Abstract
Food intake is an essential animal activity, regulated by neural circuits that motivate food localization, evaluate nutritional content and acceptance or rejection responses through the gustatory system, and regulate neuroendocrine feedback loops that maintain energy homeostasis. Excess food consumption in people is associated with obesity and metabolic and cardiovascular disorders. However, little is known about the genetic basis of natural variation in food consumption. To gain insights in evolutionarily conserved genetic principles that regulate food intake, we took advantage of a model system, Drosophila melanogaster, in which food intake, environmental conditions and genetic background can be controlled precisely. We quantified variation in food intake among 182 inbred, sequenced lines of the Drosophila melanogaster Genetic Reference Panel (DGRP). We found significant genetic variation in the mean and within-line environmental variance of food consumption and observed sexual dimorphism and genetic variation in sexual dimorphism for both food intake traits (mean and variance). We performed genome wide association (GWA) analyses for mean food intake and environmental variance of food intake (using the coefficient of environmental variation, CV E, as the metric for environmental variance) and identified molecular polymorphisms associated with both traits. Validation experiments using RNAi-knockdown confirmed 24 of 31 (77%) candidate genes affecting food intake and/or variance of food intake, and a test cross between selected DGRP lines confirmed a SNP affecting mean food intake identified in the GWA analysis. The majority of the validated candidate genes were novel with respect to feeding behavior, and many had mammalian orthologs implicated in metabolic diseases.
Introduction
Food intake is a fundamental fitness trait as it is essential for the maintenance of energy balance and survival. In humans, consumption of excessive calories is associated with an increased incidence of type 2 diabetes, obesity, cardiovascular disease, and other disorders and diseases [1–3]; while insufficient caloric intake is correlated with abnormal liver function and skin and other disorders [4, 5]. However, dissecting the genetic and environmental contributions to variation in food intake in human populations is challenging, due to difficulties in quantifying food intake [6–13] and small effects of segregating variants that together account for only a small fraction of the estimated genetic variance [14–18]. Using model organisms such as Drosophila melanogaster to delineate the genetic and neural basis of food consumption and metabolism, obesity, type 2 diabetes, and other traits and disorders connected with food consumption can identify evolutionarily conserved candidate genes and pathways relevant to human biology [19, 20].
The development of rapid and reproducible techniques for quantifying food intake has facilitated many recent studies on the genetic, neural, and environmental factors modulating food consumption in D. melanogaster. Measuring feeding frequency by quantifying proboscis extension provides an estimate of total food intake, and can be applied to the same group of flies over time [21] and automated for continuous resolution [22]. Radioactive tracers can permit measurement of food intake and fecal and egg excretion [23], while the development of the Capillary Feeding (CAFE) assay allows for continuous measurement of food intake with high resolution [24, 25].
The genetic and environmental basis of food consumption in Drosophila has many components. Drosophila utilize gustatory receptors (GRs), olfactory receptors (ORs) and odorant binding proteins (OBPs) to sense food and assess its quality as they integrate food cues with physiological state to affect feeding decisions. Expression of short neuropeptide F (sNPF) and its receptor in OR42b neurons is required for increasing food search behavior induced by starvation [26]. Food odors elicit rapid, transient physiological and transcriptional increases in circulating glucose and expression of four of the eight Drosophila Insulin-Like Peptides (DILPs) and adipokinetic hormone (AKT) [27]. OBPs are thought to transport bitter hydrophobic tastants to gustatory receptors to modulate food intake [28]. The receptor for trehalose, Gr5a, acts with the G-protein alpha subunit, Gsα, to allow sugar taste transduction [29]. Gustatory perception of food quality can distinguish among major tastant groups but not individual compounds within a modality [30]. Gustatory sugar acceptance is modulated by dopaminergic signaling [31]. Acceptance of unpalatable, often bitter, foods increases with long-term exposure, with attenuation of gustation achieved through activation of Transient Receptor Potential-Like (TRPL) localized in the GR neurons [32]. In the brain, Gr43a senses fructose circulating in the hemolymph, promotes feeding in hungry flies, and suppresses feeding in satiated flies [33]. Integration of OBPs, GRs, and subsequent neuroendocrine and motor responses can be modulated by gustatory interneurons connecting food sensing modalities with neuroendocrine and motor outputs [34].
Gustatory and olfactory sensing assess the tastants and odorants of food, while the brain assesses caloric content independent of gustatory and olfactory modalities. The brain directly senses caloric content of circulating sugar without tasting sugar via the hemolymph, and communicates necessary feeding and metabolic changes [35]. In fact, caloric sensing by the brain forms metabolic memories that allow Drosophila to balance caloric intake with metabolic state independent of taste [36]. Adult D. melanogaster increase their feeding rate under dietary restriction, thereby adjusting caloric intake to physiological needs [37]. Odorant sensing to locate and anticipate feeding, gustation to assess palatability, and caloric density assessment by the brain independent of taste are integrated with physiological state to regulate metabolism and determine feeding behavior.
The central nervous system serves as the master regulator of food intake, integrating various physiological signals with external cues. Decreases in food intake are achieved through various peptides and signaling modalities: Leucokinin (Lk) and the Leucokinin Receptor (Lkr) [38], Forkhead Box O transcription factor (FOXO) [39–41], Allatostatins [42], serotonin [43], neuronal populations of c673a [44], peripheral cAMP-responsive transcription factor (CREB) [45], hugin [46–48], Rac2 [49], and Unpaired 2 (Upd2) [50] all elicit decreased food intake. In comparison, there are fewer signaling peptides involved in increasing food intake: SP [51], sNPF [52–54], and dopamine [55] elicit feeding increases. Increases and decreases of food intake are further modulated by a complex coordination of neuronal signaling. A single pair of Feeding neurons control the initiation of food intake [56], and four GABAergic interneurons promote the cessation of feeding in response to satiety and gustatory cues [57]. Insulin, serotonergic, octopaminergic, GABAergic, and dopaminergic signaling pathways all act and interact to orchestrate physiological and metabolic cues, sensory input, and signaling peptides into the appropriate feeding responses [31, 43, 49, 57–61]. The decision to eat is accompanied by extension of the proboscis and fluid ingestion by motor neuron-driven pumping; these neurons exhibit prolonged activation to palatable tastants [62].
Despite these recent advances in understanding the integration of environmental, physiological, and neuroendocrine factors affecting initiation, continuation, and cessation of food intake, the extent to which these genes and pathways affect natural variation in food intake in Drosophila is unknown. Here, we used the inbred, sequenced lines of the D. melanogaster Genetic Reference Panel (DGRP) [63, 64] to quantify substantial genetic variation in both mean food consumption and the within-line environmental variance (measured using the coefficient of environmental variation, CV E) of food consumption [65–71]. We performed genome wide association (GWA) analyses for both traits and identified novel candidate genes, many of which we functionally validated using single nucleotide polymorphisms (SNPs) in the DGRP [72] or RNAi alleles.
Results
Quantitative genetics of mean food consumption in the DGRP
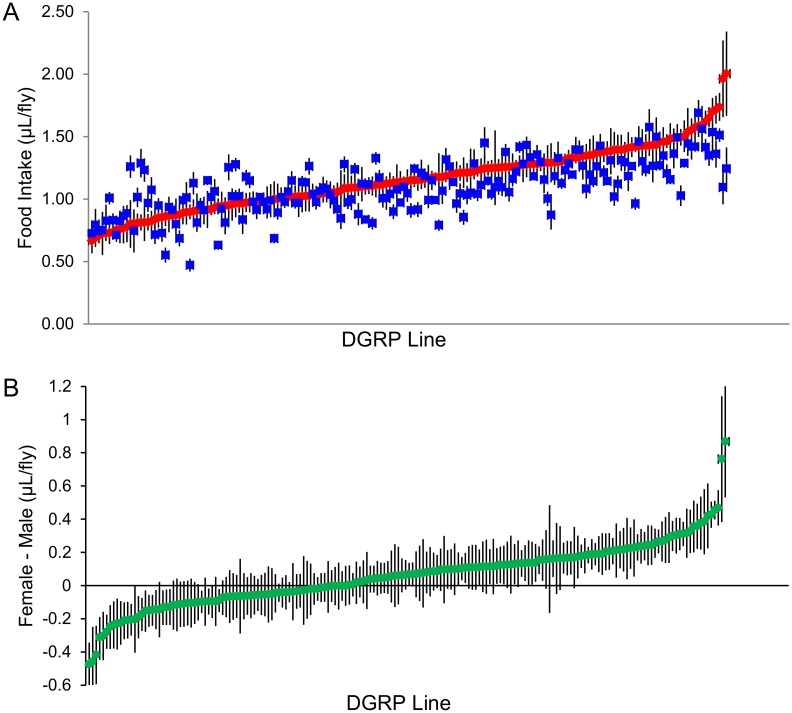
We assessed total consumption of 4% sucrose over 24 hours for 182 DGRP lines (S1 Table) using a modified version of the CAFE assay [24]. We found substantial genetic variation in food consumption among the DGRP lines, with a broad sense heritability of H 2 = 0.45 (Fig 1A, Table 1). Food intake is sexually dimorphic (P < 0.0001 for the sex term), with females generally consuming more than males. In addition, there is genetic variation among the DGRP in the magnitude of sexual dimorphism in food consumption (Fig 1B, Table 1, P < 0.0001 for the sex by line interaction term), with a cross-sex genetic correlation of r GS = 0.68. Thus, we expect the genetic architecture of mean food intake to be at least partially sex-specific.
Fig 1. Variation in mean food intake in the DGRP.
(A) Mean food intake in 182 DGRP lines, arranged in order from lowest to highest in females (red diamonds). Mean food intake of males (blue squares) are given in the same order as the female scores. (B) Sexual dimorphism of food intake. The female-male difference in mean food intake of each line is ordered from lowest to highest. All error bars are ± SE.
Table 1. Analyses of variance (ANOVA) of food intake.
| Analysis | Source of Variation | df | MS | F | P-value | σ 2 (SE) |
|---|---|---|---|---|---|---|
| Sexes | Sex | 1 | 423.60 | 33.43 | <0.0001 | Fixed |
| Pooled | Line | 183 | 125.29 | 9.89 | <0.0001 | 8.21 (1.09) |
| Sex x Line | 181 | 24.43 | 1.93 | <0.0001 | 1.92 (0.42) | |
| Error | 1867 | 12.37 | 12.66 (0.41) | |||
| Females | Line | 182 | 86.15 | 5.35 | <0.0001 | 11.42 (1.48) |
| Error | 938 | 16.09 | 16.09 (0.74) | |||
| Males | Line | 182 | 63.62 | 6.90 | <0.0001 | 8.91 (1.10) |
| Error | 929 | 9.22 | 9.21 (0.43) |
df: degrees of freedom; MS: type III mean squares; F: F-statistic; σ 2 variance component estimated using restricted maximum likelihood.
