Abstract
By enabling a tight control of cell excitation, optogenetics is a powerful approach to study the function of neurons and neural circuits. With its transparent body, a fully mapped nervous system, easily quantifiable behaviors and many available genetic tools, Caenorhabditis elegans is an extremely well-suited model to decipher the functioning logic of the nervous system with optogenetics. Our goal was to establish an efficient dual color optogenetic system for the independent excitation of different neurons in C. elegans. We combined two recently discovered channelrhodopsins: the red-light sensitive Chrimson from Chlamydomonas noctigama and the blue-light sensitive CoChR from Chloromonas oogama. Codon-optimized versions of Chrimson and CoChR were designed for C. elegans and expressed in different mechanosensory neurons. Freely moving animals produced robust behavioral responses to light stimuli of specific wavelengths. Since CoChR was five times more sensitive to blue light than the commonly used ChR2, we were able to use low blue light intensities producing no cross-activation of Chrimson. Thanks to these optogenetics tools, we revealed asymmetric cross-habituation effects between the gentle and harsh touch sensory motor pathways. Collectively, our results establish the Chrimson/CoChR pair as a potent tool for bimodal neural excitation in C. elegans and equip this genetic model organism for the next generation of in vivo optogenetic analyses.
Keywords: avoidance, behavior, mechanoreceptor, optogenetics
BY enabling the real-time manipulation of neural activity, optogenetics has revolutionized the study of neural circuit function. The blue light-activated channel channelrhodopsin-2 (ChR2), whose activation can cause cell depolarization, has been extensively used to activate neurons (Fenno et al. 2011). ChR2 can thereby be used to trigger behavior in transgenic animals, as first demonstrated in the nematode Caenorhabditis elegans (Nagel et al. 2005). C. elegans is an attractive model to decipher the nervous system functioning in vivo, notably because of its fully mapped nervous system, easily quantifiable behaviors, and the availability of powerful genetic tools (Schafer 2005). Moreover, its transparent body and its comparatively low intrinsic responsiveness to light make it an ideal model for optogenetics approaches (see Husson et al. 2013 for a review).
Independently activating different cells is a very useful approach to functionally dissect neural circuits. This can be achieved in whole field illumination by combining blue-sensitive ChR2 with “red-shifted” opsins (Zhang et al. 2008; Yizhar et al. 2011; Prigge et al. 2012; Lin et al. 2013). However, the excitation spectrum of these latter needs to be sufficiently distinct from that of ChR2 to avoid cross-activation. Independent activation of two neuron classes in vivo has been obtained in C. elegans by pairing ChR2 and the green-shifted C1V1 channelrhodopsin (Erbguth et al. 2012). However, the large overlap between their excitation spectra suggests that this opsin pair might not be straightforward to use without the risk of cross-activation or the need to extensively adjust expression levels and/or illumination parameters. Here, we show that two recently discovered channelrhodopsin variants with specific spectral properties can be used in a blue/red dual color setup to achieve bimodal neural activation and trigger avoidance behaviors in C. elegans.
Methods
Chrimson and CoChR gene synthesis and expression plasmid construction
The coding sequences for Chlamydomonas noctigama channelrhodopsin (Chrimson) and Chloromonas oogama channelrhodopsin (CoChR) (Klapoetke et al. 2014) were retrieved from the GenBank/EMBL/DDBJ database (accession nos.: KF992060 and KF992041). To create clones suitable for expression in C. elegans, we (i) optimized the codon usage, (ii) inserted three artificial introns in each clone as described previously (Redemann et al. 2011), and (iii) produced the target clones through gene synthesis (Genewiz, South Plainfield, NJ). The clones were created in the pUC57-kan backbone and were flanked with attL1 and attL2 recombination sites to be used directly as ENTRY vectors in the Multisite Gateway Cloning System (Invitrogen). These Entry clones have been submitted to Addgene. Supporting Information, Table S1 presents the plasmids used in this study.
Expression clones were created through Multisite Gateway recombinations, as reported in Table S1. For expression in the gentle touch receptor neurons (TRNs), we used the mec-4 promoter. For expression in the FLP nociceptor neurons, we used the Q-system based on three transgenes, as previously described (Schild et al. 2014).
