Abstract
The dendrite of the sensory neuron is surrounded by support cells and is composed of two specialized compartments: the inner segment and the sensory cilium. How the sensory dendrite is formed and maintained is not well understood. Hook-related proteins (HkRP) like Girdin, DAPLE, and Gipie are actin-binding proteins, implicated in actin organization and in cell motility. Here, we show that the Drosophila melanogaster single member of the Hook-related protein family, Girdin, is essential for sensory dendrite formation and function. Mutations in girdin were identified during a screen for fly mutants with no mechanosensory function. Physiological, morphological, and ultrastructural studies of girdin mutant flies indicate that the mechanosensory neurons innervating external sensory organs (bristles) initially form a ciliated dendrite that degenerates shortly after, followed by the clustering of their cell bodies. Importantly, we observed that Girdin is expressed transiently during dendrite morphogenesis in three previously unidentified actin-based structures surrounding the inner segment tip and the sensory cilium. These actin structures are largely missing in girdin mutant. Defects in cilia are observed in other sensory organs such as those mediating olfaction and taste, suggesting that Girdin has a general role in forming sensory dendrites in Drosophila. These suggest that Girdin functions temporarily within the sensory organ and that this function is essential for the formation of the sensory dendrites via actin structures.
Keywords: dendrite; cilium; sensory; degeneration, Girdin
CILIATED sensory neurons are specialized for the transmission of senses such as vision (cones and rods), smell, taste, and proprioception. The dendrite of many of these neurons is composed of two parts: the inner segment and the sensory cilium (Keil 1997; Swaroop et al. 2010) (Figure 1A). The sensory cilium is composed of a connecting cilium, which is a specialized ciliary transition zone (Malicki and Avidor-Reiss 2014), and an outer segment, which houses the sensory transduction machinery (Walker et al. 2000). The main body of the dendrite, referred to as the inner segment, serves as a link between the neuronal cell body and the sensory cilium. Although the sensory cilium is responsible for the initial detection of a sensory stimulus, the inner segment is essential for the transmission of this signal to the neuronal cell body, as well as for the proper formation and positioning of the sensory ending. Another characteristic of ciliated sensory neurons is that the function and formation of their dendrite depends on their interactions with surrounding support cells (Keil 1997; Bonilha 2014). However, how the dendrites of ciliated sensory neurons form their morphology and how support cells function in the formation of the sensory dendrite is poorly understood in molecular terms.
Figure 1.
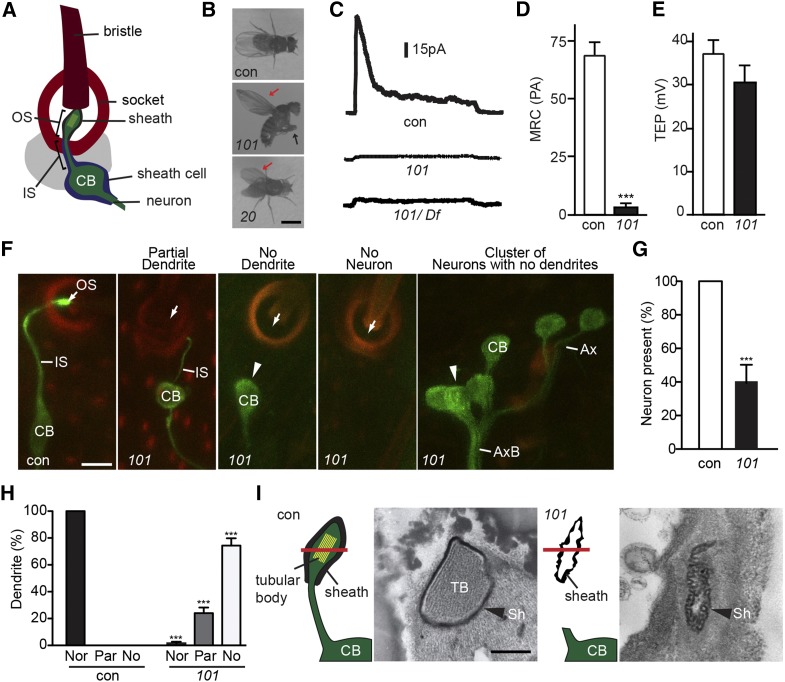
mecA mutants have mechanosensory defects. (A) Illustration of the Drosophila mechanosensory organ. (B) mecA20 and mecA101 hold their wings erect (red arrow). Additionally, mecA101 cannot stand and cross their legs (black arrow). Bar, 2 mm. (C–E) mecA101 homozygotes and mecA101 hemizygotes have an abnormally low MRC (C and D) but normal TEP (E). Shown as mean ± SD; n ≥ 11. ***P < 0.0001. con, control; 101, mecA101. (F–H) mecA101 flies have abnormally short or missing sensory dendrites labeled by GFP-tubulin (F). Sensory outer segments labeled by GFP-tubulin are virtually absent in mecA101 (white arrow). Dendrites labeled by GFP-tubulin are often absent in mecA101 (arrowhead). Green: GFP-tubulin. Red: cuticle autofluorescence. Bar, 5 μm. (G) Quantification of the percentage of mechanosensory neurons in mecA101. Shown as mean ± SD; n = 250. ***P < 0.001. (H) Quantification of the percentage of abnormally short or missing dendrites in mecA101. Shown as mean ± SD; n = 250. ***P < 0.001. Nor: normal dendrite. Par: partially short dendrite. No: no dendrite. (I) Cross-sections of adult mechanosensory organ at the level of outer segment in control (con) and in mecA101 (101). The location of cross-sections is shown by the red bar. Bar, 0.5 μm. CB, cell body; TB, tubular body; Sh, dendritic sheath. (C–E and I) bw;st (control), bw;st;mecA101 (101); F, elav-Gal4;UAS-GFP-tubulin;MKRS/TM6B (control) and elav-Gal4;UAS-GFP-tubulin;mecA101 (101).
Dysfunction or abnormal development of photoreceptor dendrites as well as their associated support cells is an underlying cause of photoreceptor degeneration and blindness (Hong et al. 2000). Yet little is known about the mechanisms underlying these pathological situations. Drosophila melanogaster sensory neurons are an attractive model for human photoreceptor degeneration for the following reasons. First, the sensory neurons in Drosophila that mediate proprioception, taste, and olfaction display similar architecture to human photoreceptors. They are bipolar, with an axon ending in a synaptic zone on one end and a single dendrite on the other end. In addition, the dendrites of these ciliated sensory neurons are composed of an inner segment that terminates in a sensory cilium composed of a connecting cilium and an outer segment on the other end (Figure 1A) (Thurm 1965; Kernan et al. 1994; Keil 1997). Second, mutations affecting the connecting cilium, such as cep290 (den Hollander et al. 2006; Cideciyan et al. 2007; Basiri et al. 2014), and sensory cilium, such as intraflagellar transport (IFT) proteins, have a similar effect in both human photoreceptor and Drosophila sensory function (Avidor-Reiss et al. 2004; Crouse et al. 2014). Third, the connecting cilium of both D. melanogaster mechanosensory neurons and human photoreceptors is uniquely long and serves as the connection between two bulky compartments: the inner and outer segments, which display much greater width and volume compared to the transition zone itself (Malicki and Avidor-Reiss 2014). This is likely to impose unusual structural requirements. Finally, both the photoreceptor neurons and Drosophila sensory ciliated neurons are developed in close proximity to support cells.
