Abstract
A major public health question is whether urbanization will transform malaria from a rural to an urban disease. However, differences about definitions of urban settings, urban malaria, and whether malaria control should differ between rural and urban areas complicate both the analysis of available data and the development of intervention strategies. This report examines the approach of the International Centers of Excellence for Malaria Research (ICEMR) to urban malaria in Brazil, Colombia, India (Chennai and Goa), Malawi, Senegal, and Uganda. Its major theme is the need to determine whether cases diagnosed in urban areas were imported from surrounding rural areas or resulted from transmission within the urban area. If infections are being acquired within urban areas, malaria control measures must be targeted within those urban areas to be effective. Conversely, if malaria cases are being imported from rural areas, control measures must be directed at vectors, breeding sites, and infected humans in those rural areas. Similar interventions must be directed differently if infections were acquired within urban areas. The hypothesis underlying the ICEMR approach to urban malaria is that optimal control of urban malaria depends on accurate epidemiologic and entomologic information about transmission.
Introduction
Although malaria is typically considered mainly a problem of the rural poor, this disease has been a concern in urban settings for centuries. Today, however, evidence suggests that economic development and various environmental changes during the twentieth century have reduced the incidence of malaria in urban contexts.1 In addition, improved housing, drainage of Anopheles breeding sites, household mosquito proofing, expanded personal protection, effective diagnosis and treatment, and other factors have contributed to the recent global decline in malaria incidence. Whether, where, and to what extent such improvements have affected urban dwellers is still being debated.1–4 Indeed, a simple PubMed search (August 17, 2014) for research articles with titles containing “urban(ization)” and “malaria” yielded more than 200 publications during the past three decades that specifically focused on malaria among urban residents. These reports studied malaria across diverse urban settings of Africa, South America, and Asia and addressed the various factors that influence the risks of infection and disease in urban locations.
Among the concerns that complicate our analysis and understanding of “urban malaria” are 1) the definitions of what constitutes urban and urban malaria; 2) the accuracy of diagnosis by microscopy, rapid diagnostic tests (RDTs), and polymerase chain reaction (PCR)–based amplification of parasite DNA; 3) epidemiologic studies to localize transmission, including the assessment of potential confounders such as travel and the spatial heterogeneity of transmission; 4) entomologic studies to localize transmission and assess the intensity of transmission; and 5) methods to assess the completeness of surveillance and reporting.
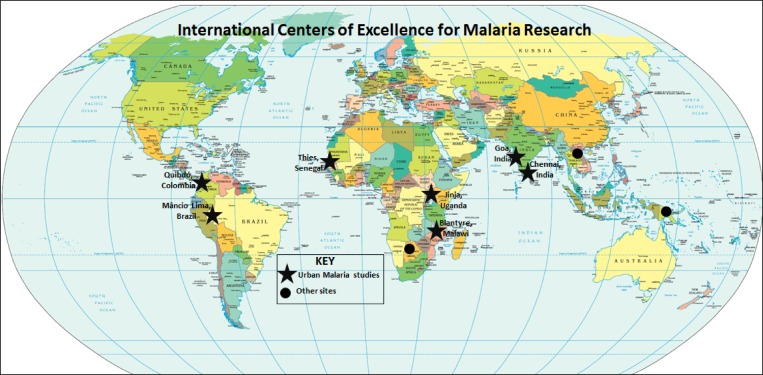
In an effort to better understand these issues in urban contexts, research is underway at locations supported by the National Institute of Health–funded International Centers of Excellence for Malaria Research (ICEMR) (Figure 1 ). This report summarizes the questions that urban malaria research is addressing and characterizes the approaches, preliminary findings, and challenges faced by ICEMR sites across the globe. In this introduction, we present many challenges of defining and studying urban malaria to highlight issues that should be considered in evaluating patterns. We recognize that not all ICEMRs are gathering data to address each of these issues, but most have been collecting or will acquire data on some of the factors. By highlighting unresolved methodological issues, we provide examples of how the ICEMRs are attempting to address them in both similar and different ways.
Figure 1.
Locations of the International Centers of Excellence for Malaria Research (ICEMR) sites, including the seven sites reporting studies on urban malaria.
Infectious disease risks in urban areas differ from those in rural settings, with urbanization having changed health patterns in those areas.3,5–11 Most of the studies that have identified differences between “urban” and “rural” environments in exposure risks and prevention opportunities recognize that those categories are poorly defined, differentially applied, and difficult to generalize. Despite problems with the definition of what constitutes urban, most urban settings in sub-Saharan Africa (SSA) are now viewed as presenting an increased risk of various infectious diseases. In particular, the importance of malaria in urban contexts has recently been recognized,12 even if it is not well understood. The burden of malaria includes not only the loss of life and productivity, but the costs of prevention and treatment that have historically been viewed primarily as problems in rural settings. Similarly, some studies have suggested lower risk in urban areas compared with neighboring rural areas,3,4,13,14 although the unresolved question of what constitutes an urban versus a rural area makes comparisons difficult. Indeed, there is an enormous diversity of ecologies, exposure patterns, and prevention opportunities at different locations, making simple claims about urban versus rural risk very doubtful. Regardless, what is clear from many studies and a few meta-analyses is that the number of malaria cases in some urban settings is considerable, due in part to the increasing number of people who are becoming residents of urban environments.
Defining urban.