We also cloned a C. elegans version of Chronos (Klapoetke et al. 2014) by using the same approach. When expressed in TRN, we observed a lower sensitivity to blue light as compared to ChR2 and these lines were not further characterized (data not shown).
C. elegans strains and growth conditions
The C. elegans strains used in this study are reported in Table S2. The AQ2313 strain was a gift from William R. Schafer (Cambridge, UK). Strains were grown at 20° on nematode growth media (NGM) agar plates with OP50 Escherichia coli as previously described (Stiernagle 2006). For optogenetic experiments, we prepared plates with or without all-trans-retinal (ATR). When ATR was included, we added 0.1% (v/v) of ATR stock (100 mM, in ethanol) to the OP50 bacteria suspension, and seeded each 6-cm NGM agar plate with 250 µl of this mix.
Transgenesis and GFP expression
Transgenic lines were created through microinjection in the gonad according to a standard procedure (Evans 2006). We used an unc-122p::RFP co-injection marker to identify transgenic animals. We initially created transgenic animals in the lite-1(ce314) background, which was previously reported to lack blue light-induced behaviors (Edwards et al. 2008). Most transgenes were maintained as extrachromosomal arrays. We integrated the FLP::CoChR transgene [mec-3p::QF, mec-4p::QS, QUAS::CoChR::GFP, unc-122p::RFP; lite-1(ce314)], using UV irradiation as previously described (Evans 2006), and backcrossed it twice with the parental KG1180 lite-1(ce314) strain to create DAG355. This strain was outcrossed with N2 to remove the lite-1(ce314) mutation, creating DAG356. At the intensities of blue light used for the study (up to 145 W/m2), we were not able to detect any intrinsic behavioral response in the absence of ATR in any strain tested (including in the lite-1(wt) background).
We verified the expression of Chrimson and CoChR thanks to GFPs fused at their C termini. Using an AxioPlan 2 fluorescence microscope (Zeiss) with a ×40 objective (air, N.A. = 0.95), we detected GFP signals in cell bodies and neurites of target neurons (see CoChR::GFP in FLP and Chrimson::GFP in PLM, Figure S2, A and B).
Light stimulation
We designed a simple system for illuminating plates under a stereomicroscope (Leica S6E). We used a projector (BenQ MS513) to project either blue or red light spots of 0.5 sec (single stimulus experiments) or 1 sec (habituation experiments) thanks to a slideshow program (Apache Open Office) on the top of worm-containing NGM agar plates with OP50 bacteria. The intensities of blue and red light stimuli were adjusted by changing the Red Green Blue (RGB) values of the projected spots. We used a spectrometer (Ocean Optics, USB4000) to (i) determine the spectra of “pure” blue and “pure” red in the RGB space as projected by the projector and (ii) calibrate the delivered light intensity (irradiance). The light intensities were periodically verified during the course of the study and did not significantly vary over this period. Blue stimuli corresponded to a ∼400- to 520-nm band and the red stimuli to an ∼600- to 725-nm band (Figure S3).
Behavioral scoring and movie recording
Scoring was performed manually, by observing single first day adult animals exposed to light stimuli. A backward movement of any length observed during a light stimulus was scored as a backing response. For the movie recording, we used a Leica M205FA stereomicroscope equipped with a DFC345FX camera. We used bright field illumination with a GFP filter that did not filter all of the blue light stimuli. This was convenient to visualize the periods of light stimulation in the resulting movie (File S1).
Optogenetics habituation protocol
To create red light-habituated FLP::CoChR;TRN::Chrimson animals, we first verified their blue light responsiveness with a single stimulus and then exposed them to repeated red light stimuli, until three subsequent stimuli gave no response. Habituated animals were then immediately challenged with one blue light stimulus. Similarly, to create blue light-habituated FLP::CoChR;TRN::Chrimson animals, we first verified their red light responsiveness with a single stimulus and then exposed them to repeated blue light stimuli, until three subsequent stimuli gave no response. Habituated animals were then immediately challenged with one red light stimulus.
