significant difference in cell proliferation in the presence of Calhex 213 (95±6% at 10 μM, n = 5, p>0.05 vs. control; Fig 4C).
Fig 4. Attenuation of cell proliferation by Calhex 231 in PASMCs from IPAH patients.
The effects of Calhex 231, a calcilytic structurally-unrelated to NPS2143, on cell proliferation were examined in normal, IPAH, and CTEPH PASMCs. Summarized data (a) show the effect of Calhex 231 on the cell viability at the concentration range between 100 nM and 10 μM in normal (A), IPAH (B), and CTEPH (C) PASMCs. Dose-response curve (b) of cell proliferation at 72 h for Calhex 231 in normal (A), IPAH (B), and CTEPH (C) PASMCs. The absorbance at several concentrations of Calhex 231 was normalized by the value in the absence of Calhex 231 (as 100%). The IC50 value of Calhex 231 for the proliferation of IPAH-PASMCs was 1.89 μM and the Hill coefficient was 1.39. Data were obtained from 5~8 experiments. **p<0.01 vs. control (100%).
Enhancement of cell proliferation by calcimimetic in PASMCs from IPAH patients
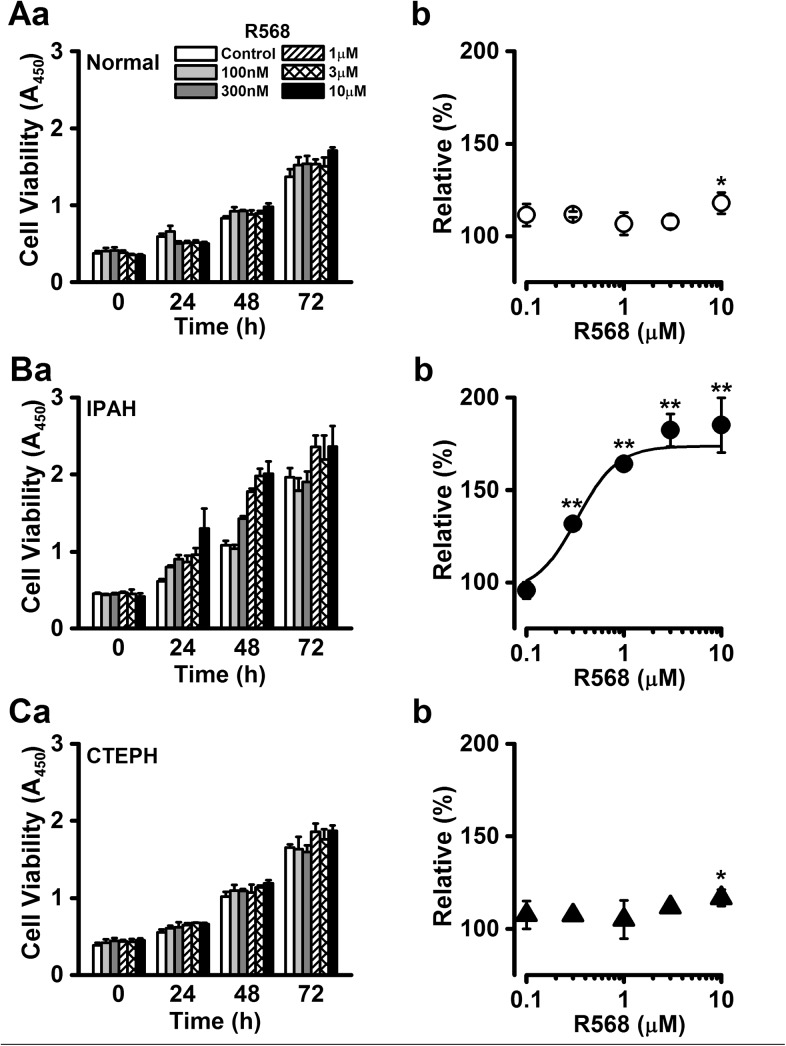
Alternatively, the effect of the calcimimetic R568, a synthetic activator and positive allosteric modulator of CaSR, on cell proliferation was examined in normal, IPAH, and CTEPH PASMCs. The proliferation rate of normal PASMCs was slightly increased by the treatment with R568 at the highest concentration of 10 μM after 48 h culture (118±6% at 48 h, n = 6, p = 0.022 vs. control; Fig 5A). The proliferation rate of IPAH-PASMCs was dramatically augmented in the presence of 10 μM R568 after 48 h culture. As the enhancing effect of R568 at 48 h was most prominent (Fig 5Ba), we evaluated the dose-response relationship of PASMC proliferation at 48 h. The R568-induced increase in cell number occurred in a concentration-dependent manner (185±15% at 10 μM, n = 11, p<0.01 vs. control; Fig 5Bb), with an EC50 value of 0.33 μM and a Hill coefficient of 2.10 (n = 7~11). Similarly to normal PASMCs, the proliferation of CTEPH-PASMCs was slightly facilitated during the exposure to R568 for 48 h (117±4% at 10 μM, n = 5, p = 0.049 vs. control; Fig 5C).