No consensus or commonly accepted definition of urban has been used by international bodies, national or local governments, planners or policy makers, let alone by health scientists.7,15 United Nations Population Division data for 228 countries indicate that 108 use administrative definitions (e.g., city resident) to define urban, 51 use population density (e.g., people per square kilometer), and 39 use functional characteristics (e.g., extent of nonagricultural economic activity), whereas no definition exists for 22 and 8 classify either all or none of their citizens as urban.3 Thus, because each national Demographic and Health Survey relies on that country's National Statistical Office for their definition of urban, it is difficult to compare with other countries because of such differences. Urban communities have been defined by characteristics such as population number, density within specific geographic areas, or ensembles of people with governance responsibilities. For example, a settlement in Uganda with more than 100 people is classified as urban, while urban locales in Nigeria are those with more than 20,000 inhabitants.16 Alternatively, settings in Ethiopia with at least 2,000 people are classified as urban, whereas in Botswana urban areas include agglomerations of at least 5,000 inhabitants, most of whom depend on nonagricultural activities.17 These examples are all from SSA. However, similar lack of clarity and consistency is seen in Latin America, Asia, and other regions. In addition, efforts to quantify or compare urban and rural malaria have begun to explore various complex metrics that view this false dichotomy as a continuum.18
A related issue involves the spatial heterogeneity of conditions within an urban boundary. Particularly in poor but rapidly developing urban areas, important spatial variation in urban features may be missed if the average or modal conditions are assumed to represent the entire setting. Consider how crowded urban slums, open city parks, dense commercial centers, or “suburban” fringe residences are often interwoven within the boundary of what is considered urban. Thus, spatial heterogeneity, hence scale, add to the confusion of what constitutes risk.19
Nevertheless, investigators often agree on a qualitative, if subjective, definition of urban as an area with a sufficient density of housing and population that the setting is not a rural environment comprising open land with many fewer people and dwellings. However, to carefully evaluate and compare different areas, a more quantitative definition of urban is needed. One example is that of Phillips.20
… a geographic region whose boundaries are specified by a municipal/national government authority; which contains one or more areas with a high concentration of businesses, housing, paved streets and roads; with a high population density; where agriculture is regulated by a municipal authority; and with a total population size that exceeds 15,000 people.
Although it may be intuitively rational, this definition illustrates some of the challenges to characterizing urban malaria risk, because it does not consider the area of land (hence human population density), the extent of infrastructure concentration, or the type and extent of crop production. These and other features of the built environment are critical to many features that contribute to malaria risk, some of which can be detected using remotely sensed (satellite image) data.21,22
Defining urban malaria.
The extent of malaria within spatial subunits can be described, but only after the definition of what constitutes malaria is clarified. Is the appropriate metric one of infection, disease, or both? What level of accuracy is needed based on the goals of surveillance? How is temporal variation (seasonality) recognized? These and other considerations will affect the presence and intensity of malaria incidence or prevalence in urban settings. Dissimilar definitions and diverse metrics make those comparisons difficult or impossible.
Defining urban malaria is also challenging because the same term is used to describe situations ranging from obviously imported cases (people infected in known endemic areas, who are then diagnosed after traveling to urban regions of non-endemic areas) to circumstances in which there is strong evidence for urban transmission in known endemic areas. In addition, substantial uncertainty often exists about the travel histories of infected people and the evidence for urban transmission within endemic areas. A better understanding of “imported” malaria is particularly important in urban settings, which constitute hubs of human mobility and migration. For those reasons, the guidance we have provided on this question (Table 1 ) emphasizes the effects of differences (gradients) in these parameters and the effects of those changes rather than a static (less flexible) classification.
Table 1.
Types of evidence used to infer whether urban malaria is the result of transmission in an urban setting
| Strength of evidence | Human surveillance | Human surveillance metrics | Entomological surveillance | Entomological indices | Parasite or vector genetic analysis |
|---|---|---|---|---|---|
| Weakest | Febrile disease at urban clinic | – | – | – | Parasite count (no. per microliter blood) |
 |
Slide or PCR confirmed diagnosis at urban clinic | PP of humans that are thick smear positive | Nearby presumed breeding sites | – | Infected humans: MOI from MSP1 genotyping, molecular barcodes, microsatellites, whole genome sequencing, SNP arrays |
| Confirmed infection in UR | Incidence of laboratory confirmed malaria (N) | CV larvae/pupae in breeding site (N) | – | – | |
| More infection in permanent URs than in travelers | PP higher in “urban” than “rural” setting; PP higher in permanent URs than travelers | CV adults in houses (vector density/immature density); cohabitation with other vectors in breeding sites | Human blood meal index (% blood-fed and ELISA positive) | Malaria parasites: frequencies of drug resistance markers such as pfcrt, dhfr, dhps, pfmdr, and kelch 13 variants | |
| – | Higher incidence (N) in permanent URs than in travelers | Blood-fed CV adults resting inside house (may be endophilic or exophilic) | HBR | – | |
| Greater PP prevalence among non-travelers | Greater PP among “non-travelers” | Sporozoite-infected adult mosquitoes | SP (% of CSP-positive adult female CVs) | – | |
| – | – | HLC or bednet trapping of CV (N) | EIR estimate | Anopheline vectors: markers for M and S chromosomal forms, PCR for species confirmation, drug resistance markers (kdr and ace-1) | |
| Strongest | Confirmed Plasmodium infection with no history of travel and Anopheles in house | ACD of elevated PP among sick and healthy people lacking travel history | Blood-fed gametocyte-infected adult mosquitoes and breeding sites nearby household | Peri-domestic breeding site(s) + sporozoite-infected adult mosquitoes + CSP-positive CVs | Similar or identical parasite clones in anopheline vectors and humans from same urban settings |
ACD = active case detection; CSP = circumsporozoite protein; CV = competent vector species; EIR = entomological inoculation rate (infective bites/person/year); ELISA = enzyme-linked immunosorbent assay; HBR = human biting rate; HH = household(s); MOI = multiplicity or clonality of infection; MSP1 = merozoite surface protein-1; PCR = polymerase chain reaction; PP = parasite prevalence in humans; SNP = single nucleotide polymorphism; SP = sporozoite prevalence; UR = urban resident(s).
The strength of evidence for Plasmodium parasites, Anopheles vectors, and relationships with humans is ranked from weakest to strongest with regard to implicating local urban transmission.
Defining “urban malaria transmission.”