Results and Discussion
Chrimson activation with red light can trigger C. elegans behavior
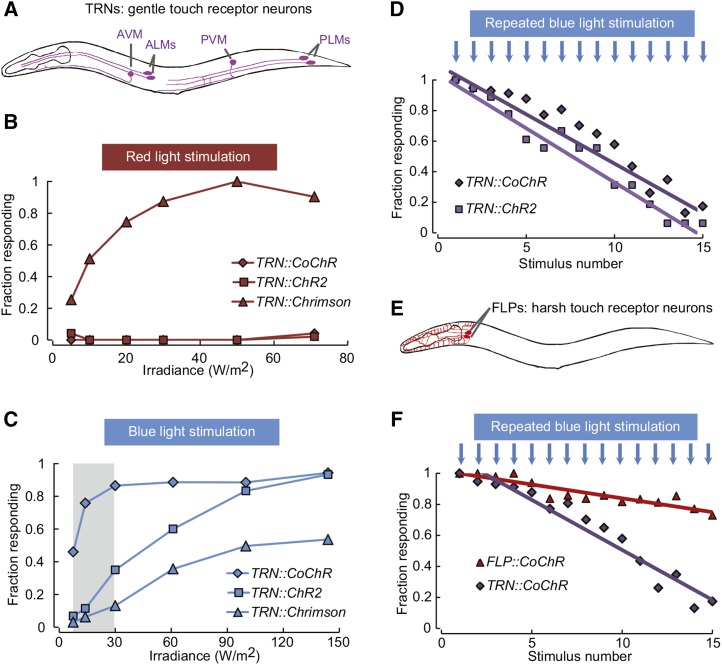
To improve our ability to independently stimulate different neuron populations in vivo, our first goal was to establish a potent red light-sensitive channelrhodopsin variant able to trigger behaviors in C. elegans. We decided to test whether Chrimson, a recently discovered red light-sensitive channelrhodopsin from C. noctigama, could trigger behaviors in C. elegans, as it did in Drosophila (Klapoetke et al. 2014). Based on Chrimson amino acid sequence, we synthesized an artificial chrimson gene suitable for expression in C. elegans. We optimized the codon usage and included three artificial introns, which are known to increase expression in C. elegans (Okkema et al. 1993). We created transgenic animals selectively expressing Chrimson in TRNs by using the mec-4 promoter (Figure 1A). Light-evoked backing behaviors were compared between these newly generated TRN::Chrimson animals and previously reported TRN::ChR2 animals (AQ2313) (Vásquez et al. 2014), which were grown in the presence or the absence of all-trans-retinal (ATR). In TRN::Chrimson animals grown with ATR, red light stimuli triggered robust backing behaviors (half activation irradiance I50 ≈ 10 W/m2, Figure 1B). In contrast, and as expected, red light we did not trigger any response in TRN::ChR2 animals. No response was observed when grown in the absence of ATR (Figure S1A, right panel). These results demonstrate that Chrimson represents a potent red light-activated opsin that is suitable for in vivo neural activation and behavior manipulation in C. elegans.
Figure 1.
Backing behavior induction with Chrimson and CoChR expressed in sensory neurons. (A) Schematic of gentle touch receptor neurons (TRNs). (B and C) Backing behaviors as a function of light intensity in transgenic animals expressing ChR2, CoChR, or Chrimson in TRNs. Each point represents data derived from 40 animals (one trial per animal). (D) Backing behavior habituation to repeated blue light stimuli (30 W/m2 for TRN::CoChR and 145 W/m2 for TRN::ChR2). Five-second interstimulus intervals were used. n = 30 animals per genotype. (E) Schematic of the FLP harsh touch receptor neurons. (F) Backing response habituation to repeated blue light stimuli (30 W/m2) in TRN::CoChR as compared to FLP::CoChR animals. n = 30 animals per genotype.