Many other physiological and life history traits have been measured in the DGRP lines, enabling us to estimate genetic correlations between these traits and food intake. In total, we tested correlations with food intake for 33 traits in females and 66 traits in males. The most significant correlation with food intake was a negative correlation with starvation resistance in both sexes (r = -0.31, P < 0.0001 for males and females) [64]. We observed nominally significant and modest correlations between food intake and several sleep traits and lifespan in both sexes [67, 73]; resistance to oxidative stress induced by paraquat [74] and menadione sodium bisulfite (MSB) in females [75]; and several nutritional index values in males [76] (S2 Table). None of the correlations between food intake and body weight or aspects of metabolism [77] approached significance, suggesting that consumption is independent of size.
Quantitative genetics of within-line variation in food consumption in the DGRP
Several studies have shown that the within-line environmental variance of inbred lines is heritable [65–69, 71]. We used Brown-Forsythe and Levene’s tests to assess whether there was heterogeneity of within-line environmental variance in food consumption among the DGRP lines. These tests are modifications of one-way ANOVA that use, respectively, the absolute deviation of each data point from the line median and mean as the response variable. Indeed, we find highly significant heterogeneity of environmental variance among the DGRP lines (Table 2), suggesting heritability of this trait. Within some inbred DGRP lines, individuals consume relatively constant amounts of food, whereas within others, individuals consume widely varying amounts of food.
Table 2. Brown-Forsythe and Levene’s tests for unequal variance.
| Test | Sex | df | F | P-value |
|---|---|---|---|---|
| Brown- | Male | 181 | 1.2246 | 0.0337 |
| Forsythe | Female | 182 | 1.4663 | 0.0002 |
| Levene | Male | 181 | 1.6288 | <.0001 |
| Female | 182 | 2.0492 | <.0001 |
df: degrees of freedom; F: F statistic.
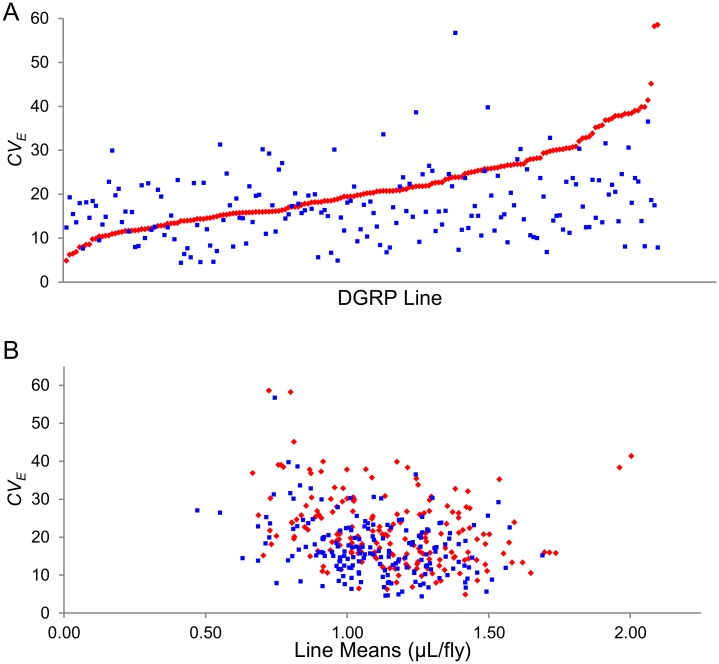
We used the coefficient of within-line variation (CV E) as the metric for within-line environmental variance (Fig 2). The correlation of CV E between males and females is r = 0.16, P = 0.031 (Fig 2). The approximate 95% confidence limits of this correlation are 0.012–0.308; thus the correlation is barely significantly different from zero and clearly different from unity, suggesting largely sex-specific genetic architecture of variation in environmental variance. The correlation between mean and CV E in food intake is significantly different from zero, but small and negative (r = -0.27, P = 0.0002 for females; and r = -0.29, P < 0.0001 for males). Therefore, only 7–8% of the total variance in CV E for food consumption can be explained by the association with the mean. We assessed whether variance in CV E could be a consequence of variation in the numbers of segregating sites in the DGRP lines [64], as it has been hypothesized that within line environmental variation may be inversely related to heterozygosity [78, 79]. This hypothesis was not supported by these data: the correlation between CV E and the number of segregating sites was not significant for females (r = 0.026, P = 0.72) or males (r = -0.106, P = 0.16; S1 Fig).
Fig 2. Variation in CV E of food intake in the DGRP.
(A) CV E of food intake, arranged in order from lowest to highest in females (red diamonds). CV E of food intake of males (blue squares) are given in the same order as the females. (B) Association of mean and CV E for food intake for females (red diamonds) and males (blue squares).
GWA analyses of food consumption
We performed GWA analyses for mean and CV E of food intake using 1,920,276 polymorphic variants with minor allele frequencies ≥ 0.05. We performed four analyses for each trait: the average of females and males, the difference between females and males (formally equivalent to the sex by line interaction), and for females and males separately. Prior to performing the GWA analyses, we tested and corrected for any effects of Wolbachia infection and common polymorphic inversions as well as polygenic relatedness [64]. For mean food intake, we observed a significant effect of Wolbachia in females (P = 0.03) and for the difference between females and males (P = 0.026); a significant effect of In(3R)K for males (P = 0.05); and a significant effect of In(3R)Mo for males (P = 0.04) and for the difference between females and males (P = 0.04) (S3 Table). None of these covariates was significant for CV E of food intake (S3 Table).
At a nominal reporting threshold of P < 10−5, we found 74 variants in or near (± 1 kb) 54 genes affecting mean food intake (S4 Table) and 160 variants in or near 101 genes affecting food intake CV E (S5 Table). Two genes, Dystrophin (Dys) and CG1136, were associated with both the mean and CV E. None of the variants were significant following a Bonferroni correction (P = 2.6 × 10−8) for multiple tests. However, 12 of the 153 genes implicated in these analyses have been previously associated with aspects of feeding (S6 Table). Among the candidate genes for mean food intake, retained (retn), CG10477 and CG42788 were previously associated with adult amino acid consumption from gene expression and GWA analyses in the DGRP [80], and Aldolase (Ald) was associated with adult transcriptional response to dietary restriction [81] (S6 Table). Among the candidate genes for CV E of food intake, previous associations have been reported for Ecdysone-inducible gene L1 (ImpL1) with larval sugar feeding [82]; CG3502, frizzled (fz) and Zwilch with amino acid intake [80]; Octopamine β3 receptor (Octβ3R) with larval hunger-driven feeding [83]; Organic anion transporting polypeptide 74D (Oatp74D) with high glucose rearing [76]; pointed (pnt) with larval nutritient control of mitochondrial biogenesis [84]; and Tyramine β hydroxylase (Tbh) with adult sweet taste-induced memory formation [85] and adult response to sucrose concentration [86] (S6 Table).
We used gene ontology (GO) enrichment analyses [87, 88] to place the top associations in context. None of the GO terms for either trait had enrichment scores passing a 5% false discovery rate (S7 Table). At nominal P-values < 0.05, the top genes associated with mean food intake were enriched for olfactory and chemosensory behavior, and epidermal growth factor (EGF) signaling (S7 Table). The top genes associated with CV E of food intake were associated with behaviors, G-protein coupled signaling, and regulation of transcription (S7 Table).
SNP-based functional validation
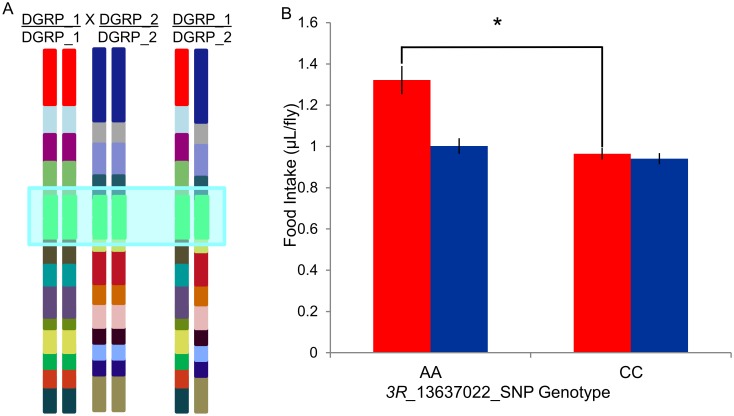
In order to assess whether a putative regulatory SNP affects feeding behavior, we chose one SNP, 3R_13637022_SNP, an A/C polymorphism 174 bp upstream of CG18012 and 241 upstream of tincar with a female-specific effect on food intake (female P-value = 5.14 × 10−6; male P-value = 2.11 × 10−4, S2 Table) to functionally validate in outbred genetic backgrounds. We selected this SNP based on its P-value and a minimum of ten minor-allele-containing DGRP lines that we had measured. We created five different F1 genotypes from 10 DGRP lines homozygous for the minor allele (C) and five F1 genotypes from 10 DGRP lines homozygous for the major allele (A), and measured their food intake (Fig 3A, S8 Table). These lines have randomized genetic backgrounds with the exception of the target polymorphism. We indeed confirmed a significant (P = 0.029; Fig 3B) female-specific effect of this polymorphism on food consumption in the predicted direction from the GWA analysis, with the major allele associated with an increase in food intake. However, the magnitude of this effect (0.36 μL), was not as large as the effect from the GWA analysis (1.37 μL). This discrepancy of effect size can be attributed to the Beavis Effect [89, 90], whereby the effect sizes from GWA analyses are overestimated as the predicted effects are generated from quantitative trait loci (QTL) that reach a critical, predetermined threshold; the distribution of QTL from which effect size is predicted is, therefore, truncated, creating an upward bias in predicted effects [89–91].
Fig 3. SNP-based functional validation.
(A) Mating scheme for generating outbred lines homozygous for major and minor SNP alleles. Two parental genotypes homozygous for the focal highlighted SNP and the tested F1 genotype are depicted. (B) 3R_13637022_SNP validation results for females (red bars) and males (blue bars) (± SE). *: P = 0.029.