Although solid evidence of human infection among urban residents is important, the critical need is to determine how much “local transmission” is ongoing in and around urban dwellings. Different kinds of observations represent stronger or weaker evidence that permits development of more or less robust inferences (Table 1). To help focus and design better interventions, it is essential to know whether people are being infected in the urban areas where infection and disease are being diagnosed. If not, then information on travel histories becomes critical to determining activities and locations of risk. For infections that are acquired in urban settings, it is then important to understand when and where transmission is occurring and how urban transmission may differ from that in rural settings. In particular, it is crucial to characterize and understand how urban microhabitats promote Anopheles vector abundance and influence their behavior of biting humans. Urban microclimate variables (e.g., temperature, relative humidity, and precipitation) are also crucial to mosquito survival, reproduction, and development, thereby influencing vector presence and abundance in urban environments.23 Beyond vector abundance, house construction, insecticide-treated net (ITN) use, and other factors that influence human–vector contact represent important factors in transmission risk. Similarly, an understanding of the amount of spatial variation that exists in urban areas with Plasmodium transmission and its magnitude are essential for knowing what kinds of control or prevention efforts should be undertaken in those settings.
A recent systematic review of factors contributing to Plasmodium transmission in urban areas of SSA24 evaluated more than 100 peer-reviewed studies from throughout the subcontinent. Most of these studies documented that cases were being steadily recorded in urban settings, at a surprisingly high incidence, although malaria incidence in urban areas was typically lower than in the surrounding rural settings. However, lower incidence in high population urban areas may represent a large number of urban malaria cases. Fewer studies have documented the vector-related characteristics (breeding sites, suitable environments, among others) found in these urban environments. Finally, only a few studies have provided direct evidence of urban transmission (vector feeding and vector infection), consistent with the difficulty of obtaining evidence for transmission in urban settings.
Epidemiologic studies and localization of urban transmission.
Epidemiologic factors consistent with the presence and importance of focal transmission include spatial patterns of focal heterogeneity in the prevalence of human infection from cross-sectional surveys or the incidence of disease from passive case detection (PCD). Most PCD occurs through health facility–based summaries of malaria treatment, whether summarized in government reports or gathered from facilities by researchers. Active case detection (ACD), however, involves researchers intentionally testing people and identifying cases through research based in the community. Although a number of reports have noted that ACD is more sensitive than PCD,25–27 the greater cost of ACD in combination with modest budgets has limited most ICEMR investigators in SSA (except Malawi) to the use of PCD for the assessment of urban malaria. In Indian cities with high population densities, the lack of adequate manpower limits the use of ACD on a routine basis. Nevertheless, follow-up contact and confirmation microscopy for Plasmodium falciparum cases is undertaken to reduce further transmission.
Travel by residents of urban areas can put them at increased risk of both infection and disease and may thus limit the accuracy of inferences about urban transmission based on the diagnosis of malaria in urban areas. These may be short-term visits (days, weeks) to more rural areas of high risk, longer-term residency shifts (months), or more permanent migration (years). Malaria parasite infections acquired outside urban settings may be diagnosed subsequently in cities or towns where health facilities are more accessible and thus attributed incorrectly to urban transmission in the absence of diligent travel histories and entomologic studies. Careful sampling in heterogeneous urban neighborhoods is necessary to obtain representative survey results,28 and questionnaire-based travel histories can be essential to determine where urban residents actually became infected.29 More generally, travel is a potential risk factor for malaria among residents in both urban and rural settings.30–33
Travel and changes in urban malaria.
Movement of a more permanent nature, in particular the movement of rural residents to urban and peri-urban areas (i.e., urbanization), is a major demographic trend with health impacts occurring in many parts of the globe.19 However, the effects of urbanization on Plasmodium transmission and malaria risk are not well understood. Decade-old reviews of studies addressing urbanization and malaria by Keiser and others,11 Tatem and Hay,7 Hay and others,3 and Utzinger and Keiser6 summarized various research projects where this issue had been investigated until then. In addition, recent work has added to our understanding of changing malaria risk in relation to migration in east Africa,33 as well as global patterns of disease caused by Plasmodium vivax.35 These studies and the work of Tatem and others1 suggest that the incidence of malaria tends to be lower in urban areas and that urbanization is accelerating as global malaria incidence is decreasing. However, because this concordance of increasing urbanization and decreasing malaria is occurring during a period of rapid scale-up of antimalarial interventions, it is difficult to ascribe causation or predict the future with confidence.
Entomologic studies informing localization and intensity of transmission.
Entomologic factors consistent with the presence and importance of urban transmission include the presence of known Anopheles vectors in collections from pyrethrum spray catches (PSCs) and human landing catches (HLCs), evidence for human blood meals in blood-fed anopheline vectors, sporozoite-infected (circumsporozoite surface protein [CSP]–positive) anopheline vectors and elevated nightly biting rates and entomologic inoculation rates (EIRs) (Table 1). However, it is often more difficult to obtain reproducible estimates of entomologic parameters in urban areas (possibly because of less intense transmission and lower vector densities) than in rural areas (which typically have more intense transmission). For example, the low numbers of vectors obtained from PSCs and HLCs in some urban areas are often associated with sporozoite prevalence of 0% (in part because of the Poisson distribution effects with small sample sizes) and thus with EIR estimates of 0.0 infective bites/person/month, although hundreds of malaria cases are being diagnosed each month in the same urban area(s). Despite these constraints of small sample sizes on EIR estimates, the presence of competent Anopheles vectors in urban settings where humans are infected is strongly suggestive of local transmission (Table 1).