CoChR is a potent blue light-sensitive channelrhodopsin suitable to trigger behavior in C. elegans
Like other opsins with red-shifted excitation peaks, the excitation spectrum of Chrimson displays a shoulder toward the blue side of the color spectrum (Klapoetke et al. 2014). To determine if this could be problematic in a ChR2/Chrimson dual color setup, we compared the ranges of blue light intensity triggering behavioral responses in TRN::Chrimson and TRN::ChR2 animals. As previously reported, TRN::ChR2 animals produced robust ATR-dependent behaviors in response to blue light (I50 ≈ 50 W/m2, Figure 1C). In comparison, the blue light sensitivity in TRN::Chrimson animals was lower and did not reach 100% responsiveness, even with 145 W/m2 (the maximal intensity in our setup). Nevertheless, the response curves between Chrimson and ChR2 animals were too close to delineate an intensity range leading to a strong ChR2 response without also causing a detectable Chrimson response.
To circumvent this limitation, we searched for a blue light-sensitive channelrhodopsin with a higher sensitivity than ChR2. We decided to test C. oogama channelrhodopsin (CoChR), which was recently discovered among natural channelrhodopsin variants (Klapoetke et al. 2014). In HEK 293FT cells, CoChR produces blue light-evoked photocurrents at least five times higher than ChR2 and is insensitive to red light (Klapoetke et al. 2014). We followed the same strategy as for Chrimson and synthesized a CoChR gene version suitable for expression in C. elegans. To evaluate whether CoChR could trigger robust neural activation, we expressed it in TRNs and assessed light-evoked backing responses. Similarly to TRN::ChR2 animals, and as expected, we observed no behavioral response to red light stimuli in TRN::CoChR (Figure 1B). In contrast, we observed robust behavioral responses to blue light stimuli (I50 ≈10 W/m2, Figure 1C). No response was detected in the absence of ATR (Figure S1, A and B, right panels). These data indicate that CoChR represents a useful alternative to ChR2 to control behavior. Indeed, like in HEK 293FT cells (Klapoetke et al. 2014), CoChR was five times more sensitive to blue light than ChR2 in C. elegans and we identified a range of blue light intensities that can trigger strong response in TRN::CoChR animals without significantly affecting the behavior of TRN::Chrimson animals (shaded area in Figure 1C).
CoChR and ChR2-mediated activation of TRN leads to similar habituation patterns
To further compare CoChR- and ChR2-expressing animals, we evaluated whether both opsins yielded similar behavioral habituation effects in animals repeatedly stimulated with light. Indeed, both repeated touch and optogenetic stimulations cause habituation effects (Rose and Rankin 2001; Husson et al. 2012). In agreement with these previous findings, we noted a steady decrease in the behavioral response of TRN::ChR2 animals and total unresponsiveness after ∼15 trials (Figure 1D). We observed a nearly identical habituation curve in TRN::CoChR animals (Figure 1D). For these experiments, we selected the lowest blue light intensities giving at least 90% of responsiveness in single trial experiments (145 W/m2 for ChR2 and 30 W/m2 for CoChR). In summary, these data indicate that the same habituation pattern can be obtained with either ChR2 or CoChR, with markedly lower light intensities in the latter case.
CoChR-mediated activation of the polymodal nociceptor FLP triggers aversive behaviors
To test whether CoChR could be used in other neurons, we expressed it in the FLP harsh touch nociceptor neurons (Figure 1E) (Li et al. 2011). Since no specific promoter is available for FLP neurons, we used a Q-system-based strategy combining several promoters (Wei et al. 2012; Schild et al. 2014). FLP::CoChR animals grown with ATR produced robust blue light-induced backing behaviors (see File S1 and Figure S1B), but no response to red light (Figure S1A). No response was produced in the absence of ATR (Figure S1, right panels). Next, we exposed FLP::CoChR animals to repeated blue light stimuli and compared their habituation curve to that of TRN::CoChR animals. The habituation in FLP::CoChR was much slower than that in TRN::CoChR animals (Figure 1F), consistent with the previously reported withstanding of nociceptive pathways to habituation (Husson et al. 2012).