Fig 5. Enhancement of cell proliferation by a calcimimetic in PASMCs from IPAH patients.
The effects of R568, a calcimimetic or positive allosteric regulator of CaSR, on cell proliferation were examined in normal, IPAH, and CTEPH PASMCs. Summarized data (a) show the effect of R568 on the cell viability in the concentration range between 100 nM and 10 μM in normal (A), IPAH (B), and CTEPH (C) PASMCs. Dose-response curve (b) of cell proliferation at 48 h for R568 in normal (A), IPAH (B), and CTEPH (C) PASMCs. The absorbance at several concentrations of R568 was normalized by the value in the absence of R568 (as 100%). The EC50 value of R568 for the proliferation of IPAH-PASMCs was 0.33 μM and the Hill coefficient was 2.10. Data were obtained from 5~11 experiments. *p<0.05 or **p<0.01 vs. control (100%).
Effects of CaSR modulators on PASMC proliferation by BrdU incorporation assay
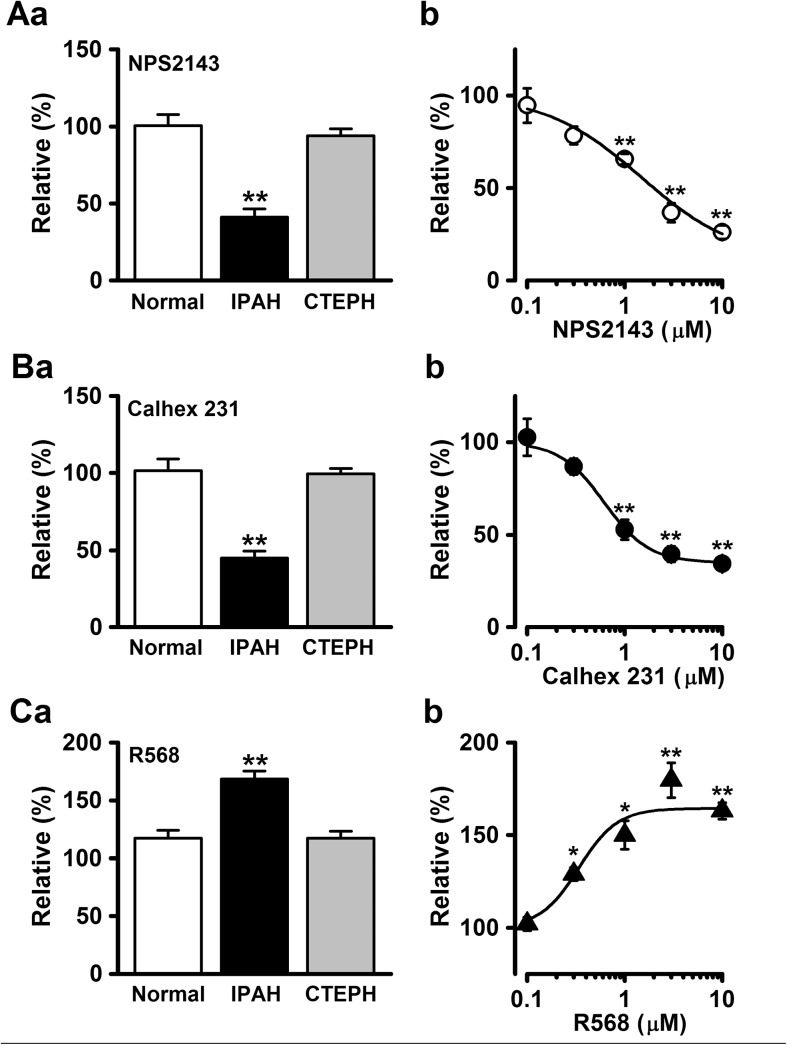
To confirm our results obtained from the MTT assay, another quantitative colorimetric assay based on the BrdU incorporation method for cell proliferation was carried out in normal, IPAH, and CTEPH PASMCs. The excessive proliferation of IPAH-PASMCs was markedly reduced by the treatment with 3 μM NPS2143 for 72 h culture (41±5%, n = 8, p<0.01 vs. control (100%); Fig 6Aa). The inhibitory effect by NPS2143 on cell proliferation occurred in a concentration-dependent manner (26±4% at 10 μM, n = 8, p<0.01; Fig 6Ab). The IC50 value of NPS2143 was 1.48 μM, and the Hill coefficient was 0.89 (n = 8). The application of 3 μM Calhex 231 also inhibited the excessive cell proliferation in IPAH-PASMCs (45±4% at 72 h, n = 9, p<0.01; Fig 6Ba), and the inhibition occurred concentration-dependently (34±4% at 10 μM, n = 8, p<0.01; Fig 6Bb), with an IC50 value of 0.62 μM and a Hill coefficient of 1.89 (n = 8). The cell proliferation in IPAH-PASMCs was enhanced by 3 μM R568 (169±7% at 48 h, n = 7, p<0.01; Fig 6Ca). The augmented cell proliferation occurred in a concentration-dependent manner (163±4% at 10 μM, n = 8, p<0.01; Fig 6Cb) with an EC50 value of 0.34 μM and a Hill