RNAi functional validation
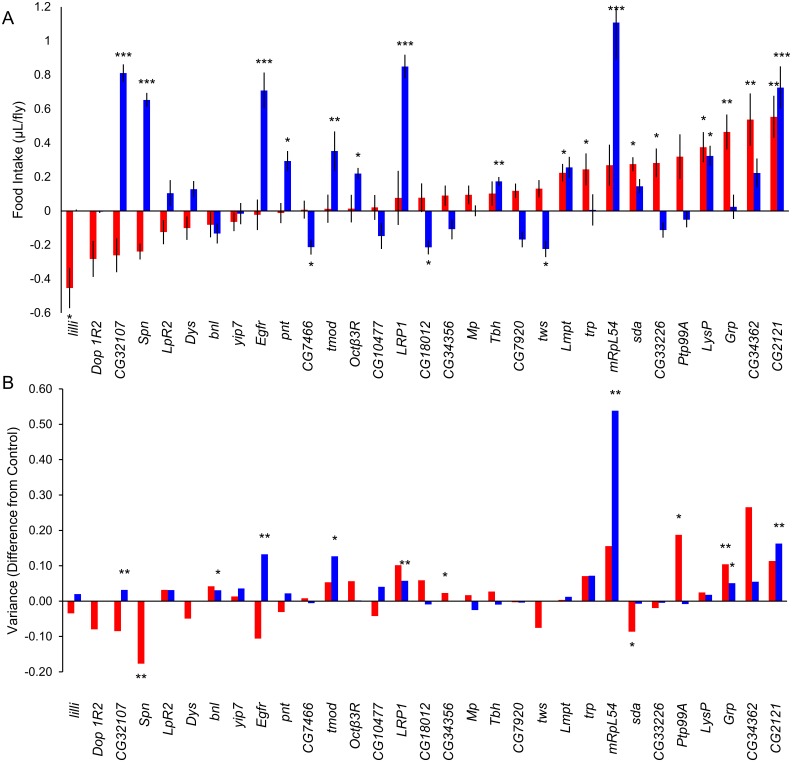
We selected 31 genes for functional validation using RNAi knockdown and a weak, ubiquitous GAL4 driver, Ubi156-GAL4. We chose these genes based on the availability of RNAi lines, patterns of adult gene expression in Drosophila (ubiquitous, or in gut, fat body, and/or brain), the existence of a human ortholog (S9 Table) and/or involvement in feeding-related processes (S6 Table). The weaker Ubi156-GAL4 driver line was used to prevent lethality often observed with stronger ubiquitin promoters, while still allowing ubiquitous expression. Although the top candidate genes from the mean and CV E GWA analyses are largely distinct, this is a consequence of using CV E as the metric for the within-line variance, as it ameliorates the dependency of the variance on the mean. However, there is a real relationship between mean and within-line variance of food consumption; therefore, we assessed both traits with the RNAi lines and controls. In total, 24 (77%) of the tested RNAi knockdown alleles affected the mean and/or within line variance relative to the control: 21 genes affected the mean, 12 affected the variance, and nine affected both the mean and variance, suggesting at least partial joint regulation of mean and within line variance of food intake (Fig 4, S9 Table). The effects of RNAi knockdown alleles on the mean and variance of food intake were highly sex-specific, as expected from the quantitative genetic and GWA analyses, and elicited both increases and decreases in both traits, suggesting RNAi knockdown does not result in ‘sick’ flies. The functionally validated genes affecting mean and/or within line variance of food intake included octopaminergic genes [Octopamine β3 receptor (Octβ3R) and Tyramine β hydroxylase (Tbh)]; EGF signaling genes [Epidermal growth factor receptor (Egfr), pointed (pnt), LDL receptor protein 1 (LRP1), and CG7466]; a hydrolase (CG33226); phosphatases [Spn, twins (tws), and Protein tyrosine phosphatase 99A (Ptp99A)]; and a calcium channel [transient receptor potential (trp)] (Fig 4, S9 Table, S10 Table).
Fig 4. RNAi functional validation.
Effects of RNAi knock down on (A) mean food intake and (B) within-genotype variance of food intake of females (red bars) and males (blue bars) for 31 candidate genes. All values are deviations from the control. Error bars are ± SE. *: P < 0.05; **: P < 0.01; ***: P < 0.0001.
Discussion
The genetic architecture of food consumption
There is substantial natural variation in food intake both within and between the sexes in the DGRP, ranging from the lowest intake in males from DGRP_307, which consume less than half a microliter over 24 hours, to the largest intake in females from DGRP_42, which consume more than four times that volume in the same time frame. The genetic architecture of mean food consumption from quantitative genetic analyses of feeding behavior in the DGRP, GWA analyses and functional validation studies is strikingly sex-specific. Perhaps surprisingly, natural variation in food intake is not highly correlated with organismal phenotypes related to body size and metabolism. Although there is a significant correlation with starvation resistance, less than 10% of the total phenotypic variation in food consumption can be explained by this association.
In addition to variation in mean food consumption, we show significant genetic variation in within-line environmental variance of food intake, adding to the growing list of quantitative traits for which there is genetic control of the magnitude of environmental variance [65–71]. We show for the first time in Drosophila that micro-environmental variance of food intake is heritable and that examining its genetic basis can yield insights into the regulation of both mean and micro-environmental variance of food intake. We tested 31 candidate genes from the GWA analyses for the mean and CV E of food intake, and validated 24 (77%) of them. The validations were not always for the trait for which they were predicted by GWA. We speculate this is because the mean and variance of food consumption are correlated. Our functional validations were for the mean and variance; whereas the GWA was for the CV E. A common biological mechanism may underlie both feeding traits, which is not always true for the mean and micro-environmental plasticity of other Drosophila quantitative traits [92]. Note also that the direction, magnitude, and sex-specificity of the effects predicted by natural variants in the GWA analyses were not the same as for the effects of RNAi knockdown. This is not unexpected because the RNAi analyses test whether reducing gene expression affects feeding behavior, whereas the variants implicated by GWA may affect the trait via other mechanisms or may be associated with increased gene expression. In addition, the genetic background of the lines used for RNAi is different from the DGRP lines. Nevertheless, we can conclude from significant effects of RNAi knockdown on feeding behavior that these genes affect the traits when their function is compromised. In the future, more precise validations will be possible by CRISPR/Cas9 allelic replacements in the DGRP lines.
Previous studies examining the genetic underpinnings of food intake in D. melanogaster revealed interconnected sensory inputs, signaling pathways, and feedback loops integrated in the central nervous system [31–33, 35–45, 49–60, 93–105]. The GWA analyses for the mean and environmental variation of food intake complement these studies and identify many novel candidate genes. Two of the functionally validated genes affecting octopaminergic signaling, Tbh and Octβ3R, have been implicated previously to affect feeding behavior using different assays [83, 85, 86]; and a third functionally validated gene, pnt, has been implicated in feeding behavior by showing that mitochondrial biogenesis is influenced by larval nutrition [84]. The remaining functionally validated genes have no prior associations with food consumption.
SNP functional validation and CG18012
We functionally validated a female-specific effect of 3R_13637022_SNP on mean food intake. This potential regulatory polymorphism located upstream of tinc and CG18012 could affect either gene. tinc encodes a transmembrane protein expressed in cardioblasts [106] and has no known mammalian orthologs [107]. CG18012 was also validated to affect mean food consumption in males using RNAi. The human ortholog of CG18012 is Chitobiosyldiphosphodolichol beta-mannosyltransferase (ALG1) (S10 Table) [107], which catalyzes the mannosylation step of lipid-linked oligosaccharide biosynthesis [108]. Mutations in ALG1 can result in congenital defects of glycosylation, caused by deficient mannosyltransferase [109]. Characteristics of the congenital defect include abnormal adipose distributions and aberrant blood coagulation resulting in thromboses and hemorrhages [109]. Thus, 3R_13637022_SNP most likely affects the gene to which it is most proximal, CG18012, although further work is necessary to understand the underlying mechanism. Note that the experimental design to test additive effects of SNPs implicated by GWA analyses in outbred DGRP genotypes can be generalized to any variant, including those in gene deserts. The observation that the SNP effect was female-specific and the RNAi-knockdown effect was male-specific is not unusual; different alleles of the same gene typically vary in the magnitude, direction and sex-specific effects on quantitative traits [reviewed in [110]].
Bioamine signaling and feeding behavior
Two functionally validated genes from RNAi knockdown of gene expression were Tyramine β hydroxylase (Tbh) and Octopamine β3 receptor (Octβ3R). These genes both affect octopamine signaling. Tbh converts tyramine to octopamine [111]. Tbh is integral in the response to different sucrose concentrations and the habituation to sucrose in adult D. melanogaster and may affect carbohydrate metabolism since Tbh mutants are starvation resistant [86]. Octβ3R is a G-protein coupled octopamine receptor [112]. Mutations in Octβ3R affect post-starvation feeding in Drosophila larvae, suggesting Octβ3R is required for this response in fasted larvae [83]. The mammalian orthologs of Octβ3R, histamine receptor H2 (HRH2) and 5-hydroxytryptamine receptor 4, G protein-coupled (HTR4) and of Tbh, dopamine beta-hydroxylase (DBH) [107], function in human food ingestion and gastric emptying [113, 114] and rat feeding and glucoregulatory responses [115, 116] and post-prandial decreases in food intake [117], respectively. We functionally validated an effect of CG34362 on food consumption using RNAi. The predicted mammalian ortholog of CG34362 is the RNA metabolic gene TIA1 granule-associated RNA binding protein (TIA1). TIA1 is down regulated in the fatty livers of obese humans and mice fed a high fat diet [118]. TIA1 functions in thyroid hormone regulation, and thyroid hormone signaling is integrated with adrenergic signaling in liver, adipose tissues, and the hypothalamus [reviewed in [119]]. Octopamine is the functional analog of mammalian norepinephrine. These results implicate an evolutionarily conserved pathway integrating metabolic information with bioamine signaling affecting feeding behavior.
EGF signaling and food intake
We expected to find genes affecting insulin-like signaling in the GWA analyses, since aspects of Drosophila feeding are affected by components of the insulin-like signaling pathway [53, 58, 120, 121]. However, we did not observe this, possibly because these genes are under strong purifying selection and do not harbor functional variants at high enough frequencies to be detected by GWA analyses in the DGRP. We did, however, detect and functionally validated four components of EGF signaling, which is known to regulate insulin-like signaling and sensitivity [122–124]. EGF signaling is involved in sleep in D. melanogaster [125–127], but its role in Drosophila food intake has not been demonstrated previously. We show from our RNAi functional validation that the EGF receptor, Egfr, affects Drosophila feeding behavior. The mammalian EGFR and components of the mammalian EGF signaling pathway are known to enhance glucose absorption in the gut [128], induce obesity in ovariectomized [129] and aged female mice [130], are decreased in adipose tissues of calorie restricted mice [131], and are associated with hyperglycemia in humans [132]. Lipid Transfer Protein (LTP) is produced by the Drosophila fat body and conveys information about dietary lipid composition to specific neurons innervating insulin-like-producing cells and thereby affecting insulin signaling [124]. LRP1, which is a promiscuous receptor in EGF signaling, allows LTP to cross the Drosophila blood brain barrier to affect insulin-like signaling [124]. Mammalian LRP1 regulates leptin signaling and energy homeostasis [133], modulates cholesterol metabolism [134], controls adipose tissue generation [135] in the development of obesity [136] with obese adipose tissue upregulation [135, 137], is associated with blood biomarkers of diet-based obesity interventions [138], controls post ingestion lipid transport and glucose regulation [139], and correlates dietary carbohydrate metabolism to metabolic syndrome [140] and fatty acid intake with body mass index [141–143].