In Drosophila, the most studied ciliated sensory neurons are those that function in mechanosensation. The cilium at the tip of a mechanosensory dendrite is formed using the typical protein machinery essential for ciliogenesis including centriolar, connecting cilium, and IFT proteins (Avidor-Reiss et al. 2004; Martinez-Campos et al. 2004; Rodrigues-Martins et al. 2007; Blachon et al. 2008, 2009; Basiri et al. 2014). The tip of the mechanosensory cilium associates with two cuticular structures: the bristle and the socket (Figure 1A), each of which is formed by a unique support cell. Also surrounding the tip of the cilium is an extracellular dendritic sheath formed by a third support cell, the sheath cell that surrounds the inner segment. This dendritic sheath mediates the connection of the sensory cilium tip to the bristle base, and since the sensory cilium tip is the point of physical contact that the sensory neuron makes with a mechanical stimulus, the maintenance of this connection is critical (Chung et al. 2001). Thus, disruption of any of components of the sensory apparatus, including those required to maintain the connection of the mechanosensory neuron to the bristle base, leads to a failure in sensing mechanical input and results in a characteristic behavioral phenotype known as unc-type uncoordination, which has been described in various ciliary mutants (Kernan et al. 1994; Avidor-Reiss et al. 2012).
In the human retina, the interactions between the dendrite of photoreceptor cells and the retinal pigment epithelium (RPE) are essential for vision. RPE cells are epithelial support cells that send an actin-based cellular extension that surrounds the photoreceptor outer segment. Similarly, in Drosophila mechanosensory organs, the sheath cell that originates from the epithelium surrounds the sensory dendrite, although it is not known if actin filaments have a specialized role in dendrite formation or function. A family of actin-binding protein is the hook-related proteins (HkRP), which participate in diverse cellular processes and include one Drosophila member named Girdin (Anai et al. 2005; Enomoto et al. 2005; Le-Niculescu et al. 2005; Simpson et al. 2005). The mammalian co-orthologs of Drosophila Girdin are HkRP1/Girdin (girders of actin filaments, also known as CCDC88A, APE, or GIV), HkRP2/DAPLE (also known as CCDC88C), and HkRP3/Gipie (also known as CCDC88B or FLJ00354) (Simpson et al. 2005; Enomoto et al. 2006). However, whether hook-related proteins have any role in the formation or function of sensory organs is not known.
Here, we report that Drosophila girdin mutants exhibit unc-type uncoordination, no mechanosensory function, and sensory dendrite degeneration after their initial formation and that Girdin is an essential component of actin structures that surrounds the tip of the sensory dendrite. This provides the first example of dendrite degeneration in ciliated sensory neurons in Drosophila and implicates Girdin in the formation of sensory dendrites. Since Drosophila mechanosensory neurons are similar structurally and functionally to human photoreceptor neurons, our finding suggests that Drosophila mechanosensory neurons can serve as a model for photoreceptor degeneration in humans.
Materials and Methods
Transgenic flies
All Drosophila stocks were cultured on standard media at 25°. For in vivo expression of Drosophila Girdin, the girdin promoter and entire gene region up to the last codon were subcloned from y1; cn1, bw1, sp1 (Bloomington Stock Center 2057) genomic DNA into a pUAS vector with an in-frame C-terminal GFP tag such that Girdin-GFP was expressed using girdin promoter in the absence of GAL4. Germline transformation was performed by BestGene Inc. (Chino Hills, CA) using w1118 flies. UAS-GFP-α1-tubulin84B line was provided by A. Spradling (Grieder et al. 2000). Ana1-tdtomato was previously described (Blachon et al. 2008, 2009).
Imaging live pupae
White pupae were collected and aged 30 hr at 25° in a humidified chamber before imaging started. Then, pupae were placed dorsal side up on a slide with a double-sided tape. A section of the pupal case was carefully removed to reveal the thorax, and the pupa was placed dorsal side down on a MatTek’s glass-bottom dish in the center. Glycerol was used to provide the attachment between the pupa and the dish. The development of sensory organ in the thorax was observed using a Leica TCS SP8 scanning confocal microscope every 10 hr over the course of 60 hr, starting at 30 hr after puparium formation (APF) until 90 hr APF. GFP excitation at 488 nm was used with a laser power of 3–5%. The green fluorescent emission photons were collected from the wavelength range of 490–550 nm, and the red autofluorescent emission photons were collected from the wavelength range of 620–750 nm. Images were processed using Adobe Creative Suite 6.
Phalloidin staining
The thorax of the early stage pupae were dissected in PBS and fixed in 4% paraformaldehyde for 15 min. Following fixation, pupae were washed in PBS for 5 min three times and stained by phalloidin for 30 min at room temperature. Rhodamine phalloidin (Cytoskeleton, Denver) was used at a 1:100 dilution in PBS containing 2% Triton X-100.
Electron microscopy
Sensory organs were processed as previously described (Avidor-Reiss et al. 2004).
Behavior and electrophysiology
Propriosensory behavior was analyzed by collecting pharate adult pupae and allowing flies to eclose in a humidified petri dish at 25°. Flies were imaged using a Canon PowerShot SX500IS camera on an Olympus MVX10 microscope. Recordings were performed on flies by mounting a glass pipette over a cut anteronotopleural bristle (Walker et al. 2000). Mechanoreceptor current was determined by applying a saturating mechanical stimulus to the bristle as previously described (Avidor-Reiss et al. 2004).
Statistical methods
Statistical analyses were done with GraphPad Prism 5. A two-tailed, unpaired Student’s t-test (with samples that do not have equal variances) was used in Figure 1, D, E, G, and H; Figure 5C; and Figure 6, A and B.
Figure 5.
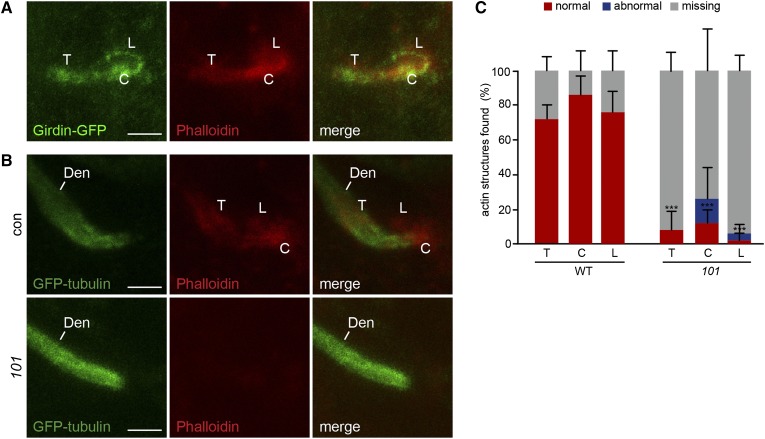
Girdin-GFP is an essential component of actin structures found at the mechanosensory organ. (A) Girdin-GFP colocalizes with filamentous actin at the dendritic tip. (B) Filamentous actin labels structures outside of the neuron expressing GFP-tubulin. These structures are absent in girdin101. (C) Percentage of normal, abnormal, or missing actin structures in wild type (WT) and in girdin101 (101) at 30 hr APF relative to neuron expressing GFP-tubulin. Shown as mean ± SD; n = 50. ***P < 0.001. The t-test was compared to the percentage of normal actin structures in wild type and in girdin101. T, tube; C, cap; L, loop; Den, Dendrite. (A) w;Girdin-GFP;girdin101. (B) elav-Gal4;UAS-GFP-tubulin;MKRS/TM6B (control) and elav-Gal4;UAS-GFP-tubulin;girdin101 (101). Bar, 2 μm.