coefficient of 2.19 (n = 8). In contrast, the proliferation of normal and CTEPH PASMCs was not significantly changed by 3 μM NPS2143 (101±7%, n = 9 and 94±4%, n = 7, respectively, p>0.05), 3 μM Calhex 231 (102±8%, n = 9 and 99±4%, n = 7, respectively, p>0.05), or 3 μM R568 (117±7%, n = 10, p = 0.085 and 117±6%, n = 14, p = 0.127, respectively).
Fig 6. Effect of CaSR modulators on cell proliferation using BrdU incorporation assay.
The proliferation rates of PASMCs from normal subjects, IPAH patients, and CTEPH patients were analyzed using quantitative colorimetric assay based on the BrdU incorporation method for cell proliferation. The effects of NPS2143 (A), Calhex 231 (B), and R568 (C) on cell proliferation were examined in normal, IPAH, and CTEPH PASMCs. Summarized data (a) show the effect of CaSR modulators at a concentration of 3 μM on cell proliferation at 48 (for R568) or 72 (for NPS2143 and Calhex 231) h in normal, IPAH, and CTEPH PASMCs. Dose-response curve (b) of cell proliferation for NPS2143, Calhex 231, and R568 in IPAH-PASMCs. The absorbance was normalized by the value in the absence of drug (as 100%). The IC50 values of NPS2143 and Calhex 231 for the proliferation of IPAH-PASMCs were 1.48 and 0.62 μM, respectively. The EC50 value of R568 was 0.34 μM. Data were obtained from 7~14 experiments. *p<0.05 or **p<0.01 vs. control (100%).
Discussion
Our previous report showed that the excessive proliferation rate of IPAH-PASMCs was attenuated by siRNA knockdown of CaSR [19]; however, pharmacological modulators are required for the development of drug therapy for PAH. In this investigation, we have shown that calcilytics (NPS2143 and Calhex 231) inhibit the excessive proliferation of PASMCs from IPAH patients, whereas a calcimimetic (R568) enhances the proliferation of PASMCs from IPAH patients as well as from normal subjects and CTEPH patients. Similar results were obtained from two different experiments for cell proliferation based on MTT test and BrdU incorporation assay. These results indicate that the activity of CaSR contributes to the PASMC proliferation.