The EGF signaling gene pnt encodes an ETS-1 transcription factor; ETS1 and ETS2 orthologs in mammals [107] are activated by EGF signaling [144, 145]. In Drosophila, persistent EGF signaling via pnt induces insulin resistance, downregulating expression of the Insulin-like receptor gene [123]. Further, pnt gene expression is correlated with larval nutrient control of mitochondrial biogenesis [84]. The mammalian orthologs are increased in the serum of obese humans [146], correlate with adipose tissue generation [147], increase obesity-related adipose tissue inflammation [148], are upregulated in diabetes [149], act in the development of diabetic retinopathy [150] and nephropathy [151], and reduce endothelial progenitor cells in diabetes [152]. While the mammalian ortholog of the EGF signaling gene CG7466, multiple EGF-like-domains 8 (MEGF8) [107], did not have a role in feeding or metabolically related mammalian traits, we did functionally validate it in Drosophila. Given the variety of metabolically related mammalian phenotypes these EGF signaling genes affect without directly affecting food consumption, we posit Drosophila as an essential tool in elucidating the roles of EGF signaling genes in directly regulating feeding behavior.
Novel insights in the genetic basis of feeding behavior
Eight of the 24 genes validated using RNAi either have mammalian orthologs with no known function in metabolically related phenotypes (CG2121, mitochondrial ribosomal protein L54 (mRpL54), tropomodulin (tmod), Ptp99A, and CG7466) or have no known mammalian orthologs (CG34356, and Gag related protein (Grp)) [107]. The remaining genes have mammalian orthologs involved in the development of various metabolically related phenotypes and diseases. The orthologs, however, typically do not affect mammalian food intake directly but rather affect the etiology of these complex phenotypes. Future analyses of the mechanisms by which these genes affect Drosophila feeding harbors the potential to better elucidate the means by which various mammalian metabolic phenotypes are also regulated.
branchless (bnl) affects variance of food intake in males. bnl encodes a growth factor that interacts with hedgehog signaling in a variety of developmental processes [153–155]. Hedgehog signaling controls fat development in Drosophila [156, 157] and mice [156] and coordinates fat development with nutrients [158]. The mammalian orthologs of bnl include a variety of fibroblast growth factors (FGFs) (S10 Table) [107]. Treatment with FGF1 restores normal blood sugar levels in a murine model of type 2 diabetes [159], and FGF1 knockout mice develop aggressive type 2 diabetes and aberrant adipose expansion [160]. Additionally, FGF1 is critical for energy homeostasis, and FGF10 plays an essential role in the development of white adipose pads [reviewed in [161]].
CG33226 is nested within Egfr. CG33226 encodes a predicted serine protease of which Granzyme B (GzmB), an immune system enzyme, is the mammalian ortholog [107]. GzmB increases inflammation in type 1 and type 2 diabetes [162] and impairs insulin secretion from islet cells [163] as part of the mechanism by which it kills pancreatic β cells in the development of type 1 diabetes [162, 164].
RNAi against LysP, which encodes the immune system enzyme glycoside hydrolase, affects mean food consumption in males and females. A mammalian ortholog of LysP is Lysozyme (Lyz) [107]. In mammals, high LYZ protein levels negatively correlate with decreased Paneth intestinal cells observed in the development of obesity [165, 166], and Lyz vascular transcript levels are upregulated in athersclerotic humans and obese juvenile swine [167]. RNAi of lilliputian (lilli) affects mean food consumption in females. The human ortholog of lilli is AF4/FMR2 family, member 1 (AFF1) [107], an adipogenic transcription factor that is also upregulated in juvenile obese adipose tissue [168].
We functionally validated Spn, tws, and Ptp99A to affect Drosophila feeding. The mammalian orthologs of these genes are protein phosphatases (S10 Table). The orthologs of Spn, protein phosphatase 1, regulatory subunit 9A (PPP1R9A) and protein phosphatase 1, regulatory subunit 9B (PPP1R9B), are associated with piglet birth weight [169] and pig food intake and human obesity [170]. The mammalian orthologs of tws, protein phosphatase 2, regulatory subunit B, alpha and delta (PPP2R2A and PPP2R2D) [107] dephosphorylate FOXO1 to cause its nuclear import during the oxidative stress accompanying pancreatic β cell death of diabetes [171], associate with increased lipid levels upon high fat feeding [172], and are regulated by microRNA-136 in the aberrant proliferation of vascular smooth muscle cells that accompanies atherosclerosis [173]. The orthologs of Ptp99A are not known to function in mammalian feeding or metabolically related phenotypes, but the novel elucidation of these phosphatases affecting feeding in Drosophila supports further Drosophila-based work to understand the evolutionarily conserved mechanisms by which they act in food intake to better understand how they act in other metabolically related traits.
RNAi of slamdance (sda) affects the mean and variance of food intake in females. The mammalian sda ortholog, alanyl aminopeptidase (ANPEP), is a receptor metalloproteinase. ANPEP has been associated with regulating networks involved in the development of type 2 diabetes [174] and may underlie pancreatic β cell fate during metabolic stress [175]. RNAi of Limpet (Lmpt) affects females food intake. The Lmpt mammalian ortholog is four and a half LIM domains 2 (FHL2) [107], which has been reported to activate Wnt signaling in the pathogenesis of diabetic nephropathy [176].
Transient receptor potential (TRP) channels are ion channels mediating a variety of sensations. In Drosophila, TRPL underlies long-term desensitization to bitter food compounds [32] and painless, the Drosophila TRPA channel, mediates larval post-feeding migration away from food [177] and avoidance of pungent tastants [178]. Here we demonstrate that trp, the Drosophila TRPC channel [107], affects total food intake in females. In mammals, TRPC channels play an important role in platelet function [179–181], affecting thrombosis and platelet aggregation [181]. TRPC3 is upregulated in platelets of type 2 diabetics [182]. TRPC channels at least partially underlie diabetic complications including cardiovascular comorbid pathophysiology via vascular tissue signaling pathway perturbation [183–185] and diabetic nephropathy [186–188].
Conclusions
We have utilized natural variation in the mean and within line variance of food consumption in the DGRP [63, 64] to identify and functionally validate novel genes and a SNP affecting food intake. Most of these genes have mammalian orthologs that are implicated in the development of many metabolically related diseases, such as type 2 diabetes, while not generally affecting food intake volume directly. These results position D. melanogaster as a unique model to elucidate the etiology of these complex phenotypes. Future work to address the tissue specificity of where and how these genes act and interact will shed further light on the extraordinary sexual dimorphism and the genetic bases of food intake and variation of micro-environmental plasticity of food intake. RNAi suppression of 17 of the confirmed candidate genes affecting mean food intake led to an increase in mean food consumption, suggesting that these genes normally act to limit food intake and that many more peptides, genes, and signaling pathways are involved in the attenuation of food intake than in its initiation or continuation [38–50]. The genetic regulation of D. melanogaster food intake behavior is similar to that of humans, further establishing this model as an integral tool for understanding why individuals eat different amounts of food.
Materials and Methods
Drosophila stocks and culture
We used 182 sequenced, wild-derived, inbred DGRP lines (S1 Table) to perform GWA analyses. We obtained RNAi transgenic fly lines (bnl 101377, CG10477 103490, CG18012 20580, CG2121 109845, CG32107 102854, CG33226 102384, CG34356 109790, CG34362 107503, CG7466 42462, CG7920 21577, Dop1R2 105324, Dys 108006, Egfr 107130, Grp 110076, lilli 106142, Lmpt 100716, LpR2 107597, LRP1 109605, LysP 110747, Mp 107319, mRpL54 105729, Octβ3R 101189, pnt 105390, Ptp99A 103931, sda 100215, Spn 105888, Tbh 107070, tmod 108389, trp 1365, tws 104167, yip7 102226) and the corresponding progenitor lines (60000 and 60010) from the Vienna Drosophila RNAi Center (VDRC) [189]. We crossed these lines to a weak, ubiquitously expressed driver, and we crossed the driver to the progenitor RNAi controls (v60000 and v60010) to serve as our experimental controls. To generate the driver, we obtained the Ubiquitin-GAL4 stock from the Bloomington Drosophila Stock Center. We replaced the major chromosomes with isogenic w 1118; Canton-S-B [190] chromosomes, except the X chromosome and chromosome 2 with the driver construct. A new driver stock, Ubiquitin-GAL4[156], was created by introducing the original Ubi-GAL4 transgene onto the Canton-S-B third chromosome using Δ2–3 mediated hopping. The X and second chromosome were then replaced with Canton-S-B chromosomes. The driver stock was generated and provided by Dr. Akihiko Yamamoto. All lines were reared in small mass cultures on cornmeal/molasses/agar medium under standard culture conditions (25°C, 12:12 hour light/dark cycle).
Behavior assays
We used mated 3–7 day old flies in all assays. After an 18-hour period of food deprivation on 1.5% agar, we placed groups of 8 flies from the same sex and genotype in individual vials containing 2 mL 1.5% agar medium and three 5 μL capillary tubes (Kimble Glass Inc.) containing a 4% sucrose solution inserted through a foam plug. Our modified version of the CAFE assay [24] includes the agar medium to prevent desiccation affecting food consumption. We placed the CAFE vials in a transparent plastic container in which high humidity is maintained with open containers of water to minimize evaporation from the capillary tubes. In addition, we assessed evaporation by placing CAFE vials with capillary tubes containing 4% sucrose but without flies in the same humid chamber. We measured total food consumption in each CAFE vial after 24 hours, between 10 A.M. and 12 P.M. We adjusted the total amount of food consumed by the average evaporation that occurred in the control vials and the total number of flies alive at the end of the assay. We performed six replicate assays per sex and DGRP line, and 10–12 replicates per sex and genotype for RNAi knock down and their respective controls and SNP-based crosses.