Figure 6.
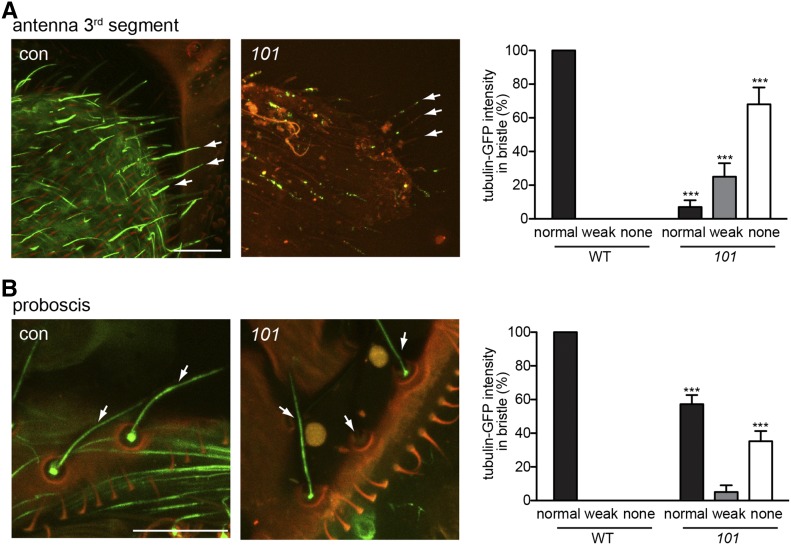
girdin101 has abnormal ciliated dendrite in olfactory and taste organs. (A and B) Cilia intensity is abnormally weak or absent (white arrow) in olfactory neurons of the third segment of antenna (A) and taste neurons in proboscis (B) expressing GFP-tubulin. Quantification of GFP-tubulin intensity in bristles from the third segment of antenna shows that cilia are mostly absent or severely defected in girdin101 (A). Quantification of GFP-tubulin intensity in bristles from proboscis shows that 36% of cilia are absent in girdin101 (B). Bar, 20 μm. Shown as mean ± SD; n = 100. ***P < 0.0001. con, control; 101, girdin101. The green fluorescent emission photons were collected from the wavelength range of 490–550 nm with a laser power of 3% for the labeling of GFP-tubulin. Illumination of bristles causes the cuticle of the bristle shaft and socket to autofluoresce in red. The merge of this autofluorescence results in orange color. (A and B) elav-Gal4;UAS-GFP-tubulin;MKRS/TM6B (control) and elav-Gal4;UAS-GFP-tubulin;girdin101 (101).
Results
mecA mutant flies cannot walk and die shortly after leaving the pupa
In Drosophila, mutants with defects in ciliated mechanosensory neurons are severely uncoordinated due to a loss of propriosensation (Kernan et al. 1994; Avidor-Reiss et al. 2012). As a result, they often fail to eclose from the pupal case, and if they do, fall into the culture media and die. Thus, we expected flies with defects in the formation of ciliated mechanosensory neurons to demonstrate an uncoordinated phenotype. As part of a screen for mechanosensory mutants (Avidor-Reiss et al. 2004), we screened mutagenized F3 lines for the presence of viable homozygous mutant pupae that failed to yield viable adult flies (Koundakjian et al. 2004). These lines were named potential mechanosensory mutants (PMM). Of the mutants identified, two (pmm20 and pmm101) did not complement and were therefore expected to belong to a single complementation group, designated as mechanosensory mutant A (mecA) (Avidor-Reiss et al. 2001). We named these alleles mecA20 and mecA101.
To observe the behavioral phenotype of the adult mecA files, pupae were collected and allowed to eclose in a humidified petri dish. mecA101 flies showed a severely uncoordinated phenotype typical of flies that completely lack functional mechanosensation such as unc, nompA, nompB, oseg1, oseg2, asl, sas-6, and sas-4 (reviewed in Avidor-Reiss et al. 2012). They were unable to stand on their legs, their legs were crossed, and their wings were held straight up (Figure 1B). mecA20 flies displayed a milder uncoordination phenotype. Their wings were held up straight, but they were able to stand on their legs and walk slowly (Figure 1B). This milder phenotype suggests that mecA20 is a hypomorph. This behavioral phenotype is similar to that of nompC and nompH mutants that show a mild uncoordination phenotype (Walker et al. 2000; Willingham and Keil 2004).
mecA mutant flies have no mechanoreceptor current
Since the behavioral phenotype displayed by mecA mutants could result from disruption of any aspect of the mechanosensory organ, we sought to further characterize the defect. In mecA101, the bristle and socket of the mechanosensory organ appear normal, suggesting that the behavioral phenotype is not due to a defect in the mechanosensory support cells forming these structures. To test if mecA mutants are defective in the function of sensory neurons, we recorded mechanoreceptor currents (MRC) and transepithelial potentials (TEP) (Walker et al. 2000). The MRC measures the peak receptor current produced by the mechanosensory cilium in response to deflection of the bristle. TEP is the voltage difference between the sensory organ endolymph, which is produced by the socket support cell and the fly hemolymph (Walker et al. 2000), whereas mutations that affect the sensory neuron are expected to show a defective MRC, those that disrupt the socket support cells often abolish the TEP (Barolo et al. 2000; Willingham and Keil 2004). We recorded MRCs and TEPs from bristles in homozygote and hemizygote mecA101 and found that they lack MRCs, but are able to maintain normal TEPs (Figure 1 C–E). The severity of the MRC is similar to that of asterlessmecD, oseg2mecE, and cep290mecH mutants that lack functional sensory cilia (Avidor-Reiss et al. 2004; Blachon et al. 2008; Basiri et al. 2014), suggesting that mecA101 flies have severe defects in the function or formation of the mechanosensory neuron.