Vector incrimination and estimation of the urban EIR36 are critical to evaluating how much infection of urban residents results from local urban transmission. As Robert and others summarized in 2003,37 the heterogeneity of EIRs across the urban-to-rural gradient, and even within individual urban settings, is considerable. In more recent studies, for example, two areas of downtown urban Dakar, Senegal, were evaluated for Plasmodium infection of Anopheles gambiae s.l. at the end of the rainy season, producing estimated annual EIRs ranging from 3 to 9.5.38 Similar studies in Libreville, Gabon, compared Anopheles abundance, feeding, and infection during rainy and dry seasons in five districts of the city, showing very heterogeneous patterns, with EIRs for An. gambiae s.l. ranging from 0 to 19.2 during the dry season and from 2.5 to 68.7 during the rainy season. Curiously, the highest EIRs in the Libreville study were found in the most central and urbanized area. In contrast, in the municipality of Kandi, Benin, Govoetchan and others39 demonstrated that while rural transmission by An. gambiae s.l. was much higher, EIRs of 7.5 PPY (per person per year) were observed in the urban areas during the rainy season. Still another study in Mbalmayo, Cameroon (population ∼65,000), demonstrated perennial transmission of P. falciparum by An. gambiae (84%), Anopheles moucheti (11%), and Anopheles funestus (5%) that produced an average annual EIR of 129 in that urban setting.40 Overall, transmission in urban areas is generally lower than in rural areas but can be surprisingly elevated in some urban environments.
Research on Urban Malaria at ICEMR Network Sites
The varied ways in which urban malaria is being studied by seven ICEMRs throughout the globe demonstrate the challenges of characterizing and comparing patterns of infection and disease risk. Investigations at these seven sites are described briefly below, with summary information on the biophysical characteristics (Table 2), study design (Table 3), parasite and vector species (Table 4), and the major research questions being examined (Table 5).
Table 2.
Summary of the geographic, demographic, and climatic characteristics of each of the seven ICEMR sites that are studying malaria in urban settings
| ICEMR (urban site, region, country) | Amazonia (Mâncio Lima City, Acre State, Brazil) | East Africa (Jinja City, Jinja District, Uganda) | India (Besant Nagar, Chennai City Tamil Nadu State, India) | Latin America (Quibdó City, Chocó Department, Colombia) | Malawi (Communities in Blantyre City, Malawi) | South Asia (Panaji City, Goa State, India) | West Africa (Madina Fall, Thiès City, Thiès Region, Senegal) |
|---|---|---|---|---|---|---|---|
| Latitude/longitude | 7.62° S, 72.89° W | 0.45° N, 33.24° E | 13.00° N, 80.27° E | 5.69° N, 76.66° W | 15.67° S, 34.97° E | 15.50° N, 73.83° E | 14.83° N, 16.92° E |
| Elevation (m) | 200 | 1,140 | 6 | 30 | 766 | 7 | 60 |
| Area (km2) | 5,453 | 12,853 | ∼21 | 3,338 (6,078) | 228 | 36 | 2.47 |
| Population | 16,795 | 31,900 | 4.7 M | 120,000 | 1.01 M | 114,405 | 320,000; 21,000 Madina Fall |
| Population density | 2.79/km2 | 2.5/km2 | 10,988/km2; 0.2 M Besant Nagar | 362,625; 36.0 (59.7)/km2 | 4,400/km2 | 3,177/km2 | 8,502/km2 |
| Climate | Dry: July–September | Wet: March–May and September–November | Tropical monsoon | Transmission throughout year | Tropical highland | Hot humid: March–May | Sudan–Savanna |
| Seasonality | Wet: October–June | Dry: December–February and June–August | Hot dry (March–June); Hot Wet (Oct–Dec) | Mild dry (May–September); Hot wet (October–April), | Monsoon: June–September; Winter: December–February | Mild dry (January–June), Hot wet (July–November) | |
| Rainfall (mm) | 2,200 | 1,334 | ∼1,400 | 8,131 (≥ 500/month) | 1,127 | 2,932 | 438 |
| Mean temperature (°C) | 27 | 23 | 33 | 27 | 22 | 27 | 24 |
| Estimates of prevalence, intensity of transmission | Prevalence: Pf = 0.5–1.3%, Pv = 1.0–4.1% | Prevalence = 8.8% (7.5–10.2%); Incidence = 0.48 (0.42–0.55) PPY | Prevalence = 18.6% (2014); 77% prevalence decline at Besant Nagar (2011–2014) | Prevalence: Pf = 2.6%; 5,064 cases (2012) with 31% from urban areas | Year-round transmission; slide prevalences: ∼5% (dry season), ∼8% (rainy season) | Slide prevalence: Pf = 0.02–0.6%, Pv = 0.2–1.3% | Prevalence < 1%, incidence uncomp-mal ≤ 1.1 × 10−3/month, 1,000–2,000 dx/year at referral clinic |
| EIR = unknown | EIR = 2.8 (1.6–4.5) PPY | EIR = unknown | EIR = unknown | EIR = unknown | EIR = 2.35 PPY | EIR = 0.0 PPY, 0.0% vectors CSP positive | |
| Aims, questions, hypotheses | Determine if in active/abandoned aquaculture produces vector breeding sites that facilitates urban transmission | XS and entomological surveys, longitudinal cohort, capacity building, technology transfer, research-policy maker links | Evaluate vector transmission, drug resistance, sexual/asexual infection, mixed species/strains, diversity of Pf/Pv; environmental conditions | Identify epidemiologic and other factors responsible for transmission | Evaluate risk factors in urban vs. rural children; HH risk for infection, asymptomatic gametocytemia and anemia | Examine parasite genetics; resistance and immunity; infection prevalence; vector identification | Obtain prevalence from XS surveys, then focus on urban areas with potential breeding sites |
| Site-specific references | 41–43 | 44–46 | 27,47–49 | 50,51 | 52–56 | 57–61 | 62,63 |
CSP = circumsporozoite surface protein; EIR = entomological inoculation rate (infectious bites PPY); HH = household; ICEMR = International Centers of Excellence for Malaria Research; M = million; Pv = Plasmodium vivax; Pf = Plasmodium falciparum; PPY = per person per year; XS = cross-sectional.
Table 3.