Quantitative genetics of mean food consumption in the DGRP
We used a linear mixed model to partition variance in mean food consumption across DGRP lines and between sexes with the following model: Y = μ + L + S + LxS + E, where Y is the phenotype, L is the random main effect of line, S is the fixed main effect of sex, and E is the error term. We also fitted reduced models of form Y = μ + L + E for each sex separately. We estimated the broad-sense heritability (H 2) pooled across sexes as H 2 = (σ 2 L + σ 2 SL)/ (σ 2 L + σ 2 SL + σ 2 E), where σ 2 L, σ 2 SL, and σ 2 E are the among-line, sex by line and within-line variance components, respectively. We estimated broad sense heritabilities for each sex as H 2 = σ 2 L/(σ 2 L + σ 2 E). We estimated the cross-sex genetic correlation as r GS = cov MF /σ LM σ LF, where cov MF is the covariance of line means between males and females, and σ LM and σ LF are the among line standard deviations for males and females.
Associations of food intake with other quantitative traits in the DGRP
We computed Pearson’s product moment correlations of line means for food consumption and other quantitative traits previously measured on the DGRP lines.
Quantitative genetics of within-line variation in food consumption in the DGRP
We assessed whether there was heterogeneity of within-line environmental variance in food consumption among the DGRP lines, using both Brown-Forsythe and Levene’s tests [65]. We used the coefficient of environmental variation for each line (CV E = 100σ E/mean), where σ E is the standard deviation of food consumption between replicate assays with each line and mean is the line mean, as our metric of within line environmental variance, to partially account for any dependence of the variance on mean food consumption.
GWA analyses for food consumption
We performed GWA analyses using the 1,920,276 SNPs and indels with minor allele frequencies ≥ 0.05 on the DGRP webserver [64] (dgrp2.gnets.ncsu.edu). We performed four analyses each for the mean and for the CV E of food consumption: the average of females and males, the difference between females and males (for assessing sexual dimorphism in the QTL effects), and females and males separately. These GWA analyses account for effects of Wolbachia infection, cryptic relatedness due to major inversions, and residual polygenic relatedness [64]. We performed gene ontology enrichment and network analyses based on the top (P < 10−5) variants associated with the mean of food consumption using DAVID bioinformatics [87, 88].
Functional validation
We functionally tested genes with top associations using RNAi knock down alleles and their respective control lines. We assessed whether differences in mean food intake between RNAi knock down alleles and the controls were significant using Dunnett’s tests (JMP 10, SAS), separately for males and females. We also assessed significant variation in environmental variance between RNAi knock down alleles and the controls with pairwise Levene’s tests (JMP 10, SAS), separately for males and females.
We performed one SNP-based test (3R_13637022_SNP near tinc and CG18012) using the DGRP lines. We randomly selected ten minor-allele containing DGRP lines and ten major-allele containing DGRP lines. Within each genotype class, we randomly selected five DGRP lines to be the male parent and five to be the female parent, thus producing 10 F1 genotypes that are homozygous for either the focal major or minor alleles and outbred elsewhere. We assessed mean food consumption with 10–12 replicates per sex per F1 genotype, and used t-tests to evaluate significant differences between the major and minor alleles, separately for males and females.
Supporting Information
Red diamonds: females. Blue squares: males.
(PDF)
(A) Raw data. (B) Summary statistics.
(XLSX)
Cells highlighted in yellow depict significant (P < 0.05) correlations. Phenotypic values from the same sources have the same color codes.
(XLSX)
df: degrees of freedom; SS: Type III sums of squares; F: F statistic; AIC: Akaike information criterion. ***: P < 0.001; **: P < 0.01; *: P < 0.05.
(DOCX)
(XLSX)
(XLSX)
Previous associations with feeding related traits are given.
(XLSX)
Only nominally enriched GO categories were observed.
(XLSX)
(A) Summary statistics. (B) Raw data. All units are μL/fly. M: male parent; F: female parent.
(XLSX)
(A) P-values from tests of differences from controls. (B) Raw data. (C) Summary statistics.
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of General Medical Sciences R01 GM45146 to TFCM, and by the National Institute on Alcohol Abuse and Alcoholism R01 AA016560 to TFCM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Goncalves H, Gonzalez DA, Araujo CP, Muniz L, Tavares P, Assuncao MC, et al. Adolescents' perception of causes of obesity: unhealthy lifestyles or heritage? J Adolesc Health. 2012;51(6 Suppl):S46–52. Epub 2013/01/11. S1054-139X(12)00359-X [pii] 10.1016/j.jadohealth.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naja F, Hwalla N, Itani L, Salem M, Azar ST, Zeidan MN, et al. Dietary patterns and odds of Type 2 diabetes in Beirut, Lebanon: a case—control study. Nutr Metab (Lond). 2012;9(1):111 Epub 2012/12/29. 1743-7075-9-111 [pii] 10.1186/1743-7075-9-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azadbakht L, Haghighatdoost F, Karimi G, Esmaillzadeh A. Effect of consuming salad and yogurt as preload on body weight management and cardiovascular risk factors: a randomized clinical trial. Int J Food Sci Nutr. 2012. Epub 2012/12/20. 10.3109/09637486.2012.753039 [DOI] [PubMed] [Google Scholar]
- 4. Harris RH, Sasson G, Mehler PS. Elevation of liver function tests in severe anorexia nervosa. Int J Eat Disord. 2012. Epub 2013/01/03. 10.1002/eat.22073 [DOI] [PubMed] [Google Scholar]
- 5. Strumia R. Eating disorders and the skin. Clin Dermatol. 2013;31(1):80–5. Epub 2012/12/19. S0738-081X(11)00337-3 [pii] 10.1016/j.clindermatol.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 6. Kim WW, Mertz W, Judd JT, Marshall MW, Kelsay JL, Prather ES. Effect of making duplicate food collections on nutrient intakes calculated from diet records. Am J Clin Nutr. 1984;40(6 Suppl):1333–7. [DOI] [PubMed] [Google Scholar]
- 7. Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48(10):373–9. [DOI] [PubMed] [Google Scholar]
- 8. Schoeller DA. Limitations in the assessment of dietary energy intake by self-report. Metabolism. 1995;44(2 Suppl 2):18–22. [DOI] [PubMed] [Google Scholar]
- 9. Kaczkowski CH, Jones PJ, Feng J, Bayley HS. Four-day multimedia diet records underestimate energy needs in middle-aged and elderly women as determined by doubly-labeled water. J Nutr. 2000;130(4):802–5. [DOI] [PubMed] [Google Scholar]
- 10. Seale JL, Klein G, Friedmann J, Jensen GL, Mitchell DC, Smiciklas-Wright H. Energy expenditure measured by doubly labeled water, activity recall, and diet records in the rural elderly. Nutrition. 2002;18(7–8):568–73. [DOI] [PubMed] [Google Scholar]
- 11. Bray GA, Flatt JP, Volaufova J, Delany JP, Champagne CM. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr. 2008;88(6):1504–10. 10.3945/ajcn.2008.26289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champagne CM, Han H, Bajpeyi S, Rood J, Johnson WD, Lammi-Keefe CJ, et al. Day-to-day variation in food intake and energy expenditure in healthy women: the Dietitian II Study. J Acad Nutr Diet. 2013;113(11):1532–8. 10.1016/j.jand.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 13. Basiotis PP, Welsh SO, Cronin FJ, Kelsay JL, Mertz W. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J Nutr. 1987;117(9):1638–41. [DOI] [PubMed] [Google Scholar]
- 14. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. 10.1038/nature08494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Visscher PM, McEvoy B, Yang J. From Galton to GWAS: quantitative genetics of human height. Genet Res (Camb). 2010;92(5–6):371–9. 10.1017/S0016672310000571 [DOI] [PubMed] [Google Scholar]
- 16. Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42(7):570–5. 10.1038/ng.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vattikuti S, Guo J, Chow CC. Heritability and genetic correlations explained by common SNPs for metabolic syndrome traits. PLoS Genet. 2012;8(3):e1002637 10.1371/journal.pgen.1002637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17(R2):R143–50. 10.1093/hmg/ddn268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bharucha KN. The epicurean fly: using Drosophila melanogaster to study metabolism. Pediatr Res. 2009;65(2):132–7. Epub 2008/11/04. 10.1203/PDR.0b013e318191fc68 [DOI] [PubMed] [Google Scholar]
- 20. Lai CQ, Parnell LD, Arnett DK, García-Bailo B, Tsai MY, Kabagambe EK, et al. WDTC1, the ortholog of Drosophila adipose gene, associates with human obesity, modulated by MUFA intake. Obesity (Silver Spring). 2009;17(3):593–600. 10.1038/oby.2008.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063 10.1371/journal.pone.0006063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Itskov PM, Moreira JM, Vinnik E, Lopes G, Safarik S, Dickinson MH, et al. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun. 2014;5:4560 10.1038/ncomms5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng C, Du Y, Alberico T, Seeberger J, Sun X, Zou S. Gender-specific prandial response to dietary restriction and oxidative stress in Drosophila melanogaster . Fly (Austin). 2011;5(3):174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104(20):8253–6. Epub 2007/05/15. 0702726104 [pii] 10.1073/pnas.0702726104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deshpande SA, Carvalho GB, Amador A, Phillips AM, Hoxha S, Lizotte KJ, et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 2014;11(5):535–40. 10.1038/nmeth.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145(1):133–44. 10.1016/j.cell.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lushchak OV, Carlsson MA, Nässel DR. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci. 2015. 10.1007/s00018-015-1884-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swarup S, Morozova TV, Sridhar S, Nokes M, Anholt RR. Modulation of feeding behavior by odorant-binding proteins in Drosophila melanogaster . Chem Senses. 2014;39(2):125–32. 10.1093/chemse/bjt061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ueno K, Kohatsu S, Clay C, Forte M, Isono K, Kidokoro Y. Gsalpha is involved in sugar perception in Drosophila melanogaster . J Neurosci. 2006;26(23):6143–52. 10.1523/JNEUROSCI.0857-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masek P, Scott K. Limited taste discrimination in Drosophila. Proc Natl Acad Sci U S A. 2010;107(33):14833–8. 10.1073/pnas.1009318107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73(5):941–50. 10.1016/j.neuron.2011.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang YV, Raghuwanshi RP, Shen WL, Montell C. Food experience-induced taste desensitization modulated by the Drosophila TRPL channel. Nat Neurosci. 2013;16(10):1468–76. 10.1038/nn.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151(5):1113–25. 10.1016/j.cell.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris DT, Kallman BR, Mullaney BC, Scott K. Representations of Taste Modality in the Drosophila Brain. Neuron. 2015;86(6):1449–60. 10.1016/j.neuron.2015.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108(28):11644–9. 10.1073/pnas.1017096108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Liu G, Yan J, Li B, Cai D. Metabolic learning and memory formation by the brain influence systemic metabolic homeostasis. Nat Commun. 2015;6:6704 10.1038/ncomms7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster . Nat Methods. 2005;2(11):813–5. 10.1038/nmeth798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, Waters C, et al. The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr Biol. 2010;20(11):969–78. 10.1016/j.cub.2010.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kramer JM, Davidge JT, Lockyer JM, Staveley BE. Expression of Drosophila FOXO regulates growth and can phenocopy starvation. BMC Dev Biol. 2003;3:5 10.1186/1471-213X-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2(2):239–49. [DOI] [PubMed] [Google Scholar]