mecA mutant flies have abnormally short or missing sensory dendrites
To directly observe the morphology of the mechanosensory neurons in mecA flies, a Gal4-UAS driver was used to express GFP-tubulin under the control of a pan-neuronal promoter, allowing for the visualization of mechanosensory neurons. In this system, GFP-tubulin labels the sensory neuron cell body, axon, and dendrite and is enriched in the sensory cilium (Avidor-Reiss et al. 2004) (Figure 1F). We found that mechanosensory neurons of mecA101 late pupae exhibit several phenotypes. First, 60% of the thoracic mechanosensory organs are missing their neurons (Figure 1, F and G). Of the remaining 40% of mechanosensory organs that have sensory neurons, 74% of these neurons have no dendrites and 24% have abnormally short dendrites that fail to innervate the bristle base (Figure 1, F and H). In addition, the cell bodies of those sensory neurons that lack dendrites are often clustered together (Figure 1F). The mechanosensory organ defects displayed by mecA101 are more severe than those observed in mutations that specifically affect components of the sensory cilium; in such mutations the dendrite extends to the base of the socket, and absence or clustering of neuronal cell bodies is not observed (Avidor-Reiss et al. 2004; Blachon et al. 2008; Basiri et al. 2014). However, the mecA phenotype resembles that of nompA, which also often fails to innervate the bristle base. Still, mechanosensory dendrites and cell bodies are not missing in nompA (Chung et al. 2001).
We next examined the ultrastructure of mecA101 sensory neurons by serial-section electron microscopy. From their distal tip, wild-type sensory neurons contain a sensory cilium (outer segment), followed by a connecting cilium and two basal bodies (centrioles). These basal bodies exist at the distal end of the dendrite (inner segment) (Keil 1997). Within the specialized mechanosensory cilium, a rich array of tightly packed microtubules (tubular body) is surrounded by the plasma membrane and delimited by the dendritic sheath (Figure 1I). We examined serial sections from the bristle shaft to the dendrite of mecA101 pupae and found that, unlike wild type, we were unable to identify any components of the sensory neuron at the base of the bristle. Furthermore, we observed that the dendritic sheath, which did not contain a sensory cilium with the tubular body, displayed dramatic defects (Figure 1I). This phenotype is distinct from that observed in centriole or cilium mutants, which have inner segments but fail to form the centrioles and the cilium (Avidor-Reiss et al. 2004; Blachon et al. 2008, 2009). The absence of the tubular body in mecA101 is unlikely due to defects in tubular body formation itself since DCX-EMAP mutant flies that cause specific defects in tubular body formation show no other anatomical defects (Bechstedt et al. 2010). This ultrastructural phenotype is consistent with our morphological studies using GFP-tubulin and indicates that the dendrite is absent or dramatically shortened in mecA101.
The mecA gene encodes Girdin
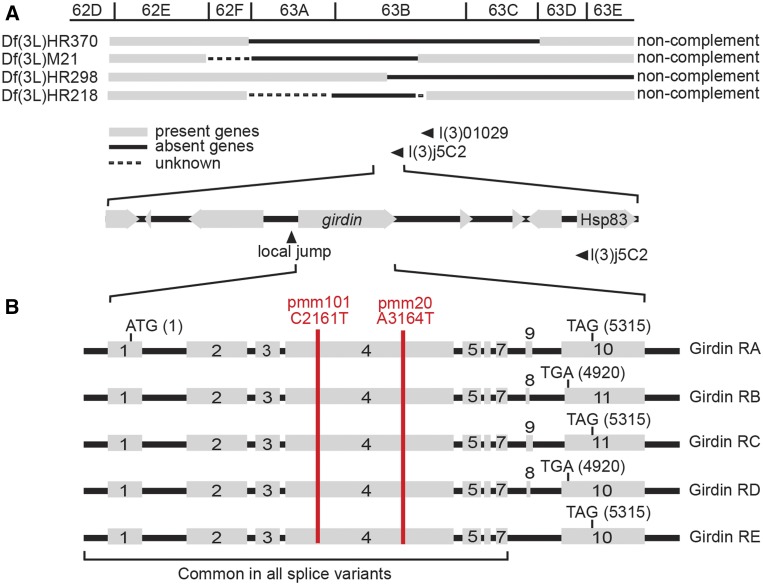
We used positional cloning to identify the underlying mutation in mecA. We found that mecA alleles fail to complement the deficiencies Df(3L)HR370, Df(3L)M21, Df(3L)HR298, placing it at the center of the 63B segment (Figure 2A). Male recombination and local P-element jumping experiments identified a mutant where the element duplicated to the girdin (Figure 2A). Sequencing these genes found an independent nonsense mutation in mecA101 and a missense mutation in mecA20 (Figure 2B). The mecA101 mutation changed the amino acid glutamine (Q) at position 505 to an early stop codon, resulting in premature termination of translation. The mecA20 mutation changed aspartic acid (D) to valine (V) at position 839. Examining the alignment of the Girdin protein family in humans, Caenorhabditis elegans, and Drosophila shows that the amino acids in these positions have a conserved charged residue (aspartic acid, D; glutamic acid, E; arginine, R), suggesting that the amino acid change from charged to uncharged (valine) is incompatible. The finding that mecA101 produced a shorter protein compared to mecA20 is consistent with the more severe behavioral phenotype in mecA101 and suggests that the mecA20 missense mutation produces a partially functioning protein.
Figure 2.
The mecA gene encodes Girdin. (A) Positional cloning of mecA mutants found mutations in girdin (CG12734). mecA mutants do not complement the deficiencies HR370, M21, HR298, and HR218 and map to the left of l(3)01029 and l(3)j5C2. (B) Alternative splicing at the C terminus results in five Drosophila Girdin mRNA isoforms named Girdin RA through Girdin RE. Exons 1–7 are common in all isoforms. Two separate mutations, named mecA101 and mecA20, were found in exon 4 (red line). Nonsense mutation was introduced in mecA101 at the base 2161 that changed cytosine (C) to thymine (T), resulting in a premature stop codon. A missense mutation was introduced in mecA20 at the base 3164 that changed adenine (A) to thymine (T), resulting in an amino acid change from aspartic acid (D) to valine (V).
Drosophila Girdin has five splicing isoforms—Girdin-A, -B, -C, -D, and -E—that share the first 1229 amino acids and have a distinct C terminus (St Pierre et al. 2014). Since in both mecA101 and mecA20 mutations were introduced in a region that is common to all splice variants, both mecA alleles are expected to affect all Girdin isoforms. Both human and Drosophila Girdin have been reported to have N termini that are similar to the microtubule-binding domain of the Hook protein, an extensive central coiled-coil domain that is conserved in family-HkRP and more diverse C termini that specify the binding partners, such as actin (Simpson et al. 2005; Enomoto et al. 2006; Puseenam et al. 2009). The mutations in both mecA101 and mecA20 are found in the central coiled-coil domain.
To confirm that Girdin is responsible for the observed phenotypes, we generated a transgenic rescue construct that consists of the girdin promoter and gene up to the stop codon of Girdin-A. To allow localization, we also fused a GFP at the C terminus of Girdin-A in this rescue construct. Introducing the Girdin-GFP to the genetic background of the mecA101 mutant rescued the behavioral phenotype and generated viable flies, demonstrating that Girdin is responsible for the observed mechanosensory phenotype. This finding confirms that Girdin is essential for proprioception and indicates that Girdin-GFP is a functional reporter. We therefore renamed our mecA mutants girdin101 and girdin20.