[Ca2+]cyt plays an important role in regulation of the proliferation and migration of PASMCs. An increase in [Ca2+]cyt in PASMCs is an important stimulus for PASMC proliferation and pulmonary vascular remodeling under physiological and pathological conditions [6, 7, 10]. We recently found that CaSR is upregulated in lung tissues and PASMCs isolated from IPAH patients, as well as in animals with experimental pulmonary hypertension [19, 26, 27]. This upregulated CaSR plays a pivotal role in the increased resting [Ca2+]cyt and augmented Ca2+ influx, as well as the enhanced cell proliferation in IPAH-PASMCs. Importantly, the intraperitoneal injection of the calcilytic NPS2143 prevented the development of pulmonary hypertension and right ventricular hypertrophy in monocrotaline-induced pulmonary hypertensive rats and hypoxia-induced pulmonary hypertensive mice. Functionally upregulated CaSR in PASMCs may be involved in a novel pathogenic mechanism underlying excessive pulmonary vascular remodeling in IPAH patients. In this study, we have direct evidence in vitro that calcilytics specifically inhibit the excessive proliferation of IPAH-PASMCs. Interestingly, calcilytics did not affect the cell growth of normal PASMCs. Pharmacological blockade of the upregulated CaSR with calcilytics may be a novel therapeutic approach for PAH patients who do not respond to conventional drug therapy. On the other hand, a calcimimetic enhanced the proliferation of IPAH-PASMCs. Even for normal and CTEPH PASMCs, cell growth was slightly but significantly increased by a calcimimetic. This result is in agreement with our previous observation that CaSR is expressed at low levels in the plasma membrane of normal PASMCs [19].
CaSR belongs to the G-protein-coupled receptor subfamily C [20, 21, 28]. The activation of CaSR coupled to Gq proteins stimulates phospholipase C that hydrolyzes phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 induces Ca2+ release from the sarcoplasmic reticulum mediated by IP3 receptors. In vascular smooth muscle cells, the activation of CaSR increases [Ca2+]cyt and thereafter induces vasoconstriction under physiological conditions [29–31]. Therefore, CaSR is thought to be involved in the regulation of myogenic tone, peripheral vascular resistance, and arterial blood pressure [32–34]. Alternatively, CaSR also regulates multiple signal pathways such as mitogen-activated protein kinase (MAPK) cascades: MAPK kinase (MEK), extracellular signal-regulated kinases (ERK1/2), and c-Jun N-terminal kinase (JNK). Recently obtained evidence suggests that CaSR in vascular myocytes contributes to cell proliferation and apoptosis through MAPK and phospholipase C cascades [35–38]. It is likely that [Ca2+]cyt increase mediated by CaSR activation triggers MAPK cascades to accelerate PASMC proliferation under physiological and pathological conditions. Therefore, the blockade of CaSR by calcilytics caused attenuation of excessive cell proliferation in IPAH-PASMCs. Related to the decrease in cell viability by the treatment with calcilytics, these cells apparently exhibited altered morphological features, leading to outcomes such as cell damage and death. Calcilytics may cause apoptosis and necrosis followed by morphological damage involving cell death. However, further experiments are necessary to elucidate the mechanism underlying cell proliferation and death via the CaSR pathway.