- 41. Jünger MA, Rintelen F, Stocker H, Wasserman JD, Végh M, Radimerski T, et al. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2(3):20 10.1186/1475-4924-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang C, Chin-Sang I, Bendena WG. The FGLamide-allatostatins influence foraging behavior in Drosophila melanogaster . PLoS One. 2012;7(4):e36059 10.1371/journal.pone.0036059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neckameyer WS. A trophic role for serotonin in the development of a simple feeding circuit. Dev Neurosci. 2010;32(3):217–37. 10.1159/000304888 [DOI] [PubMed] [Google Scholar]
- 44. Al-Anzi B, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S. Obesity-blocking neurons in Drosophila. Neuron. 2009;63(3):329–41. 10.1016/j.neuron.2009.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iijima K, Zhao L, Shenton C, Iijima-Ando K. Regulation of energy stores and feeding by neuronal and peripheral CREB activity in Drosophila. PLoS One. 2009;4(12):e8498 10.1371/journal.pone.0008498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schoofs A, Hückesfeld S, Schlegel P, Miroschnikow A, Peters M, Zeymer M, et al. Selection of motor programs for suppressing food intake and inducing locomotion in the Drosophila brain. PLoS Biol. 2014;12(6):e1001893 10.1371/journal.pbio.1001893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Melcher C, Bader R, Pankratz MJ. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J Endocrinol. 2007;192(3):467–72. 10.1677/JOE-06-0066 [DOI] [PubMed] [Google Scholar]
- 48. Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3(9):e305 10.1371/journal.pbio.0030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goergen P, Kasagiannis A, Schiöth HB, Williams MJ. The Drosophila small GTPase Rac2 is required for normal feeding and mating behaviour. Behav Genet. 2014;44(2):155–64. 10.1007/s10519-014-9643-0 [DOI] [PubMed] [Google Scholar]
- 50. Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123–37. 10.1016/j.cell.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr Biol. 2006;16(7):692–6. 10.1016/j.cub.2006.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279(49):50781–9. 10.1074/jbc.M407842200 [DOI] [PubMed] [Google Scholar]
- 53. Hong SH, Lee KS, Kwak SJ, Kim AK, Bai H, Jung MS, et al. Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in Drosophila and mammals. PLoS Genet. 2012;8(8):e1002857 10.1371/journal.pgen.1002857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kapan N, Lushchak OV, Luo J, Nässel DR. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol Life Sci. 2012. 10.1007/s00018-012-1097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A. 2011;108(2):834–9. 10.1073/pnas.1010930108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013. Epub 2013/06/12. nature12208 [pii] 10.1038/nature12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pool AH, Kvello P, Mann K, Cheung SK, Gordon MD, Wang L, et al. Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron. 2014;83(1):164–77. 10.1016/j.neuron.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Y, Luo J, Carlsson MA, Nässel DR. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. J Comp Neurol. 2015. 10.1002/cne.23768 [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Pu Y, Shen P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013;3(3):820–30. 10.1016/j.celrep.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 60. Yang Z, Yu Y, Zhang V, Tian Y, Qi W, Wang L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc Natl Acad Sci U S A. 2015;112(16):5219–24. 10.1073/pnas.1417838112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nässel DR, Kubrak OI, Liu Y, Luo J, Lushchak OV. Factors that regulate insulin producing cells and their output in Drosophila. Front Physiol. 2013;4:252 10.3389/fphys.2013.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Manzo A, Silies M, Gohl DM, Scott K. Motor neurons controlling fluid ingestion in Drosophila. Proc Natl Acad Sci U S A. 2012;109(16):6307–12. 10.1073/pnas.1120305109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482(7384):173–8. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang W, Massouras A, Inoue Y, Peiffer J, Rámia M, Tarone A, et al. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 2014. 10.1101/gr.171546.113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgante F, Sørensen P, Sorensen DA, Maltecca C, Mackay TFC. Genetic Architecture of Micro-Environmental Plasticity in Drosophila melanogaster . Sci Rep. 2015;5 10.1038/srep09785 Available: http://www.nature.com/srep/2015/150506/srep09785/abs/srep09785.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mackay TF, Lyman RF. Drosophila bristles and the nature of quantitative genetic variation. Philos Trans R Soc Lond B Biol Sci. 2005;360(1459):1513–27. 10.1098/rstb.2005.1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harbison ST, McCoy LJ, Mackay TF. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics. 2013;14:281 10.1186/1471-2164-14-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hall MC, Dworkin I, Ungerer MC, Purugganan M. Genetics of microenvironmental canalization in Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2007;104(34):13717–22. 10.1073/pnas.0701936104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sangster TA, Salathia N, Undurraga S, Milo R, Schellenberg K, Lindquist S, et al. HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc Natl Acad Sci U S A. 2008;105(8):2963–8. 10.1073/pnas.0712200105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ordas B, Malvar RA, Hill WG. Genetic variation and quantitative trait loci associated with developmental stability and the environmental correlation between traits in maize. Genet Res (Camb). 2008;90(5):385–95. 10.1017/S0016672308009762 [DOI] [PubMed] [Google Scholar]
- 71. Ansel J, Bottin H, Rodriguez-Beltran C, Damon C, Nagarajan M, Fehrmann S, et al. Cell-to-cell stochastic variation in gene expression is a complex genetic trait. PLoS Genet. 2008;4(4):e1000049 10.1371/journal.pgen.1000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Geiger-Thornsberry GL, Mackay TF. Association of single-nucleotide polymorphisms at the Delta locus with genotype by environment interaction for sensory bristle number in Drosophila melanogaster . Genet Res. 2002;79(3):211–8. [DOI] [PubMed] [Google Scholar]
- 73. Ayroles JF, Carbone MA, Stone EA, Jordan KW, Lyman RF, Magwire MM, et al. Systems genetics of complex traits in Drosophila melanogaster . Nat Genet. 2009;41(3):299–307. 10.1038/ng.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weber AL, Khan GF, Magwire MM, Tabor CL, Mackay TF, Anholt RR. Genome-wide association analysis of oxidative stress resistance in Drosophila melanogaster . PLoS One. 2012;7(4):e34745 10.1371/journal.pone.0034745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jordan KW, Craver KL, Magwire MM, Cubilla CE, Mackay TF, Anholt RR. Genome-wide association for sensitivity to chronic oxidative stress in Drosophila melanogaster . PLoS One. 2012;7(6):e38722 10.1371/journal.pone.0038722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Unckless RL, Rottschaefer SM, Lazzaro BP. A genome-wide association study for nutritional indices in Drosophila. G3 (Bethesda). 2015;5(3):417–25. 10.1534/g3.114.016477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jumbo-Lucioni P, Ayroles JF, Chambers MM, Jordan KW, Leips J, Mackay TF, et al. Systems genetics analysis of body weight and energy metabolism traits in Drosophila melanogaster . BMC Genomics. 2010;11:297 10.1186/1471-2164-11-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lewontin RC. The interaction of selection and linkage. II. Optimum models. Genetics. 1964;50:757–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lerner IM. Genetic Homeostasis. Oliver, Boyd, editors. New York City: Wiley; 1954. [Google Scholar]
- 80. Toshima N, Hara C, Scholz CJ, Tanimura T. Genetic variation in food choice behaviour of amino acid-deprived Drosophila. J Insect Physiol. 2014;69:89–94. 10.1016/j.jinsphys.2014.06.019 [DOI] [PubMed] [Google Scholar]
- 81. Whitaker R, Gil MP, Ding F, Tatar M, Helfand SL, Neretti N. Dietary switch reveals fast coordinated gene expression changes in Drosophila melanogaster . Aging (Albany NY). 2014;6(5):355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zinke I, Schütz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002;21(22):6162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang T, Branch A, Shen P. Octopamine-mediated circuit mechanism underlying controlled appetite for palatable food in Drosophila. Proc Natl Acad Sci U S A. 2013;110(38):15431–6. 10.1073/pnas.1308816110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Baltzer C, Tiefenböck SK, Marti M, Frei C. Nutrition controls mitochondrial biogenesis in the Drosophila adipose tissue through Delg and cyclin D/Cdk4. PLoS One. 2009;4(9):e6935 10.1371/journal.pone.0006935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol. 2015;25(6):751–8. 10.1016/j.cub.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Scheiner R, Steinbach A, Claßen G, Strudthoff N, Scholz H. Octopamine indirectly affects proboscis extension response habituation in Drosophila melanogaster by controlling sucrose responsiveness. J Insect Physiol. 2014;69:107–17. 10.1016/j.jinsphys.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 87. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 89.Beavis WD, editor The power and deceit of QTL experiments: lessons from comparative QTL studies. Proceedings of the forty-ninth annual corn and sorghum industry research conference; 1994; Chicago, IL.