Independent control of separate neurons in the same animal with Chrimson and CoChR
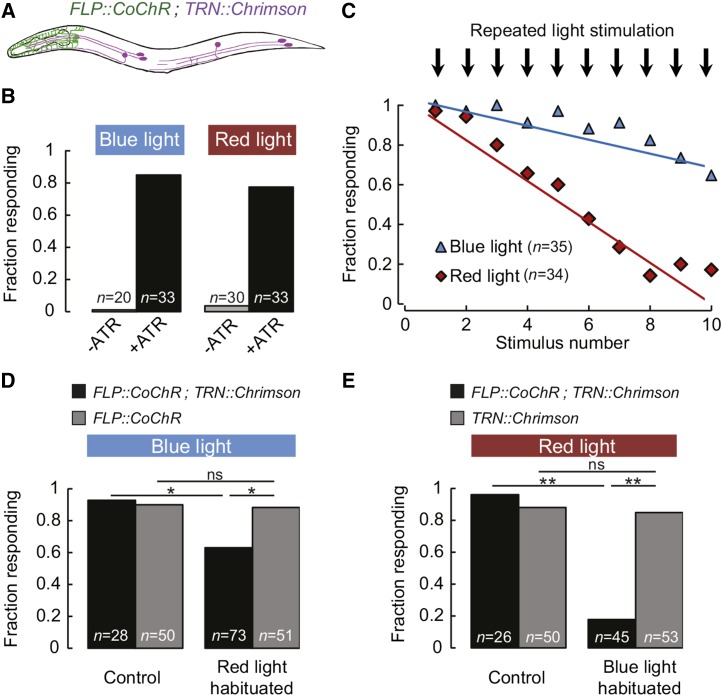
Next, we wanted to test if the Chrimson/CoChR pair could be used in the same animals to independently control two neuron populations. To that end, we created transgenic animals combining the FLP::CoChR and TRN::Chrimson transgenes (Figure 2A). Unlike transgenic animals expressing each single transgene, these double transgene animals produced robust backward locomotion in response to both blue and red light (Figure 2B). The responses were dependent on the presence of ATR in the growth media (Figure 2B). Because the backward locomotion responses triggered by FLP or TRN are very similar, it was important to verify that the behavioral responses induced by blue and red light were indeed mediated by FLP and TRN activation, respectively. To differentiate between them, we used the differential habituation properties of these separate neural pathways. Indeed, TRN-dependent behavior habituates rapidly to repeated stimulations, whereas the nociceptive response mediated by FLP is prone to a much slower habituation process (Figure 1F). We exposed FLP::CoChR;TRN::Chrimson animals to repeated blue or red light stimuli and compared the habituation curves. We confirmed a slow habituation for blue light stimuli and a rapid habituation for red light stimuli, corresponding to FLP::CoChR and TRN::Chrimson, respectively (Figure 2C). Together with the results obtained with animals expressing single transgenes, these data suggest that the Chrimson/CoChR pair can be used in single animals to independently control distinct neurons in a dual color, whole-animal illumination setup.
Figure 2.
Bimodal excitation of separate classes of Chrimson- and CoChR-expressing neurons in single animals. (A) Schematic of an animal carrying the FLP::CoChR;TRN::Chrimson double transgene. (B) Blue and red light-evoked backing behavior in FLP::CoChR;TRN::Chrimson in the presence or absence of ATR. (C) Backing behavior habituation to either repeated blue light stimuli (30 W/m2) or repeated red light stimuli (30 W/m2). Three-second interstimulus intervals were used. Number of animals is indicated. (D and E) Cross-habituation analysis in FLP::CoChR;TRN::Chrimson animals. FLP::CoChR and TRN::Chrimson single transgene control animals were used as indicated. Backing behavior in animals fully habituated with one color and tested with the other color, as compare to control treatment without habituation. Numbers of animals tested are indicated. *P < 0.01; **P < 0.001; ns, not significant by Fisher’s exact tests.