In pharmacological research associated with CaSR, NPS2143, Calhex 231, and R568 are widely used as CaSR modulators [39, 40]. A prototypical calcimimetic, R568 (2-chloro-N-[(1R)-1-(3-methoxyphenyl)ethyl]benzenepropanamine) (Fig 1B), is a phenylalkylamine compound structurally derived from fendiline, a blocker of voltage-dependent Ca2+ channels [41]. On the other hand, the first calcilytic, NPS2143 (2-chloro-6-[(2R)-3-[[1,1-dimethyl-2-(2-naphthalenyl)ethyl]amino-2-hydroxypropoxy]benzonitrile), was not obtained by direct derivatization based on phenylalkylamine calcimimetics, but by optimization of a positive compound in high-throughput screening [42] (Fig 1A). Later on, Calhex 231 (4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide) was developed as a calcilytic structurally unrelated to NPS 2143 by another high-throughput screening [43]. In this study, three structurally-unrelated CaSR modulators influenced the proliferation of IPAH-PASMCs; namely, inhibition or enhancement by calcilytics or calcimimetic, respectively. Furthermore, the IC50 and EC50 values of these modulators on the proliferation of IPAH-PASMCs were close to the effective concentration ranges of CaSR in recombinant HEK293 cells [40–43] and vascular smooth muscle cells [19, 27, 44, 45]. To our knowledge, there are few reports about adverse effects of these compounds. Taken together, our data strongly support our conclusion that these compounds interact with CaSR to respond in PASMCs.
On the basis of our understanding of the pathological mechanisms of PAH, drug therapy for PAH has progressed in recent years via the development of several specific drugs that offer an effective alternative to voltage-dependent Ca2+ channel blockers (calcium blockers), such as nifedipine and diltiazem [2, 28]. Currently, three therapeutic classes are widely used for the treatment of PAH: endothelin receptor antagonists (bosentan and ambrisentan), phosphodiesterase type 5 inhibitors (sildenafil and tadalafil), and prostacyclins (also known as prostaglandin I2) (epoprostenol, treprostinil, and iloprost). Moreover, numerous potential candidates have either been submitted for approval or are in development. Despite recent major therapeutic advances, current treatments of PAH do not achieve a cure of life-threatening disease. In this study, we have demonstrated that the PASMC proliferation is modulated by the activity of CaSR, which is upregulated in IPAH patients, and that this is a novel pathogenic mechanism underlying the augmented PASMC proliferation in IPAH patients. These results also provide an important pathway for developing therapeutic interventions for PAH. Calcilytics are potential drug candidates for the treatment of osteoporosis and other bone metabolism diseases [39, 40]. Therefore, the accumulation of clinical data about calcilytics may be useful for clinical trials and the application of calcilytics as therapeutic drugs for PAH.
Our previous reports showed that a calcilytic inhibited the development of pulmonary hypertension in monocrotaline-induced pulmonary hypersensitive rats and hypoxia-induced pulmonary hypertensive mice [19, 26]. In this study, we clearly demonstrate that calcilytics attenuate the excessive proliferation of PASMCs from IPAH patients, whereas calcilytics do not affect cell survival in PASMCs from normal subjects. Taken these findings together, calcilytics are a potential candidate for therapeutic drugs for PAH patients.
Acknowledgments
We thank Dr. Jason X.-J. Yuan (University of Arizona, Tucson, USA) for providing PASMC lines from IPAH and CTEPH patients. We also thank Dr. Hisao Yamamura (Nagoya City University, Nagoya, Japan) for reading of this manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (25860068 and 15K18881; to A.Y.).