- 90. Beavis WD. QTL analyses: power, precision, and accuracy. Molecular dissection of complex traits. 1998:145–62. [Google Scholar]
- 91. Xu S. Theoretical basis of the Beavis effect. Genetics. 2003;165(4):2259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ayroles JF, Buchanan SM, O'Leary C, Skutt-Kakaria K, Grenier JK, Clark AG, et al. Behavioral idiosyncrasy reveals genetic control of phenotypic variability. Proc Natl Acad Sci U S A. 2015;112(21):6706–11. 10.1073/pnas.1503830112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Belle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): a major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123(1):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A. 2012;109(10):3967–72. 10.1073/pnas.1200778109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gasque G, Conway S, Huang J, Rao Y, Vosshall LB. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci Rep. 2013;3:srep02120 10.1038/srep02120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20(11):1000–5. 10.1016/j.cub.2010.03.061 [DOI] [PubMed] [Google Scholar]
- 97. Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, et al. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr Biol. 2008;18(14):1062–6. 10.1016/j.cub.2008.06.059 [DOI] [PubMed] [Google Scholar]
- 98. Kubrak OI, Kučerová L, Theopold U, Nässel DR. The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS One. 2014;9(11):e113051 10.1371/journal.pone.0113051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mueller LD, Barter TT. A model of the evolution of larval feeding rate in Drosophila driven by conflicting energy demands. Genetica. 2015;143(1):93–100. 10.1007/s10709-015-9818-5 [DOI] [PubMed] [Google Scholar]
- 100. Luo J, Lushchak OV, Goergen P, Williams MJ, Nässel DR. Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS One. 2014;9(6):e99732 10.1371/journal.pone.0099732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kaun KR, Riedl CA, Chakaborty-Chatterjee M, Belay AT, Douglas SJ, Gibbs AG, et al. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J Exp Biol. 2007;210(Pt 20):3547–58. 10.1242/jeb.006924 [DOI] [PubMed] [Google Scholar]
- 102. Masek P, Worden K, Aso Y, Rubin GM, Keene AC. A Dopamine-modulated neural circuit regulating aversive taste memory in Drosophila. Curr Biol. 2015. 10.1016/j.cub.2015.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ostojic I, Boll W, Waterson MJ, Chan T, Chandra R, Pletcher SD, et al. Positive and negative gustatory inputs affect Drosophila lifespan partly in parallel to dFOXO signaling. Proc Natl Acad Sci U S A. 2014;111(22):8143–8. 10.1073/pnas.1315466111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J Exp Biol. 2007;210(Pt 8):1424–34. 10.1242/jeb.02755 [DOI] [PubMed] [Google Scholar]
- 105. Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101(6):647–56. [DOI] [PubMed] [Google Scholar]
- 106. Hirota Y, Sawamoto K, Okano H. tincar encodes a novel transmembrane protein expressed in the Tinman-expressing cardioblasts of Drosophila. Mechanisms of Development. 2002;119(Suppl. 1):S279–S83. [DOI] [PubMed] [Google Scholar]
- 107. Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 2015;43(Database issue):D1079–85. 10.1093/nar/gku1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jaeken J, Lefeber D, Matthijs G. Clinical utility gene card for: ALG1 defective congenital disorder of glycosylation. Eur J Hum Genet. 2015. 10.1038/ejhg.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morava E, Vodopiutz J, Lefeber DJ, Janecke AR, Schmidt WM, Lechner S, et al. Defining the phenotype in congenital disorder of glycosylation due to ALG1 mutations. Pediatrics. 2012;130(4):e1034–9. 10.1542/peds.2011-2711 [DOI] [PubMed] [Google Scholar]
- 110. Mackay TF. Mutations and quantitative genetic variation: lessons from Drosophila. Philos Trans R Soc Lond B Biol Sci. 2010;365(1544):1229–39. 10.1098/rstb.2009.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhou C, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008;11(9):1059–67. 10.1038/nn.2164 [DOI] [PubMed] [Google Scholar]
- 112. Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94(2):547–60. 10.1111/j.1471-4159.2005.03251.x [DOI] [PubMed] [Google Scholar]
- 113. Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134(7):1842–60. 10.1053/j.gastro.2008.05.021 [DOI] [PubMed] [Google Scholar]
- 114. Ozawa T, Yoshikawa N, Tomita T, Akita Y, Mitamura K. The influence of feeding on gastric acid suppression in Helicobacter pylori-positive patients treated with a proton pump inhibitor or an H2-receptor antagonist after bleeding from a gastric ulcer. J Gastroenterol. 2003;38(9):844–8. 10.1007/s00535-003-1159-y [DOI] [PubMed] [Google Scholar]
- 115. Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-beta-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology. 2006;147(7):3428–34. 10.1210/en.2006-0235 [DOI] [PubMed] [Google Scholar]
- 116. Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89(4):490–500. 10.1016/j.physbeh.2006.05.036 [DOI] [PubMed] [Google Scholar]
- 117. Potes CS, Turek VF, Cole RL, Vu C, Roland BL, Roth JD, et al. Noradrenergic neurons of the area postrema mediate amylin's hypophagic action. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R623–31. 10.1152/ajpregu.00791.2009 [DOI] [PubMed] [Google Scholar]
- 118. Pihlajamäki J, Boes T, Kim EY, Dearie F, Kim BW, Schroeder J, et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94(9):3521–9. 10.1210/jc.2009-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–82. 10.1152/physrev.00030.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Söderberg JA, Carlsson MA, Nässel DR. Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, Drosulfakinin. Front Endocrinol (Lausanne). 2012;3:109 10.3389/fendo.2012.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ryuda M, Tsuzuki S, Matsumoto H, Oda Y, Tanimura T, Hayakawa Y. Identification of a novel gene, anorexia, regulating feeding activity via insulin signaling in Drosophila melanogaster . J Biol Chem. 2011;286(44):38417–26. 10.1074/jbc.M111.267344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. McNeill H, Craig GM, Bateman JM. Regulation of neurogenesis and epidermal growth factor receptor signaling by the insulin receptor/target of rapamycin pathway in Drosophila. Genetics. 2008;179(2):843–53. 10.1534/genetics.107.083097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang W, Thompson BJ, Hietakangas V, Cohen SM. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet. 2011;7(12):e1002429 10.1371/journal.pgen.1002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brankatschk M, Dunst S, Nemetschke L, Eaton S. Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling. Elife. 2014;3 10.7554/eLife.02862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Potdar S, Sheeba V. Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. J Neurogenet. 2013;27(1–2):23–42. 10.3109/01677063.2013.791692 [DOI] [PubMed] [Google Scholar]
- 126. Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10(9):1160–7. 10.1038/nn1957 [DOI] [PubMed] [Google Scholar]
- 127. Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324(5923):105–8. 10.1126/science.1166657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bird AR, Croom WJ Jr., Fan YK, Daniel LR, Black BL, McBride BW, et al. Jejunal glucose absorption is enhanced by epidermal growth factor in mice. J Nutr. 1994;124(2):231–40. Epub 1994/02/01. [DOI] [PubMed] [Google Scholar]
- 129. Kurachi H, Adachi H, Ohtsuka S, Morishige K, Amemiya K, Keno Y, et al. Involvement of epidermal growth factor in inducing obesity in ovariectomized mice. Am J Physiol. 1993;265(2 Pt 1):E323–31. Epub 1993/08/01. [DOI] [PubMed] [Google Scholar]
- 130. Adachi H, Kurachi H, Homma H, Adachi K, Imai T, Sakata M, et al. Involvement of epidermal growth factor in inducing adiposity of age female mice. J Endocrinol. 1995;146(3):381–93. Epub 1995/09/01. [DOI] [PubMed] [Google Scholar]
- 131. Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila). 2008;1(1):65–76. 10.1158/1940-6207.CAPR-08-0022 [DOI] [PubMed] [Google Scholar]
- 132. Memon AA, Bennet L, Zöller B, Wang X, Palmer K, Sundquist K, et al. Circulating human epidermal growth factor receptor 2 (HER2) is associated with hyperglycaemia and insulin resistance. J Diabetes. 2015;7(3):369–77. 10.1111/1753-0407.12184 [DOI] [PubMed] [Google Scholar]
- 133. Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, Herz J, et al. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9(1):e1000575 10.1371/journal.pbio.1000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56(1):66–78. 10.1016/j.neuron.2007.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D, et al. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One. 2009;4(10):e7422 10.1371/journal.pone.0007422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Constantinou C, Mpatsoulis D, Natsos A, Petropoulou PI, Zvintzou E, Traish AM, et al. The low density lipoprotein receptor modulates the effects of hypogonadism on diet-induced obesity and related metabolic perturbations. J Lipid Res. 2014;55(7):1434–47. 10.1194/jlr.M050047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Clemente-Postigo M, Queipo-Ortuño MI, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones FJ, Cardona F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 2011;6(9):e24783 10.1371/journal.pone.0024783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Konieczna J, Sánchez J, van Schothorst EM, Torrens JM, Bunschoten A, Palou M, et al. Identification of early transcriptome-based biomarkers related to lipid metabolism in peripheral blood mononuclear cells of rats nutritionally programmed for improved metabolic health. Genes Nutr. 2014;9(1):366 10.1007/s12263-013-0366-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, et al. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117(11):3271–82. 10.1172/JCI31929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Delgado-Lista J, Perez-Martinez P, Solivera J, Garcia-Rios A, Perez-Caballero AI, Lovegrove JA, et al. Top single nucleotide polymorphisms affecting carbohydrate metabolism in metabolic syndrome: from the LIPGENE study. J Clin Endocrinol Metab. 2014;99(2):E384–9. 10.1210/jc.2013-3165 [DOI] [PubMed] [Google Scholar]
- 141. Smith CE, Tucker KL, Lee YC, Lai CQ, Parnell LD, Ordovás JM. Low-density lipoprotein receptor-related protein 1 variant interacts with saturated fatty acids in Puerto Ricans. Obesity (Silver Spring). 2013;21(3):602–8. 10.1002/oby.20001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Smith CE, Ngwa J, Tanaka T, Qi Q, Wojczynski MK, Lemaitre RN, et al. Lipoprotein receptor-related protein 1 variants and dietary fatty acids: meta-analysis of European origin and African American studies. Int J Obes (Lond). 2013;37(9):1211–20. 10.1038/ijo.2012.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Frazier-Wood AC, Kabagambe EK, Borecki IB, Tiwari HK, Ordovas JM, Arnett DK. Preliminary evidence for an association between LRP-1 genotype and body mass index in humans. PLoS One. 2012;7(2):e30732 10.1371/journal.pone.0030732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. O'Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78(1):137–47. [DOI] [PubMed] [Google Scholar]