In girdin101 mutant, the sensory dendrites initially form normally but later degenerate
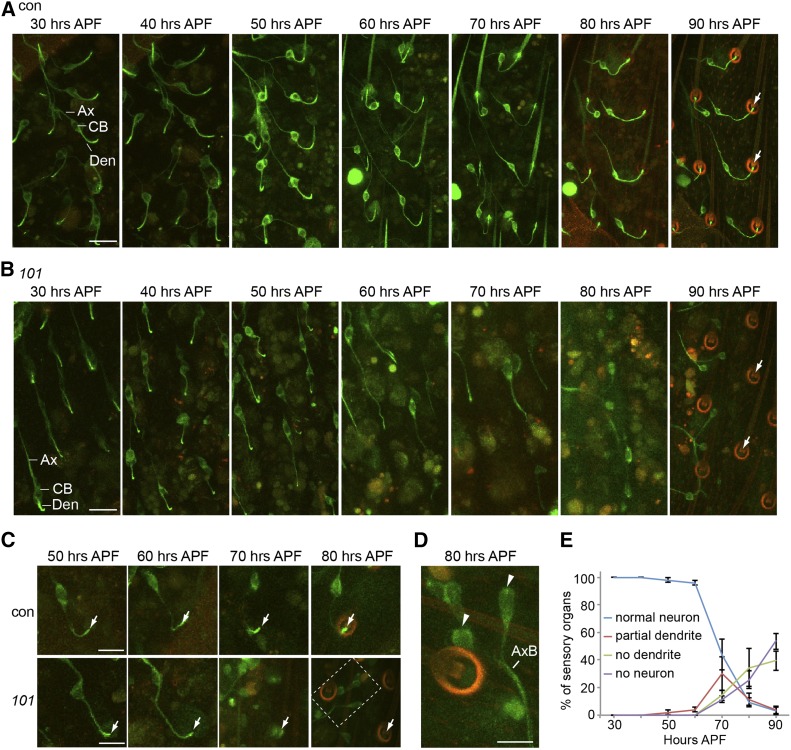
To test if Girdin is essential for the initiation of dendrite formation or if it is instead important later in dendrite formation, we used GFP-tubulin to visualize the development of mechanosensory neurons during pupal maturation. We also took advantage of cuticle autofluorescence to visualize the external components of the mechanosensory organ, including the socket and bristle shaft. This autofluorescence first appears ∼50 hr (APF), when the socket and bristle shaft autofluoresce at emission wavelengths similar to that of GFP (Figure 3A and Figure 4A). At 70–90 hr APF, this autofluorescence becomes more apparent, and its emission expands to a longer wavelength, allowing it to be easily discerned from GFP. In later stages, bristle shaft autofluorescence is diminished.
Figure 3.
Girdin is essential for sensory dendrite formation. (A and B) The development of sensory neuron in the thorax was monitored using confocal microscopy of GFP-tubulin. Starting from 30 hr APF, images were taken every 10 hr up to 90 hr APF in wild type (A) and girdin101 (B). Bar, 20 μm. (C) A single neuron was followed during development using live confocal microscopy of GFP-tubulin. Bar, 10 μm. A 488-nm laser with 5% power was applied for GFP fluorescent excitation; emission photons were collected from the wavelength 490–550 nm for the green and 620–750 nm for the red autofluoresce. The merge of this autofluorescence results in orange color. Ax, axon; CB, cell body; Den, dendrite. (D) Magnification of dashed-line box is at 80 hr APF in girdin101. Sensory outer segments labeled by GFP-tubulin are virtually absent in girdin101 (white arrow). Dendrites labeled by GFP-tubulin are absent in girdin101 (arrowhead). Green: GFP-tubulin. Red: cuticle autofluorescence. Bar, 5 μm. (E) Quantification of the percentage of normal neuron (having a dendrite with a cilium at the expected location for each stage), abnormally short or missing dendrites, or missing neurons in girdin101 over sensory organ development starting at 30 hr APF until 90 hr APF. Shown as mean ± SD; n = 250. (A–D) elav-Gal4;UAS-GFP-tubulin;MKRS/TM6B (control) and elav-Gal4;UAS-GFP-tubulin;girdin101 (101).
Figure 4.
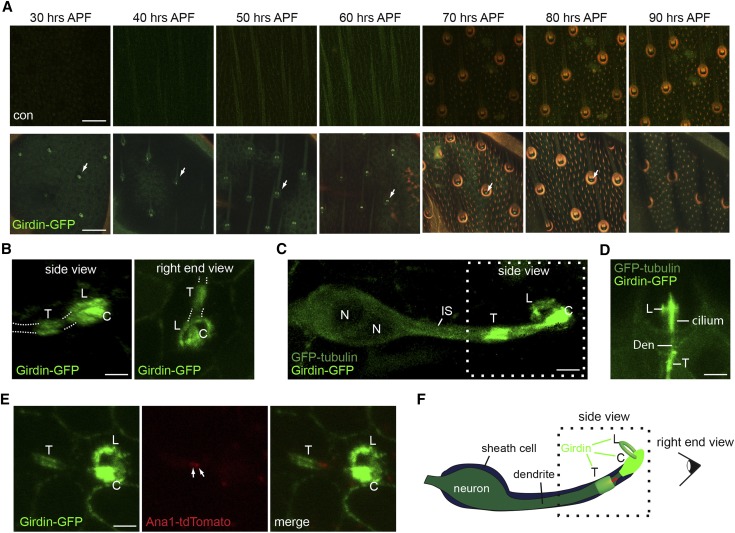
Girdin-GFP transiently localizes to sensory organ during development. (A) Live confocal imaging of Girdin-GFP in the thorax was monitored over the course of 30–90 hr APF. The images were taken every 10 hr. The same sensory organ is followed over the course of 60 hr. Illumination of bristles causes the cuticle of the bristle shaft and socket to autofluoresce in green ∼30 and 40 hr APF, respectively, and in red ∼70 hr APF. The merge of this autofluorescence results in orange color. Bar, 20 μm. (B) Live confocal 3D reconstruction images of Girdin-GFP in the thorax at 30 hr APF. Bar, 2 μm. T, tube; C, cap; L, loop. (C) Live confocal 3D reconstruction images of Girdin-GFP and GFP-tubulin in the thorax at 30 hr APF. Bar, 2 μm. (D) Live confocal imaging of Girdin-GFP and GFP-tubulin in the thorax at 60 hr APF. Bar, 2 μm. (E) Live confocal imaging of Girdin-GFP and Ana1-tdTomato in the thorax at 30 hr APF. Bar, 2 μm. (F) Schematic drawing of Girdin-GFP and Ana1-tdTomato localizations in a position relative to the sensory neuron and sheath cell. (A and B) w;Girdin-GFP;girdin101. (C and D) elav-Gal4;UAS-GFP-tubulin/Girdin-GFP;girdin101. (E) w;Girdin-GFP/Ana1-tdTomato;girdin101.