Summary of the study design, questions, methods, human sampling, and analytical characteristics of each of the seven ICEMR sites conducting research on malaria in urban settings
| ICEMR (urban site, region, country) | Amazonia (Mâncio Lima City, Acre State, Brazil) | East Africa (Jinja City, Jinja District, Uganda) | India (Besant Nagar, Chennai City Tamil Nadu State, India) | Latin America (Quibdó City, Chocó Department, Colombia) | Malawi (Communities in Blantyre City, Blantyre District Malawi) | South Asia (Panaji City, Goa State, India) | West Africa (Madina Fall, Thiès City, Thiès Region, Senegal) |
|---|---|---|---|---|---|---|---|
| Types of epidemiologic studies | Prospective, observational | Incidence and prevalence surveillance | Epidemiologic, experimental, and laboratory-based genetic studies | Descriptive epidemiologic surveillance | Ecologic, environmental and laboratory based | Epidemiologic, experimental, and laboratory based | Community-based longitudinal cohort study |
| Outcome(s) being measured | Time/GIS pattern by genotype; clonal expansion; vector breeding sites; larval genetic markers to estimate migration | Infection incidence (0.55–0.36/year); prevalance (9.8–6.7%); EIR 4.0–1.8; prevalence of anemia | Prevalance of infection; MOI; genetic diversity of Pv; role of sub-patent Pf in transmission; vector resting preference; HBI; vectorial capacity and EIR | Prevalance and origin of infection (local or imported); vector presence and predominance; human–vector contact | Urban vs. rural behavioral, SES, environmental risk | MOI, disease severity, genetic variation, drug resistance, var gene expression, RBC invasion, human malaria vectors | Infection duration and prevalence; antibodies to vaccine antigens (in study cohort); home visits to evaluate/review symptoms |
| Prevalence of parasitemia (including gametocytemia) in healthy subjects | |||||||
| Exposure(s) or predictor(s) being measured | Environmental: breeding sites; behavior: travel to rural sites and other malaria-endemic areas | LLINs, HH structure, vegetation, temperature, and humidity | Environmental micro-climate, temperature, and humidity (larval and adult vector studies); vector saliva antibodies | Humidity, rainfall, temperature, breeding sites, vector contact by antibodies to vector saliva | Housing, vector proximity, prevention methods | Geographic (migration, travel history), occupational (i.e., migrants, construction workers) | Intensity of vector-to-human transmission; vector breeding sites (GIS) |
| Demographic, pregnancy, season | |||||||
| Study designs being used | PID at government HF | Longitudinal cohort | XS surveys (ACD, and RCD); PCD at clinic and longitudinal cohort designs | XS studies (ACD, RCD) and interventions | Case–control HF + follow-up | PCD at tertiary care government hospital | Cross-sectional, household-based prospective study design |
| Community XS/school surveys | XS, HH-based designs | ||||||
| Sources of subjects | Government HFs | Community HHs | Community HHs | HHs, HFs | HFs, HHs | HFs | Community HHs |
| Community HHs | |||||||
| Selection or sampling method of subjects | Persons seeking malaria diagnosis and treatment at government HFs | Sample of 100 HHs with children + caregiver > 18 | Epidemiologic study (random sampling); environmental based (transmission study) | Recruitment of cases at HFs | Any visitor to HF fitting the inclusion criteria | Positives at HFs | Random sample of HHs initially |
| Sample of 200 HHs + three schools | Active case detection at two sites with 900 people per site | All HH members | |||||
| Definition and determination of urban | Households are considered urban if residents call them urbano | Not predefined | Based on current guidelines from the MoH (UMS of India) and the city of Chennai for the community of Besant Nagar | Urban as defined by a government; examine effects of sociodemographic and geographic factors (including GIS) | Government city limits; multiple definitions and criteria | Defined by Indian government administrative criterion, including agglomerations | City population of 320,000 with 1,000–2,000 Pf cases of uncomplicated malaria diagnosed each year at regional clinic |
| Random HHs in city | |||||||
| Age(s) of subject(s) | Persons of all ages | 6 months–11 years | ≤ 1 to ≤ 70 year old (Eco-Epidemiology Project) | > 2 year old | ≤ 15 years old | Subjects ≤ 1–65 years old | Persons of all ages |
| Person > 18 years old | All ages | ||||||
| Sample period | January 2015–January 2016 | August 2011–present | XS: December 2013–December 2014 | June 2014 (pilot) | April 2012–present | Apr 2012–present | September 2012–present |
| Clin: 2013–present | April 2012–present | ||||||
| RCD: 2014–present | |||||||
| Mosq: 2013–2014 | |||||||
| Long: February 2013–January 2014 | |||||||
| Sample frequency | 5 days/week (Monday–Friday) when HFs open | During illness and at 3 months checkup | Clin, RCD: ∼weekly; XS: quarterly, Long: monthly | Twice yearly | All year, 5 days/week | Daily (one time-point per subject) | Twice yearly XS surveys |
| 6 weeks × 2/year | |||||||
| Contextual information or confounders | To be defined | Socioeconomic status, housing | Travel (as a potential confounding factor) | KAP surveys, case investigations (for travel and occupational histories) | KAP, SES, travel (confounders) | Travel, clinical presentation, previous antimalarial treatment | HH conditions (contextual); preventive measures (confounder) |
| SES, prevention, ITN, IPTp, location (confounders) | |||||||
| Unit(s) of analysis | Individual people; HHs parasite: clones breeding sites | Individual people; HHs | Individual people census clusters (XS surveys) | Individuals, HHs, study sites | Individual people, HH, HF, urban–rural environments | Individual people: parasite isolates/clones; vectors at construction sites | Individual people (longitudinal cohort studies), HHs (XS surveys), communities |
| Individual people, HH, EA | |||||||
| Analytical approaches being used | Spatial analysis: human infection genotype clusters near breeding sites | Statistical, modeling and spatial analysis | Spatial analysis; time series | Descriptive and inferential statistics. spatial analysis and statistical modeling | Logistic regression, multilevel, spatial | ICEMR analyses are specialized for each biological question | Initially descriptive, hypotheses based on seasonality, spatial clustering |
| Regression, multilevel statistic |
ACD = active case detection; EA = Enumeration Area; GIS = geographic information system; HBI = human biting index; HF = health facility; HH = household; IPTp = intermittent preventive treatment in pregnancy; ITN = insecticide-treated net; KAP = knowledge, attitudes and practices; LLINs = long-lasting insecticidal nets; MoH = Ministry of Health; MOI = multiplicity of infection; ICEMR = International Centers of Excellence for Malaria Research; PCD = passive case detection; PID = passive infection detection; Pv = Plasmodium vivax; Pf = Plasmodium falciparum; RBC = red blood cell; RCD = reactive case detection; SES = socioeconomic status; UMS = Urban Malaria Scheme; XS = cross-sectional.