Cross-habituation analysis with dual color optogenetics
To illustrate the utility of the Chrimson/CoChR pair for the analysis of neural function in vivo, we analyzed habituation cross-talks between the harsh and gentle touch neural pathways. Because harsh touch simultaneously stimulates nociceptors (such as FLP) and TRNs, the specific contribution of these neurons in harsh touch-evoked habituation is difficult to address. The FLP::CoChR;TRN::Chrimson animals create an opportunity to decipher the relative contribution of the two types of neurons. First, we habituated the TRN pathway with repeated red light stimulations in FLP::CoChR;TRN::Chrimson animals and evaluated whether FLP activation with blue light was still triggering backing behaviors. In red light-habituated animals, we still observed a response to blue light in 64% of the animals (Figure 2D). However, this fraction was significantly lower than the fraction of responding animals in the nonhabituated control (92%, P < 0.01 by Fisher’s exact test). Thus, habituating the TRN pathway slightly reduces, but does not abolish, FLP-elicited backing behavior. The blue light responsiveness in FLP::CoChR single transgene animals was not affected by repeated red light stimulations (Figure 2D). Second, we performed the mirror experiment and habituated the FLP-mediated behavior with repeated blue light stimuli in FLP::CoChR;TRN::Chrimson animals. When stimulated with red light, blue light-habituated animals produced a drastically reduced response as compared to the unhabituated control (Figure 2E, P < 0.001). Thus, habituating the FLP-dependent harsh touch pathway is sufficient to almost totally abolish the response to TRN activation. The red light responsiveness in TRN::Chrimson single transgene animals was not affected by repeated blue light stimulations (Figure 2E). This indicates that the cross-habituation effect in the double transgene animals is unlikely to result from repeated weak activations of Chrimson by blue light.
Taken together, these findings are consistent with a model in which (i) most of the habituation in the nociceptive pathway is accounted for by downstream interneurons shared by both gentle and harsh touch pathways, and (ii) the habituation in the gentle touch pathway is mainly accounted for by TRN-specific effects and to a lesser extent by habituation effects in common downstream interneurons. While additional experiments are required to identify the relevant interneurons, our results highlight how the dual color optogenetics approach can highlight functional neural interplays within the sensory motor circuit.
Benefits, limitations, and future prospects
The present study brings significant improvements to the C. elegans optogenetics toolbox. Indeed, the C. elegans-optimized version of CoChR created here represents a potent alternative to ChR2. Similarly to earlier observations of photocurrents in HEK 293FT cells (Klapoetke et al. 2014), we observed a fivefold higher sensitivity to light in animals expressing CoChR (I50 ≈10 W/m2) as compared to ChR2 (I50 ≈ 50 W/m2). A higher sensitivity constitutes an important advantage when targeting neurons for which strong promoters are not available. ChR2 has been commonly activated with light intensities between 500 and 5000 W/m2 (Husson et al. 2013). However, blue light stimuli above 500 W/m2 elicit an intrinsic, lite-1-dependent photophobic response (Edwards et al. 2008). Using low blue light intensities with CoChR could remove the contribution of endogenous light-evoked behaviors or the need to use a light-insensitive lite-1 mutant background. This should facilitate the design and/or interpretation of optogenetic experiments. In addition, it may ease the joint use of blue sensitive opsins with red-shifted calcium indicators (Akerboom et al. 2013).
Chrimson had previously been used to control behavior in Drosophila (Klapoetke et al. 2014). Here, we introduce a Chrimson version optimized for expression in C. elegans and show that it triggers robust behavioral responses when activated with red light. One limitation of Chrimson in a dual color setup is that it can also be activated to some extent by blue light. In our experimental setup, the cross-talk between Chrimson and ChR2 prompted us to favor the use of CoChR. However, it should be noted that we have used in this study a simple video projector to produce light stimuli consisting in relatively broad wavelength bands (see Methods). A more sophisticated system design using LED with narrower emission spectra or adding band pass filters to a video projector could improve our ability to selectively activate blue- and red-sensitive opsins. We anticipate that, if narrower wavelength bands are used, the Chrimson/ChR2 pair could become viable in a dual color setup. However, the Chrimson/CoChR pair remains more versatile and its use would become particularly adapted when the illumination properties cannot be fully optimized and/or if high opsin expression levels are more difficult to achieve.