Thoracic mechanosensory neurons first appear ∼20 hr APF. The inner segment appears by 24 hr APF, and the cilium appears by 26 hr APF (Hartenstein and Posakony 1989). We followed the formation of the same individual thoracic mechanosensory neurons using confocal imaging in live preparations (Figure 3, A and B). In wild-type pupae at ∼30–40 hr APF, we observed mechanosensory neurons with dendrites extended from their cell bodies to their target bristle base. At ∼50 hr, the inner segment and cilium are labeled with GFP-tubulin at the dendritic tip. At ∼80 and 90 hr, GFP-tubulin strongly labels the cilium and is diminished within the inner segment.
In girdin101, we found that at 30–50 hr APF all the sensory neurons had an axon and dendrite with an intense GFP-tubulin at its distal end (Figure 3B). Later, at ∼60–90 hr APF, many of these dendrites were degenerated, with many of the neurons having only axons and cell bodies (Figure 3B). Similar results were observed when we followed the development of a single neuron from 40 to 80 hr APF (Figure 3C). In wild type, the morphology of the dendrite was maintained throughout the development. However, in girdin101, the dendrites began to degenerate at ∼60–70 hr APF, and the cell bodies formed clusters. At 80 hr APF, we observed clusters of neurons with missing dendrites (Figure 3D). Quantification of the timing of the dendritic degeneration shows that starting at ∼60 hr APF, most of the neurons began to lose their dendrites (Figure 3E). At 90 hr APF, only 3% of the neurons maintained their morphology with intact dendrites (Figure 3E). This phenotype is rescued by insertion of Girdin-GFP, suggesting that Girdin plays an important role in the formation of the dendrite (supporting information, Figure S1C).
Girdin-GFP is enriched temporarily at the mechanosensory organ
Girdin is expressed in various tissues throughout development (Puseenam et al. 2009; Yamaguchi et al. 2010). To determine Girdin localization in sensory neurons, we followed Girdin-GFP labeling under the control of endogenous girdin promoter throughout the development of thoracic mechanosensory organs. To enhance the signal and prevent mislocalization due to competition with endogenous Girdin protein, Girdin-GFP localization was determined in the girdin101 mutant background. Girdin was observed in various cell types, including thoracic epithelial cells, where it labeled the plasma membrane, and sperm cells, where it labeled the cytoplasm (Figure S1A). In the differentiating thoracic mechanosensory organ, we found that Girdin-GFP is expressed intensely from 30 hr APF until it disappeared at ∼90 hr APF (Figure 4A). Throughout this time, the plasma membrane of epithelial cells in the thorax was labeled consistently by low levels of Girdin-GFP. At 30 hr APF, Girdin-GFP was enriched in foci that were organized in rows reminiscent of the arrangement of the mature sensory organs (Figure 4A). Later, we found Girdin-GFP to be associated with the bristle sensory organs observed via autofluorescence (Figure 4A). Examination of the Girdin-GFP signal using 3D analysis identifies three foci at the dendritic tip: one looks like a tube, another like a cap structure, and the third like a loop (Figure 4, B–F; Figure S1B).
To get insight to localization of the three Girdin-GFP foci observed within the sensory organ, we examined Girdin localization relative to neurons expressing GFP-tubulin. Since both Girdin and tubulin were tagged with GFP, we were unable to determine if the two proteins colocalized. Still, we used difference in signal morphology to ask if Girdin is located outside the neuron. At 30 hr APF, GFP-tubulin labels both the neuron and the sheath cell (Figure 4C). At that time, Girdin-GFP localizes around the neuron at three distinct regions: a tube-like structure located just before the dendrite tip and a cap-like and loop-like structures that surround the dendritic tip (Figure 4C). These structures of Girdin-GFP were also observed at 60 hr APF (Figure 4D). Next, to better define this localization, we examined the localization of Girdin-GFP relative to the centriolar marker Ana1-tdTomato, which marks the junction between the inner segment and sensory cilium. We found that Ana1-tdTomato labeled the distal tip of the tube-like structure, suggesting that the tube-like structure of Girdin-GFP localizes at or around the distal tip of the inner segment (Figure 4, E and F). In addition, the cap-like and loop-like structures localize at or around the cilium (Figure 4, E and F).
Girdin-GFP is an essential component of actin structures found at the mechanosensory organ
Since Girdin is an actin-binding protein (Enomoto et al. 2005; Puseenam et al. 2009), we also examined the localization of Girdin-GFP relative to phalloidin labeling of filamentous actin. We found that in the sensory organs filamentous actin forms three foci that are similar to Girdin-GFP in morphology colocalizing with Girdin-GFP (Figure 5A). These observations suggest that Girdin is a component of previously undescribed actin-based structures found near the dendritic tip. Next, we tested the role of Girdin in these actin structures using phalloidin staining relative to neuron-expressing GFP-tubulin. As expected in control cells, the actin structures were located around the dendritic tip at 30 hr APF (Figure 5B). However, those structures were absent or dramatically impaired in girdin101 (Figure 5, B and C). Together, these observations suggest that Girdin is essential for organizing actin structures found at the dendritic tip of the mechanosensory neuron during its morphogenesis.
Girdin101 has defects in other ciliary organs
Next we sought to determine whether Girdin is essential for the development of ciliated sensory neurons in general, or if its function is instead specific to thoracic mechanosensory neurons. To address this question, we studied GFP-tubulin in olfactory organs in the third segment of the antenna and taste organs in the proboscis in girdin101 flies. Unlike mechanosensory neurons, where GFP-tubulin labels sensory cilia at the base of the bristle, sensory cilia in olfactory and taste sensory neurons are found inside the bristle shaft (Figure 6, A and B). In both the olfactory and taste organs, GFP-tubulin labeling was abnormal or absent in girdin101. In the third segment of antenna, <10% of sensory cilia were labeled by GFP-tubulin (Figure 6A). In proboscis, only 60% of sensory neurons were labeled normally by GFP-tubulin (Figure 6B). We also found that Girdin-GFP localized to olfactory and taste sensory organs (Figure S1D). This suggests that Girdin is essential for ciliated sensory neurons in general. Taken together, our results suggest that Girdin is essential for sensory dendrite morphology and its formation in various sensory organs.
Discussion
In this study, we report the identification and characterization of girdin mutants and their role in dendrite formation. Here, we show a novel function of Girdin during sensory neuron development in Drosophila, suggesting that Girdin is essential for morphogenesis of sensory dendrites. This conclusion stems from several lines of evidence. First, girdin101 exhibits complete unc-type uncoordination, a phenotype that is present only in flies with mutations in the sensory neuron or its attachment to the bristle. Second, girdin101 flies have no MRC but a normal TEP, the socket and the bristle appear normal, and the dendrite sheath is present, suggesting that, although the support cells of the sensory organ are present, the neuron itself is defective. Third, in girdin101, GFP-tubulin labeling exhibits dramatic defects in dendrite and cilium morphogenesis but a normal morphology of the cell body and axon. Finally, Girdin-GFP is present in relatively high intensity in the sensory organ at the general location of the dendritic tip during its morphogenesis.