References
- 1. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54: S43–54. 10.1016/j.jacc.2009.04.012 . [DOI] [PubMed] [Google Scholar]
- 2. Montani D, Gunther S, Dorfmüller P, Perros F, Girerd B, Garcia G, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013; 8: 97 10.1186/1750-1172-8-97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987; 107: 216–23. 10.7326/0003-4819-107-2-216 . [DOI] [PubMed] [Google Scholar]
- 4. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010; 137: 376–87. 10.1378/chest.09-1140 . [DOI] [PubMed] [Google Scholar]
- 5. Hassoun PM, Mouthon L, Barberá JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009; 54: S10–9. 10.1016/j.jacc.2009.04.006 . [DOI] [PubMed] [Google Scholar]
- 6. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009; 54: S20–31. 10.1016/j.jacc.2009.04.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang XR, Lin MJ, Sham JS. Physiological functions of transient receptor potential channels in pulmonary arterial smooth muscle cells. Adv Exp Med Biol. 2010; 661: 109–22. 10.1007/978-1-60761-500-2_7 . [DOI] [PubMed] [Google Scholar]
- 8. Yamamura A, Yamamura H, Zeifman A, Yuan JX-J. Activity of Ca2+-activated Cl- channels contributes to regulating receptor- and store-operated Ca2+ entry in human pulmonary artery smooth muscle cells. Pulm Circ. 2011; 1: 269–79. 10.4103/2045-8932.83447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamamura H, Yamamura A, Ko EA, Pohl NM, Smith KA, Zeifman A, et al. Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca2+ entry in human pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2014; 306: C871–8. 10.1152/ajpcell.00221.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta. 2007; 1772: 895–906. 10.1016/j.bbadis.2007.02.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ko EA, Wan J, Yamamura A, Zimnicka AM, Yamamura H, Yoo HY, et al. Functional characterization of voltage-dependent Ca2+ channels in mouse pulmonary arterial smooth muscle cells: divergent effect of ROS. Am J Physiol Cell Physiol. 2013; 304: C1042–52. 10.1152/ajpcell.00304.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004; 101: 13861–6. 10.1073/pnas.0405908101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, et al. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L1202–10. 10.1152/ajplung.00214.2006 . [DOI] [PubMed] [Google Scholar]
- 14. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, et al. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004; 95: 496–505. 10.1161/01.RES.0000138952.16382.ad . [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006; 98: 1528–37. 10.1161/01.RES.0000227551.68124.98 . [DOI] [PubMed] [Google Scholar]
- 16. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008; 295: L104–13. 10.1152/ajplung.00058.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wan J, Yamamura A, Zimnicka AM, Voiriot G, Smith KA, Tang H, et al. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav1.2 and Cav3.2. Am J Physiol Lung Cell Mol Physiol. 2013; 305: L154–64. 10.1152/ajplung.00313.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith KA, Voiriot G, Tang H, Fraidenburg DR, Song S, Yamamura H, et al. Notch activation of Ca2+ signaling mediates hypoxic pulmonary vasoconstriction and pulmonary hypertension. Am J Respir Cell Mol Biol. 2015; 53: 355–67. 10.1165/rcmb.2014-0235OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA, et al. Enhanced Ca2+-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res. 2012; 111: 469–81. 10.1161/CIRCRESAHA.112.266361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003; 4: 530–8. 10.1038/nrm1154 . [DOI] [PubMed] [Google Scholar]
- 21. Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011; 32: 3–30. 10.1210/er.2009-0043 . [DOI] [PubMed] [Google Scholar]
- 22. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993; 366: 575–80. 10.1038/366575a0 . [DOI] [PubMed] [Google Scholar]
- 23. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001; 81: 239–97. . [DOI] [PubMed] [Google Scholar]
- 24. Ogawa A, Firth AL, Yao W, Madani MM, Kerr KM, Auger WR, et al. Inhibition of mTOR attenuates store-operated Ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009; 297: L666–76. 10.1152/ajplung.90548.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamura H, Ugawa S, Ueda T, Morita A, Shimada S. TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol Cell Physiol. 2008; 295: C296–301. 10.1152/ajpcell.00499.2007 . [DOI] [PubMed] [Google Scholar]