Table 4.
Summary of the Plasmodium and Anopheles species present, vector sampling, and laboratory methods at each of the seven ICEMR sites that are investigating malaria in urban settings
| ICEMR (urban site, region, country) | Amazonia (Mâncio Lima City, Acre State, Brazil) | East Africa (Jinja City, Jinja District, Uganda) | India (Besant Nagar, Chennai City, Tamil Nadu State, India) | Latin America (Quibdó City, Chocó Department, Colombia) | Malawi (Communities in Blantyre City, Blantyre District, Malawi) | South Asia (Panaji City, Goa State, India) | West Africa (Madina Fall, Thiès City, Thiès Region, Senegal) |
|---|---|---|---|---|---|---|---|
| Plasmodium species | vivax, falciparum (malariae) | falciparum (predominant) | vivax (predominant), falciparum | falciparum (61%) | falciparum, malariae, ovale | vivax (predominant), falciparum | falciparum |
| Anopheles species | triannulatus, darlingi | gambiae s.s. (36%), arabiensis (64%) | stephensi | nuñeztovari, darling, albimanus, neivai, pseudopunctipennis | gambiae s.s., arabiensis, funestus | stephensi | arabiensis, funestus |
| Vector sampling | Larvae: dipping | Larvae: dipping | Larvae: dipping | Adults: HLC | Larvae: dipping (outdoor ground) | Adults: CDC light traps in sleeping areas of construction sites | Adults: HLC for biting rates, EIRs |
| Adults: CDC light traps | Adult HLC and PSC for biting rates, EIR | Transects from rural to peri-urban and to urban areas | Adults: light traps | PSCs likewise for biting rates, EIRs | |||
| Vector testing | ID: morphology, planned: PCR | ID: morphology, PCR | ID: morphology for spp., egg ridge for races or ecological variation | ID: morphology, PCR | ID: morphology, PCR for species | ID: morphology, PCR | ID: PCR |
| Blood meal: PCR | Infection: sporozoite CSP by ELISA | Blood meal for host | Infection: sporozoite CSP by ELISA | Infection: sporozoite CSP by ELISA | Genotype: PCR | Infection: sporozoite CSP ELISA | |
| Infection: sporozoite PCR | Infection: sporozoite CSP by ELISA | Blood meal: PCR | Blood meal: ELISA | ||||
| Plasmodium: PCR | |||||||
| Laboratory and field testing | Humans: smears, PCR, nested PCR | Humans: RDT, thick smear microscopy, Hemocue for Hb, immunologic studies | Humans: RDT, Hemocue for Hb | Humans: thick smear, RDT | Humans: slide microscopy, RDT | Humans: RDT, thick and thin smear, PCR, qPCR, sequencing, flow cytometry | Humans: RDT |
| Parasite typing based on SNPs or microsatellites | Laboratory: thick and thin smear and PCR | Laboratory: PCR-based parasite species ID | Laboratory: qRT-PCR | Laboratory: thick smear or PCR for diagnosis of human infection |
CDC = Centers for Disease Control and Prevention; CSP = circumsporozoite surface protein; EIR = entomologic inoculation rate; ELISA = enzyme-linked immunosorbent assay; Hb = hemoglobin; HF = health facility; HLC = human landing catches; ICEMR = International Centers of Excellence for Malaria Research; PSC = pyrethroid spray capture; qRT-PCR = quantitative reverse transcription polymerase chain reaction; RDT = rapid diagnostic test; SNP = single nucleotide polymorphism test.
Table 5.
Research questions of common interest across the ICEMR network at urban sites in South America, Asia, and sub-Saharan Africa
| Evaluation of malaria in urban areas | Malaria in urban areas ranges from foci of intense transmission to obvious importations in travelers returning from highly endemic areas |
| Because the intensity of transmission is typically lower in urban than rural areas, proof of urban malaria transmission is uncommon | |
| This inadvertently means that the term “urban malaria” is often applied empirically to all persons whose malaria was diagnosed in urban areas | |
| Although difficult, the proof of transmission (or the lack of transmission) in urban areas is an essential priority for malarial control | |
| This is because proof of transmission simultaneously provides both new information and potential malaria control strategies | |
| Descriptive epidemiology | What are the age distributions of malarial infection (parasitemia, positive smears) and disease (uncomplicated and complicated/severe malaria)? |
| Is there evidence that children, adults, or others are protected from (or at increased risk of) infection or disease? | |
| Seasonality | Does the prevalence of infection (parasitemia) decrease during the dry season and increase with the return of seasonal rains? |
| How are seasonal patterns such as rainfall related to the incidence of disease? | |
| When does the incidence of malarial disease peak in relation to the intensity of transmission and the peak prevalence of infection? | |
| Length of residence | The effects of prolonged residence in this or other malaria-endemic areas |
| Do persons who have lived in this or other malaria-endemic areas for ≥ 10 years acquire either the semi-immune state (protection against serious disease) or sterile immunity (protection against both infection and disease)? | |
| Entomologic factors | What vectors are present at different study sites during the different times (seasons) of the year? |
| How do their biting rates and EIRs relate to the frequency of human infection and disease? | |
| Are these characteristics in more urban settings similar to or different from what has been found in rural areas? | |
| Complex malaria | Why are infections with more than one parasite genotype most common with Plasmodium falciparum in sub-Saharan Africa? |
| Conversely, why are infections with more than one parasite species (e.g., P. falciparum and Plasmodium vivax) more common in India, other parts of Asia, and South America? | |
| Why are Plasmodium ovale and Plasmodium malariae infections much less common than P. falciparum in Africa? |
EIRs = entomologic inoculation rates; ICEMR = International Centers of Excellence for Malaria Research.