Our finding suggests a role for Girdin in dendrite formation and neuron cell body positioning. During the early stages of mechanosensory neuron development, the morphology of the cell body, dendrite, and cilium all appear normal. Only later in development, the ciliated dendrite begins shortening. This degeneration continues until the dendrites are fully absent and the cell bodies become clustered. Since the cell bodies in girdin101 tend to form a cluster after their dendrites degenerate, it is possible that the dendrite is essential for positioning of the sensory neuron cell body. Alternatively, since Girdin is important in cell migration in many cell types (Enomoto et al. 2006; Ghosh et al. 2008), it is possible that the mislocalization of the cell body is due to a function of Girdin in cell migration. One possible explanation for the clustering of the cell bodies is that cell bodies migrate along their axons until they bundle. In C. elegans, dendrites of ciliated sensory cells are extended in a retrograde fashion where the cell body migrates away from the dendritic tip, which remains anchored in place (Heiman and Shaham 2009). A similar mechanism that regulates cell-body positioning during mechanosensory neuronal development may also occur in Drosophila.
Girdin shares some similarities with NompA: both are essential for fly viability, coordinated behavior, and MRC, while both are not essential for bristle or socket formation and TEP. Both have a similar dendrite formation defect in which the tip of the sensory dendrite terminates at a significant distance from the bristle base and socket. Finally, both NompA and Girdin localize inside the sensory organ at the vicinity of the dendritic tip (Chung et al. 2001). Taking together, these phenotypes and localization suggest that Girdin is associated with the function of NompA to attach the dendritic tip to the base of the bristle for the mechanical transduction of mechanosensory signals. However, since Girdin is expressed only transiently in the sensory organ, it is likely to have only a developmental role in forming the mechanosensory organ rather than a direct role in mechanosensation as is proposed for NompA. Also, since girdin101 sensory neurons showed a more severe defect in dendrite morphology than nompA, Girdin might have additional functions beyond temporarily connecting the dendrite to the bristle shaft. One such additional function of Girdin may be to stabilize the dendrite as it differentiates.
Girdin is an intracellular, actin-binding protein that associates with the plasma membrane or cytoplasm of epithelial cells (Enomoto et al. 2005; Puseenam et al. 2009). In the sensory organ cells, Girdin-GFP is present in three actin structures surrounding the tip of the inner segment and the sensory cilium. Phalloidin staining relative to neurons expressing GFP-tubulin suggests that the cap-like and loop-like structures of actin are found outside the sensory neuron and in the support cells. Since actin colocalizes with Girdin-GFP, this suggests that Girdin-GFP is a component of the support cells at these two locations. On the other hand, the tube-like structure of actin and Girdin-GFP appears to localize near the inner segment and may be part of the neuron or sheath cell.
The Girdin-GFP–labeled tube-like structure is found at the tip of the inner segment, a specialized location that has cell–cell contacts with the sheath cell (Foelix et al. 1989). The Girdin-GFP–labeled cap-like structure is found at the tip of the cilium where the dendritic sheath forms during neuron differentiation (Keil 2012). The Girdin-GFP–labeled loop-like structure is found away from the neuronal tip as determined by neurons expressing GFP-tubulin, and the loop-like structure diameter is too large to fit within the dimensions of either the sensory neuron or the sheath cell. Therefore, it is likely that the loop-like structure is inside either the shaft or the socket support cells, which are bigger and are found at that location. Which of these three Girdin-GFP/actin structures is essential for normal dendrite formation is currently not known.
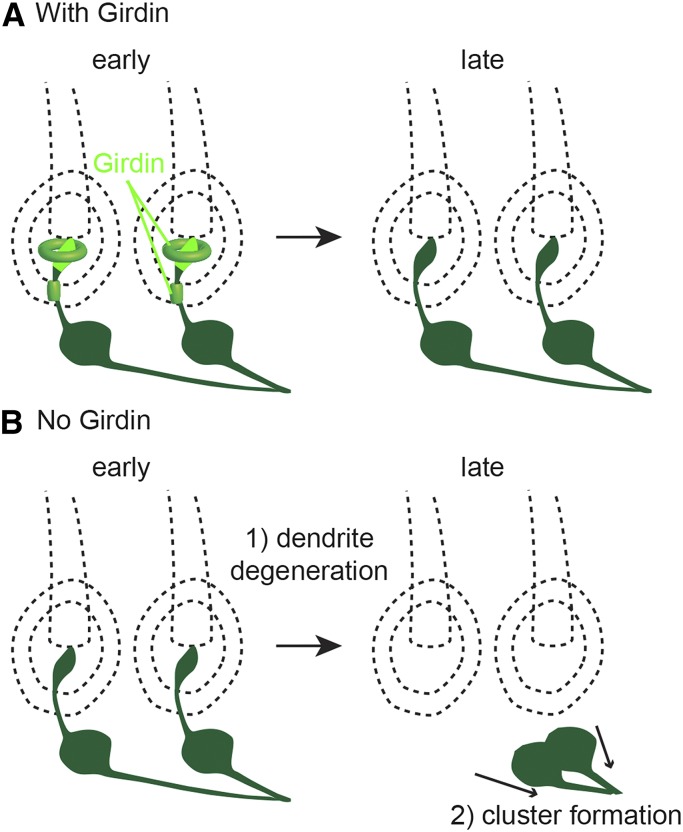
Based on our findings and known functions of Girdin, we propose a model for Girdin function in sensory organs during sensory neuron development (Figure 7, A and B). We show that Girdin functions near the tip of the dendrite at three locations. In these locations, Girdin regulates the formation of actin structures that may temporarily stabilize the dendritic tip and is essential for its connection to the dendritic sheath (Figure 7A). In girdin mutants, dendrite degeneration is likely due to a failure in complete development of the dendrite after its initiation. Once the tip of the dendrite is destabilized and disappears, the neuron cell body recedes toward the axon until it meets other sensory cell bodies and forms clusters where the individual axon meets to form a bundle (Figure 7B). This model can serve to direct further studies of Girdin.
Figure 7.
A model for Girdin function during sensory neuron development. (A and B) Schematic drawings of sensory neuron development at early (30–40 hr APF) and late (>90 hr APF) stages.
Because of the similarities between fly sensory neurons and human photoreceptor cells, Girdin may have a similar function in the formation of rod and cone dendrites. Rod and cone photoreceptors are capable of surviving throughout a life span, yet they are sensitive to various insults that result in their degeneration, leading to gradual loss of visual acuity and blindness. Since RPE cells surround the tip of the photoreceptors and are essential for forming and maintaining the morphology of the photoreceptors (Hamel et al. 1993; Bonilha 2014), studying the role of Girdin in RPE cell lines may provide more insights into understanding how RPE cells regulate the morphology of photoreceptor cells. By better understanding the mechanisms of how ciliated a sensory neuron functions and forms its structure in Drosophila, our hope is to assist in confronting retinal diseases and provide insight to treat them.