In conclusion, we report here a successful adaptation of Chrimson and CoChR for neural activation and remote behavior control in freely moving C. elegans. To our knowledge, this is the first report where red and blue light can be combined to independently control two neural populations in the same living animal and trigger behavior. We have illustrated the utility of the approach with a cross-habituation analysis in sensory motor pathways. We anticipate that the Chrimson/CoChR pair will permit reseachers to address many open questions about the fundamental functioning of neural circuits and that C. elegans could become an avenue for dual color optogenetics.
Supplementary Material
Acknowledgments
We are grateful to Simon Sprecher for sharing equipment; Miriam B. Goodman, Marc Hammarlund, and Erik Jorgensen for the gift of plasmids; and William R. Schafer for the gift of the AQ2313 strain. Some strains were from the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). This research was supported by a Swiss National Science Foundation professorship (PP00P3_150681) to D.A.G. Author contributions: L.C.S. conducted experiments. D.A.G. designed the study, conducted experiments, analyzed data, and wrote the article.
Footnotes
Communicating editor: P. Sengupta
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.177956/-/DC1.
Literature Cited
- Akerboom J., Carreras Calderón N., Tian L., Wabnig S., Prigge M., et al. , 2013. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Charlie N. K., Milfort M. C., Brown B. S., Gravlin C. N., et al. , 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbguth K., Prigge M., Schneider F., Hegemann P., Gottschalk A., 2012. Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans. PLoS ONE 7: e46827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. C., 2006. Transformation and microinjection (April 6, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.108.1. http://www.wormbook.org. [Google Scholar]
- Fenno L., Yizhar O., Deisseroth K., 2011. The development and application of optogenetics. Annu. Rev. Neurosci. 34: 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson S. J., Costa W. S., S. Wabnig, J. N. Stirman, J. D. Watson et al, 2012. Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr. Biol. 22: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson S. J., Gottschalk A., Leifer A. M., 2013. Optogenetic manipulation of neural activity in C. elegans: from synapse to circuits and behaviour. Biol. Cell 105: 235–250. [DOI] [PubMed] [Google Scholar]
- Klapoetke N. C., Murata Y., Kim S. S., Pulver S. R., Birdsey-Benson A., et al. , 2014. Independent optical excitation of distinct neural populations. Nat. Methods 11: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kang L., Piggott B. J., Feng Z., Xu X. Z. S., 2011. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat. Commun. 2: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Y., Knutsen P. M., Muller A., Kleinfeld D., Tsien R. Y., 2013. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16: 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Brauner M., Liewald J. F., Adeishvili N., Bamberg E., et al. , 2005. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15: 2279–2284. [DOI] [PubMed] [Google Scholar]
- Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A., 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135: 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M., Schneider F., Tsunoda S. P., Shilyansky C., Wietek J., et al. , 2012. Color-tuned channelrhodopsins for multiwavelength optogenetics. J. Biol. Chem. 287: 31804–31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redemann S., Schloissnig S., Ernst S., Pozniakowsky A., Ayloo S., et al. , 2011. Codon adaptation-based control of protein expression in C. elegans. Nat. Methods 8: 250–252. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Rankin C. H., 2001. Analyses of habituation in Caenorhabditis elegans. Learn. Mem. 8: 63–69. [DOI] [PubMed] [Google Scholar]
- Schafer W. R., 2005. Deciphering the neural and molecular mechanisms of C. elegans behavior. Curr. Biol. 15: R723–R729. [DOI] [PubMed] [Google Scholar]
- Schild L. C., L. Zbinden, H. W. Bell, Y. V. Yu, P. Sengupta et al, 2014. The balance between cytoplasmic and nuclear CaM kinase-1 signaling controls the operating range of noxious heat avoidance. Neuron 84: 983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans. WormBook 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez V., Krieg M., Lockhead D., Goodman M. B., 2014. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Reports 6: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Potter C. J., Luo L., Shen K., 2012. Controlling gene expression with the Q repressible binary expression system in Caenorhabditis elegans. Nat. Methods 9: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O., Fenno L. E., Prigge M., Schneider F., Davidson T. J., et al. , 2011. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Prigge M., Beyriere F., Tsunoda S. P., Mattis J., et al. , 2008. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11: 631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.