Amazonia ICEMR, Mâncio Lima City, Brazil.
Mâncio Lima City on the Moa River is the westernmost city in Brazil, has a low population density of 1.4–3.0 inhabitants/km2, an average daily temperature of 27°C and 2,200 mm (220 cm = 7.2 feet) of rain per year. With a 0.5–1.3% prevalence of P. falciparum infection (based on thick smears) and a 1.4–4.1% prevalence of P. vivax infection (based on thick smears), malaria is an important public health problem and a potentially serious obstacle to socioeconomic development. With distinct seasons (dry from July to September, wet from October to June) and limited entomologic information available, potential high priority questions to consider include the use of long-lasting insecticidal nets (LLINs) and the availability of free artemisinin combination therapy (ACT) treatment of persons who have uncomplicated malaria. Because interventions that interrupt transmission are likely to be the most effective, the most important question in terms of malaria control may be when vector-borne transmission and the prevalence of parasitemia begin to increase each year.
East Africa ICEMR, Jinja City, Uganda.
Annual rainfall and the average daily temperature were lower in Jinja City, Uganda, than in either Quibdó City, Colombia, or Mâncio Lima City (1,334 versus 8,131 and 2,200 mm; 23°C versus 27° C and 27° C, respectively). Nevertheless, the prevalence of P. falciparum infection was higher in Jinja City (8.8% versus 2.6% and 0.5–1.3%) and the EIR in Jinja City was substantial (2.8 infectious bites PPY [per person per year]) although no EIR data were available for comparison from Quibdó City or Mâncio Lima City. Preliminary results from Jinja City indicate that the malaria control strategies implemented have been associated with reductions in both the prevalence of infection (from 9.8% to 6.7%) and the EIR (from 4.0 to 1.8 PPY) and thus suggest that those interventions have been effective. The greater prevalence of infection in Jinja City than in Quibdó City or Mâncio Lima City indicates that other factors in addition to annual rainfall and average daily temperature also affect the prevalence of human infection in these malaria-endemic areas.
India ICEMR, Besant Nagar Community in Chennai, India.
The city of Chennai has the highest population density of the seven ICEMR urban sites with 9,524 persons/km2 in the study site of Besant Nagar, substantial annual rainfall (1,400 mm/year), and the highest average daily temperature (33°C).47 Therefore, the prevalence of infection (parasitemia) in Besant Nagar (18.6%) was higher than in either Mâncio Lima City or Jinja City (0.5–1.3% and 8.8%, respectively). Chennai city accounts for 57–78% of all malaria cases recorded in the state of Tamil Nadu, with 93.6–99.7% of cases due to P. vivax. Anopheles stephensi, the vector responsible for transmission in the urban Chennai context, breeds in clean/clear stored water in overhead tanks, wells, cisterns, curing tanks/pits, underground tanks or sumps, earthen pots, roof gutters, and other such artificial containers.23
Latin America ICEMR, Quibdó City, Colombia.
An important characteristic of the environment in Quibdó City on the banks of the Atrato River in Colombia is the overwhelming amount of rainfall each year in this tropical rain forest area (8,200 mm = 323 inches or 27 feet of rain per year). This means that 25 of every 30 days of each month are rainy days, with one continuous tropical rainy season throughout the year and an average daily temperature of 27°C. The prevalence of infection in the community (from preliminary studies based on positive blood smears for asexual P. falciparum parasites) is 2.6%. Paradoxically, the most intriguing question raised by the data from this urban site is “Why is the prevalence of parasitemia in the community only 2.6%? Why is it not higher?” Factors potentially relevant to this question include variation in the human population (sickle cell, glucose-6-phosphate dehydrogenase), in the vector (zoophilic versus anthropophilic biting behavior, extrinsic incubation periods and conditions), and in the parasite population (var gene expression, single nucleotide polymorphisms related to antimalarial resistance).
Malawi ICEMR, Communities within Blantyre, Malawi.
The most striking feature of these data was the high population density within the urban communities studied in Blantyre, Malawi (consistent with extensive urbanization). Among the seven ICEMR urban sites, the population density in these urban sections of Blantyre (4,400 persons/km2) was exceeded only in Besant Nagar (10,988 persons/km2). Although total annual rainfall was similar (1,127 mm in Blantyre versus 1,400 mm in Chennai), the prevalence of infection was greater in Chennai than Blantyre (18.6% versus 5%, respectively, during the dry season and 8% in the rainy season). One of the unresolved questions raised by these data is why the prevalence of malaria in the urbanized areas of Blantyre (5–8%) is not closer to the similarly urbanized area of Chennai (19%). To confirm or reject candidate hypotheses, potential explanations (such as differences in the vector, parasite, or human populations) will need to be tested in additional communities at other sites with similar population densities.
South Asia ICEMR, Panaji City in Goa, India.