Supplementary Material
Acknowledgments
We thank Marcus Basiri and Jarema Malicki for commenting and advice on the manuscript. This work was supported by grant 1121176 (Molecular and Cellular Biosciences) from the National Science Foundation and grant R01GM098394 from the National Institute of General Medical Sciences. The authors declare no competing financial interests.
Footnotes
Communicating editor: I. K. Hariharan
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.178954/-/DC1.
Literature Cited
- Anai M., Shojima N., Katagiri H., Ogihara T., Sakoda H., et al. , 2005. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J. Biol. Chem. 280: 18525–18535. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss, T., A. D. Polyanovsky, R. W. Hardy, and C. S. Zuker, 2001 The molecular basis of mechanosensory transduction in Drosophila melanogaster. Program and Abstracts. 42nd Annual Drosophila Research Conference, Washington, DC. [Google Scholar]
- Avidor-Reiss T., Maer A. M., Koundakjian E., Polyanovsky A., Keil T., et al. , 2004. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T., Gopalakrishnan J., Blachon S., Polyanovsky A., 2012. Centriole duplication and inheritance in Drosophila melanogaster, pp. 3–31 in The Centrosome: Cell and Molecular Mechanisms of Functions and Dysfunctions in Disease, edited by Schatten H. Humana Press, New York. [Google Scholar]
- Barolo S., Walker R. G., Polyanovsky A. D., Freschi G., Keil T., et al. , 2000. A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103: 957–969. [DOI] [PubMed] [Google Scholar]
- Basiri M. L., A. Ha, A. Chadha, N. M. Clark, A. Polyanovsky et al, 2014. A migrating ciliary gate compartmentalizes the site of axoneme assembly in Drosophila spermatids. Curr. Biol. 24: 2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S., Albert J. T., Kreil D. P., Muller-Reichert T., Gopfert M. C., et al. , 2010. A doublecortin containing microtubule-associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat. Commun. 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Gopalakrishnan J., Omori Y., Polyanovsky A., Church A., et al. , 2008. Drosophila asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics 180: 2081–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachon S., Cai X., Roberts K. A., Yang K., Polyanovsky A., et al. , 2009. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 182: 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha V. L., 2014. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp. Eye Res. 126: 38–45. [DOI] [PubMed] [Google Scholar]
- Chung Y. D., Zhu J., Han Y., Kernan M. J., 2001. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron 29: 415–428. [DOI] [PubMed] [Google Scholar]
- Cideciyan A. V., Aleman T. S., Jacobson S. G., Khanna H., Sumaroka A., et al. , 2007. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum. Mutat. 28: 1074–1083. [DOI] [PubMed] [Google Scholar]
- Crouse J. A., Lopes V. S., Sanagustin J. T., Keady B. T., Williams D. S., et al. , 2014. Distinct functions for IFT140 and IFT20 in opsin transport. Cytoskeleton (Hoboken) 71: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander A. I., Koenekoop R. K., Yzer S., Lopez I., Arends M. L., et al. , 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 79: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A., Murakami H., Asai N., Morone N., Watanabe T., et al. , 2005. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell 9: 389–402. [DOI] [PubMed] [Google Scholar]
- Enomoto A., Ping J., Takahashi M., 2006. Girdin, a novel actin-binding protein, and its family of proteins possess versatile functions in the Akt and Wnt signaling pathways. Ann. N. Y. Acad. Sci. 1086: 169–184. [DOI] [PubMed] [Google Scholar]
- Foelix R. F., Stocker R. F., Steinbrecht R. A., 1989. Fine structure of a sensory organ in the arista of Drosophila melanogaster and some other dipterans. Cell Tissue Res. 258: 277–287. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Garcia-Marcos M., Bornheimer S. J., Farquhar M. G., 2008. Activation of Galphai3 triggers cell migration via regulation of GIV. J. Cell Biol. 182: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieder N. C., de Cuevas M., Spradling A. C., 2000. The fusome organizes the microtubule network during oocyte differentiation in Drosophila. Development 127: 4253–4264. [DOI] [PubMed] [Google Scholar]
- Hamel C. P., Tsilou E., Pfeffer B. A., Hooks J. J., Detrick B., et al. , 1993. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J. Biol. Chem. 268: 15751–15757. [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W., 1989. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107: 389–405. [DOI] [PubMed] [Google Scholar]
- Heiman M. G., Shaham S., 2009. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137: 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D. H., Pawlyk B. S., Shang J., Sandberg M. A., Berson E. L., et al. , 2000. A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc. Natl. Acad. Sci. USA 97: 3649–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil T. A., 1997. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 39: 506–531. [DOI] [PubMed] [Google Scholar]
- Keil T. A., 2012. Sensory cilia in arthropods. Arthropod Struct. Dev. 41: 515–534. [DOI] [PubMed] [Google Scholar]
- Kernan M., Cowan D., Zuker C., 1994. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron 12: 1195–1206. [DOI] [PubMed] [Google Scholar]
- Koundakjian E. J., Cowan D. M., Hardy R. W., Becker A. H., 2004. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Niculescu H., Niesman I., Fischer T., DeVries L., Farquhar M. G., 2005. Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J. Biol. Chem. 280: 22012–22020. [DOI] [PubMed] [Google Scholar]
- Malicki J., Avidor-Reiss T., 2014. From the cytoplasm into the cilium: bon voyage. Organogenesis 10: 138–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J. W., 2004. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 165: 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puseenam A., Yoshioka Y., Nagai R., Hashimoto R., Suyari O., et al. , 2009. A novel Drosophila Girdin-like protein is involved in Akt pathway control of cell size. Exp. Cell Res. 315: 3370–3380. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Bettencourt-Dias M., Riparbelli M., Ferreira C., Ferreira I., et al. , 2007. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr. Biol. 17: 1465–1472. [DOI] [PubMed] [Google Scholar]
- Simpson F., Martin S., Evans T. M., Kerr M., James D. E., et al. , 2005. A novel hook-related protein family and the characterization of hook-related protein 1. Traffic 6: 442–458. [DOI] [PubMed] [Google Scholar]
- St Pierre S. E., Ponting L., Stefancsik R., McQuilton P.; FlyBase Consortium, 2014. FlyBase 102: advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42: D780–D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A., Kim D., Forrest D., 2010. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 11: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm U., 1965. An insect mechanoreceptor. I. Fine structure and adequate stimulus. Cold Spring Harb. Symp. Quant. Biol. 30: 75–82. [DOI] [PubMed] [Google Scholar]
- Walker R. G., Willingham A. T., Zuker C. S., 2000. A Drosophila mechanosensory transduction channel. Science 287: 2229–2234. [DOI] [PubMed] [Google Scholar]
- Willingham A. T., Keil T., 2004. A tissue specific cytochrome P450 required for the structure and function of Drosophila sensory organs. Mech. Dev. 121: 1289–1297. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Suyari O., Nagai R., Takahashi M., 2010. dGirdin a new player of Akt /PKB signaling in Drosophila melanogaster. Front. Biosci. 15: 1164–1171 (Landmark Ed). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.