- 145. Brunner D, Dücker K, Oellers N, Hafen E, Scholz H, Klämbt C. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370(6488):386–9. 10.1038/370386a0 [DOI] [PubMed] [Google Scholar]
- 146. Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46(11):1483–8. 10.1007/s00125-003-1221-6 [DOI] [PubMed] [Google Scholar]
- 147. Birsoy K, Berry R, Wang T, Ceyhan O, Tavazoie S, Friedman JM, et al. Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development. 2011;138(21):4709–19. 10.1242/dev.067710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kulyté A, Belarbi Y, Lorente-Cebrián S, Bambace C, Arner E, Daub CO, et al. Additive effects of microRNAs and transcription factors on CCL2 production in human white adipose tissue. Diabetes. 2014;63(4):1248–58. 10.2337/db13-0702 [DOI] [PubMed] [Google Scholar]
- 149. Vairaktaris E, Goutzanis L, Kalokerinos G, Yannopoulos A, Yapijakis C, Vassiliou S, et al. Diabetes increases both N-ras and ets-1 expression during rat oral oncogenesis resulting in enhanced cell proliferation and metastatic potential. In Vivo. 2007;21(4):615–21. [PubMed] [Google Scholar]
- 150. Du ZJ, Kamei M, Suzuki M, Tano Y, Wang BR, Hui YN. Coordinated expression of Ets-1, pERK1/2, and VEGF in retina of streptozotocin-induced diabetic rats. Ophthalmic Res. 2007;39(4):224–31. 10.1159/000104831 [DOI] [PubMed] [Google Scholar]
- 151. Kato M, Dang V, Wang M, Park JT, Deshpande S, Kadam S, et al. TGF-β induces acetylation of chromatin and of Ets-1 to alleviate repression of miR-192 in diabetic nephropathy. Sci Signal. 2013;6(278):ra43 10.1126/scisignal.2003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Seeger FH, Chen L, Spyridopoulos I, Altschmied J, Aicher A, Haendeler J. Downregulation of ETS rescues diabetes-induced reduction of endothelial progenitor cells. PLoS One. 2009;4(2):e4529 10.1371/journal.pone.0004529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Barrett AL, Krueger S, Datta S. Branchless and Hedgehog operate in a positive feedback loop to regulate the initiation of neuroblast division in the Drosophila larval brain. Dev Biol. 2008;317(1):234–45. 10.1016/j.ydbio.2008.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Butí E, Mesquita D, Araújo SJ. Hedgehog is a positive regulator of FGF signalling during embryonic tracheal cell migration. PLoS One. 2014;9(3):e92682 10.1371/journal.pone.0092682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mukherjee T, Choi I, Banerjee U. Genetic analysis of fibroblast growth factor signaling in the Drosophila eye. G3 (Bethesda). 2012;2(1):23–8. 10.1534/g3.111.001495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140(1):148–60. 10.1016/j.cell.2009.12.027 [DOI] [PubMed] [Google Scholar]
- 157. Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3(1):25–34. 10.1016/j.cmet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 158. Rodenfels J, Lavrynenko O, Ayciriex S, Sampaio JL, Carvalho M, Shevchenko A, et al. Production of systemically circulating Hedgehog by the intestine couples nutrition to growth and development. Genes Dev. 2014;28(23):2636–51. 10.1101/gad.249763.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513(7518):436–9. 10.1038/nature13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, et al. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485(7398):391–4. 10.1038/nature10998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ohta H, Itoh N. Roles of FGFs as Adipokines in Adipose Tissue Development, Remodeling, and Metabolism. Front Endocrinol (Lausanne). 2014;5:18 10.3389/fendo.2014.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Thomas HE, Trapani JA, Kay TW. The role of perforin and granzymes in diabetes. Cell Death Differ. 2010;17(4):577–85. 10.1038/cdd.2009.165 [DOI] [PubMed] [Google Scholar]
- 163. Estella E, McKenzie MD, Catterall T, Sutton VR, Bird PI, Trapani JA, et al. Granzyme B-mediated death of pancreatic beta-cells requires the proapoptotic BH3-only molecule bid. Diabetes. 2006;55(8):2212–9. 10.2337/db06-0129 [DOI] [PubMed] [Google Scholar]
- 164. Thomas HE, Kay TW. Intracellular pathways of pancreatic β-cell apoptosis in type 1 diabetes. Diabetes Metab Res Rev. 2011;27(8):790–6. 10.1002/dmrr.1253 [DOI] [PubMed] [Google Scholar]
- 165. Hodin CM, Verdam FJ, Grootjans J, Rensen SS, Verheyen FK, Dejong CH, et al. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J Pathol. 2011;225(2):276–84. 10.1002/path.2917 [DOI] [PubMed] [Google Scholar]
- 166. Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, et al. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am J Physiol Gastrointest Liver Physiol. 2015;308(2):G100–11. 10.1152/ajpgi.00287.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Padilla J, Jenkins NT, Lee S, Zhang H, Cui J, Zuidema MY, et al. Vascular transcriptional alterations produced by juvenile obesity in Ossabaw swine. Physiol Genomics. 2013;45(11):434–46. 10.1152/physiolgenomics.00038.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Aguilera CM, Gomez-Llorente C, Tofe I, Gil-Campos M, Cañete R, Gil Á. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int J Mol Sci. 2015;16(4):7723–37. 10.3390/ijms16047723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhang L, Zhou X, Michal JJ, Ding B, Li R, Jiang Z. Genome wide screening of candidate genes for improving piglet birth weight using high and low estimated breeding value populations. Int J Biol Sci. 2014;10(3):236–44. 10.7150/ijbs.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Do DN, Strathe AB, Ostersen T, Jensen J, Mark T, Kadarmideen HN. Genome-wide association study reveals genetic architecture of eating behavior in pigs and its implications for humans obesity by comparative mapping. PLoS One. 2013;8(8):e71509 10.1371/journal.pone.0071509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yan L, Guo S, Brault M, Harmon J, Robertson RP, Hamid R, et al. The B55α-containing PP2A holoenzyme dephosphorylates FOXO1 in islet β-cells under oxidative stress. Biochem J. 2012;444(2):239–47. 10.1042/BJ20111606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Joseph BK, Liu HY, Francisco J, Pandya D, Donigan M, Gallo-Ebert C, et al. Inhibition of AMP Kinase by the Protein Phosphatase 2A Heterotrimer, PP2APpp2r2d. J Biol Chem. 2015;290(17):10588–98. 10.1074/jbc.M114.626259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang CF, Kang K, Li XM, Xie BD. MicroRNA-136 Promotes Vascular Muscle Cell Proliferation Through the ERK1/2 Pathway by Targeting PPP2R2A in Atherosclerosis. Curr Vasc Pharmacol. 2014. [DOI] [PubMed] [Google Scholar]
- 174. Locke JM, Hysenaj G, Wood AR, Weedon MN, Harries LW. Targeted allelic expression profiling in human islets identifies cis-regulatory effects for multiple variants identified by type 2 diabetes genome-wide association studies. Diabetes. 2015;64(4):1484–91. 10.2337/db14-0957 [DOI] [PubMed] [Google Scholar]
- 175. Singh H, Farouk M, Bose BB, Singh P. Novel genes underlying beta cell survival in metabolic stress. Bioinformation. 2013;9(1):37–41. 10.6026/97320630009037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Li SY, Huang PH, Tarng C, Lin TP, Yang WC, Chang YH, et al. Four-and-a-Half LIM Domains Protein 2 Is a Coactivator of Wnt Signaling in Diabetic Kidney Disease. J Am Soc Nephrol. 2015. 10.1681/ASN.2014100989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Xu J, Sornborger AT, Lee JK, Shen P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat Neurosci. 2008;11(6):676–82. 10.1038/nn.2119 [DOI] [PubMed] [Google Scholar]
- 178. Al-Anzi B, Tracey WD, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16(10):1034–40. 10.1016/j.cub.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 179. Dionisio N, Redondo PC, Jardin I, Rosado JA. Transient receptor potential channels in human platelets: expression and functional role. Curr Mol Med. 2012;12(10):1319–28. [DOI] [PubMed] [Google Scholar]
- 180. Ramanathan G, Gupta S, Thielmann I, Pleines I, Varga-Szabo D, May F, et al. Defective diacylglycerol-induced Ca2+ entry but normal agonist-induced activation responses in TRPC6-deficient mouse platelets. J Thromb Haemost. 2012;10(3):419–29. 10.1111/j.1538-7836.2011.04596.x [DOI] [PubMed] [Google Scholar]
- 181. Vemana HP, Karim ZA, Conlon C, Khasawneh FT. A critical role for the transient receptor potential channel type 6 in human platelet activation. PLoS One. 2015;10(4):e0125764 10.1371/journal.pone.0125764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zbidi H, López JJ, Amor NB, Bartegi A, Salido GM, Rosado JA. Enhanced expression of STIM1/Orai1 and TRPC3 in platelets from patients with type 2 diabetes mellitus. Blood Cells Mol Dis. 2009;43(2):211–3. 10.1016/j.bcmd.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 183. Mita M, Ito K, Taira K, Nakagawa J, Walsh MP, Shoji M. Attenuation of store-operated Ca2+ entry and enhanced expression of TRPC channels in caudal artery smooth muscle from Type 2 diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol. 2010;37(7):670–8. 10.1111/j.1440-1681.2010.05373.x [DOI] [PubMed] [Google Scholar]
- 184. Chung AW, Au Yeung K, Chum E, Okon EB, van Breemen C. Diabetes modulates capacitative calcium entry and expression of transient receptor potential canonical channels in human saphenous vein. Eur J Pharmacol. 2009;613(1–3):114–8. 10.1016/j.ejphar.2009.04.029 [DOI] [PubMed] [Google Scholar]
- 185. Wuensch T, Thilo F, Krueger K, Scholze A, Ristow M, Tepel M. High glucose-induced oxidative stress increases transient receptor potential channel expression in human monocytes. Diabetes. 2010;59(4):844–9. 10.2337/db09-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zhang D, Freedman BI, Flekac M, Santos E, Hicks PJ, Bowden DW, et al. Evaluation of genetic association and expression reduction of TRPC1 in the development of diabetic nephropathy. Am J Nephrol. 2009;29(3):244–51. 10.1159/000157627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. He F, Peng F, Xia X, Zhao C, Luo Q, Guan W, et al. MiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia. 2014;57(8):1726–36. 10.1007/s00125-014-3282-0 [DOI] [PubMed] [Google Scholar]
- 188. Chen K, Jin X, Li Q, Wang W, Wang Y, Zhang J. Association of TRPC1 gene polymorphisms with type 2 diabetes and diabetic nephropathy in Han Chinese population. Endocr Res. 2013;38(2):59–68. 10.3109/07435800.2012.681824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–6. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- 190. Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, Patel PH, et al. Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr Biol. 2003;13(16):1388–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red diamonds: females. Blue squares: males.
(PDF)
(A) Raw data. (B) Summary statistics.
(XLSX)
Cells highlighted in yellow depict significant (P < 0.05) correlations. Phenotypic values from the same sources have the same color codes.
(XLSX)
df: degrees of freedom; SS: Type III sums of squares; F: F statistic; AIC: Akaike information criterion. ***: P < 0.001; **: P < 0.01; *: P < 0.05.
(DOCX)
(XLSX)
(XLSX)
Previous associations with feeding related traits are given.
(XLSX)
Only nominally enriched GO categories were observed.
(XLSX)
(A) Summary statistics. (B) Raw data. All units are μL/fly. M: male parent; F: female parent.
(XLSX)
(A) P-values from tests of differences from controls. (B) Raw data. (C) Summary statistics.
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.