- 26. Guo Q, Huang JA, Yamamura A, Yamamura H, Zimnicka AM, Fernandez R, et al. Inhibition of the Ca2+-sensing receptor rescues pulmonary hypertension in rats and mice. Hypertens Res. 2014; 37: 116–24. 10.1038/hr.2013.129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamura A, Yamamura H, Guo Q, Zimnicka AM, Wan J, Ko EA, et al. Dihydropyridine Ca2+ channel blockers increase cytosolic [Ca2+] by activating Ca2+-sensing receptors in pulmonary arterial smooth muscle cells. Circ Res. 2013; 112: 640–50. 10.1161/CIRCRESAHA.113.300897 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamura A. Pathological function of Ca2+-sensing receptor in pulmonary arterial hypertension. J Smooth Muscle Res. 2014; 50: 8–17. 10.1540/jsmr.50.8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol. 2000; 175: 203–12. 10.1007/s00232001068 . [DOI] [PubMed] [Google Scholar]
- 30. Chow JY, Estrema C, Orneles T, Dong X, Barrett KE, Dong H. Calcium-sensing receptor modulates extracellular Ca2+ entry via TRPC-encoded receptor-operated channels in human aortic smooth muscle cells. Am J Physiol Cell Physiol. 2011; 301: C461–8. 10.1152/ajpcell.00389.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li GW, Wang QS, Hao JH, Xing WJ, Guo J, Li HZ, et al. The functional expression of extracellular calcium-sensing receptor in rat pulmonary artery smooth muscle cells. J Biomed Sci. 2011; 18: 16 10.1186/1423-0127-18-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohanian J, Gatfield KM, Ward DT, Ohanian V. Evidence for a functional calcium-sensing receptor that modulates myogenic tone in rat subcutaneous small arteries. Am J Physiol Heart Circ Physiol. 2005; 288: H1756–62. 10.1152/ajpheart.00739.2004 . [DOI] [PubMed] [Google Scholar]
- 33. Fryer RM, Segreti JA, Widomski DL, Franklin PH, Banfor PN, Koch KA, et al. Systemic activation of the calcium sensing receptor produces acute effects on vascular tone and circulatory function in uremic and normal rats: focus on central versus peripheral control of vascular tone and blood pressure by cinacalcet. J Pharmacol Exp Ther. 2007; 323: 217–26. 10.1124/jpet.107.123901 . [DOI] [PubMed] [Google Scholar]
- 34. Smajilovic S, Yano S, Jabbari R, Tfelt-Hansen J. The calcium-sensing receptor and calcimimetics in blood pressure modulation. Br J Pharmacol. 2011; 164: 884–93. 10.1111/j.1476-5381.2011.01317.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006; 348: 1215–23. 10.1016/j.bbrc.2006.07.192 . [DOI] [PubMed] [Google Scholar]
- 36. Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, et al. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007; 293: F946–55. 10.1152/ajprenal.00474.2006 . [DOI] [PubMed] [Google Scholar]
- 37. Sun YH, Li YQ, Feng SL, Li BX, Pan ZW, Xu CQ, et al. Calcium-sensing receptor activation contributed to apoptosis stimulates TRPC6 channel in rat neonatal ventricular myocytes. Biochem Biophys Res Commun. 2010; 394: 955–61. 10.1016/j.bbrc.2010.03.096 . [DOI] [PubMed] [Google Scholar]
- 38. Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, et al. The calcium-sensing receptor mediates hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells through MEK1/ERK1,2 and PI3K pathways. Basic Clin Pharmacol Toxicol. 2011; 108: 185–93. 10.1111/j.1742-7843.2010.00639.x . [DOI] [PubMed] [Google Scholar]
- 39. Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007; 8: 169–84. 10.2174/138945007779315614 . [DOI] [PubMed] [Google Scholar]
- 40. Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011; 63: 59–126. 10.1124/pr.109.002501 . [DOI] [PubMed] [Google Scholar]
- 41. Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A. 1998; 95: 4040–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nemeth EF, Delmar EG, Heaton WL, Miller MA, Lambert LD, Conklin RL, et al. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001; 299: 323–31. . [PubMed] [Google Scholar]
- 43. Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca2+-sensing receptor. J Biol Chem. 2003; 278: 49487–94. 10.1074/jbc.M308010200 . [DOI] [PubMed] [Google Scholar]
- 44. Awumey EM, Bridges LE, Williams CL, Diz DI. Nitric-oxide synthase knockout modulates Ca2+-sensing receptor expression and signaling in mouse mesenteric arteries. J Pharmacol Exp Ther. 2013; 346: 38–47. 10.1124/jpet.113.205534 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, Ruat M, et al. The expression and function of Ca2+-sensing receptors in rat mesenteric artery; comparative studies using a model of type II diabetes. Br J Pharmacol. 2008; 154: 652–62. 10.1038/bjp.2008.108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.