As estimated from annual rainfall and average daily temperatures, environmental conditions in Panaji City, India, were basically similar to those in Chennai (2,932 and 1,605 mm annual rainfall, 27° and 29°C average daily temperatures, respectively). Goa is one of four sites being studied in the south Asia ICEMR and is potentially the most relevant for understanding urban malaria in south Asia. Goa is India's smallest state and is composed of contiguous urban centers including the capital city of Panaji.64 With a gross domestic product per capita 2.5 times that of the whole country and as much as 10 times greater than states such as Assam, Jharkhand, and Orissa, infrastructure projects in Goa attract migrant workers from the east and northeast of India.65–67 These states are rural and have the highest malaria endemicity in the country.68 In addition, tourism is Goa's largest industry, with many domestic and international tourists visiting year-round.69
One potentially important factor in the spread of malaria in India, particularly P. falciparum, is human migration. For south Asia malaria control experts, the link between human migration and the importation of malaria into urban settings is of enormous interest. Migrants may not have access to government health services and may therefore be exposed to preventive and treatment strategies different than national malaria control recommendations (e.g., use of ITNs and correct choice of antimalarial drug/drug regimens).70 However, the greatest worry is that migrants may facilitate the movement of drug resistance from northeast India to large urban centers in the west and southwest parts of India.71–73
The south Asia ICEMR is interested primarily in understanding how parasite populations vary in genetic plasticity across India and how that variation affects drug resistance, pathogenesis, and the ability of the parasite to overcome innate and acquired immunity. Those questions are now being addressed in part through passive surveillance at a public tertiary care hospital (Goa Medical College and Hospital), via vector collections in semi-enclosed living quarters at construction sites and with the aid of parasite collections in other states (Assam and Jharkhand).
West Africa ICEMR, Community of Madina Fall in Thiès, Senegal.
The most striking feature of the data from Madina Fall is the discrepancy between the substantial numbers of cases diagnosed at the regional clinic for this area of Senegal (1,000–2,000 cases of uncomplicated P. falciparum malaria per year) and apparently contradictory factors such as the low prevalence of infection in the community (< 1% based on thick smears), the virtual absence of sporozoite-infected (CSP-positive) vectors, and—as a result—low or undetectable EIRs (0.0 infectious bites/person/month or year) at times when hundreds of cases are being diagnosed at a clinic within the same community each month.
The most interesting potential explanation for the greater numbers of malaria cases in Madina Fall than the surrounding urban areas is a gradient from primarily urban to primarily rural environments, which progresses from smaller to larger numbers of vectors and from lower to higher prevalences of human infection in association with active breeding sites in the most rural areas of Madina Fall where there is irrigation and vegetable farming. This hypothesis, if confirmed, suggests that malaria control strategies for such hot spots should focus on active breeding sites and should begin before the number of adult vectors and the prevalence of human infection begin to increase after the seasonal rains begin.
Implications of ICEMR Analyses of Urban Malaria
The practical implications of better understanding the patterns and causes of Plasmodium infection and malarial disease in urban areas are many. Depending on how much transmission is occurring at the urban site versus elsewhere, prevention and control strategies may need to be modified extensively. For example, indoor residual spraying (IRS) will have no effect on malaria transmission if mosquitoes are not biting indoors. In India, vector control measures in urban settings are based primarily on larval source management, which is achieved by preventing ovipositioning by An. stephensi in artificial water storage containers. However, this approach becomes more difficult and less successful in other settings where natural, rain-fed bodies of water or irrigation channels serve as breeding sites. In those settings, additional preventive strategies may include specific education to remind travelers to use ITNs, LLINs when away from their homes. As a result, the effectiveness of ITNs74 and of ITNs versus IRS75 may vary substantially in urban versus rural settings.
Similarly, treatment may be ineffective and inappropriate if antimalarial drugs are taken for malaria-like symptoms in the absence of a positive smear or RDT. Conversely, testing for malaria may be delayed or unavailable if it has been assumed that malaria is rare or nonexistent in urban areas.
The small-scale spatial heterogeneity in urban transmission associated with clusters of people living near parks, water bodies, small-scale agricultural land, or peri-urban fringes also makes prevention difficult if neighborhood-specific risks are not known or considered. For example, urban breeding sites for An. gambiae in SSA (e.g., small urban gardens) and An. stephensi in India (e.g., urban water storage tanks) are microhabitats that are often unrecognized in large urban areas. This observation highlights the importance of identifying “hot spots” of transmission76 that require intervention at the local level. In addition, the generally lower frequency of malaria in urban settings also creates challenges for surveillance. This is because the lower prevalences of infection and lower incidences of disease in those areas mean that foci of transmission are more difficult to detect. As suggested during a recent study of urban malaria in Ouagadougou, Burkina Faso,48 irregularly or sparsely constructed dwellings near irrigation networks are locations where preventive strategies focused on urban children can be very effective.
The frequency of Plasmodium infection and the frequency and severity of malarial disease among urban residents vary by ICEMR regional epidemiologies and vector ecologies. The goal of these studies is to identify patterns across the urban sites within the ICEMR network that can be used to improve malaria control. However, in each case, detailed knowledge about local conditions will likewise be essential to reduce the intensity of urban transmission. We hypothesize that the urban sites that succeed—those that markedly reduce the frequency of both malarial infection and disease—will be the sites that most clearly define the ways in which transmission at their site is similar to and different from urban transmission at other urban ICEMR sites.
ACKNOWLEDGMENTS
We thank the following scientists from each of the seven participating ICEMRs for contributing information that was used in this report: Paulo Ribolla (Amazonia); Agaba Katreebe, Grant Dorsey (east Africa); Lalitha Ramanathapuram, Jane Carlton (India); Martha Quinones, Socrates Herrera (Latin America); Atupele Kapito-Tembo, Themba Mzilahowa, Miriam Laufer, Terrie Taylor (Malawi); Ashwani Kumar, Edwin Gomes, Jagadish Mahanta, P. K. Mohapatra, Pradipsinh Rathod (south Asia); and Jules Gomis, Jean-Louis Ndiaye (west Africa).
Footnotes
Financial support: This study was supported by grants from the National Institute of Allergy and Infectious Diseases to each of the seven ICEMR sites whose research is represented in this report.
Authors' addresses: Mark L. Wilson, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, E-mail: wilsonml@umich.edu. Donald J. Krogstad, Department of Tropical Medicine, Tulane School of Public Health and Tropical Medicine, New Orleans, LA, E-mail: krogstad@tulane.edu. Emmanuel Arinaitwe, Infectious Diseases Research Collaboration, Mulago Hospital Campus, Kampala, Uganda, E-mail: earinaitwe@idrc-uganda.org. Myriam Arevalo-Herrera, Caucaseo Scientific Research Center, School of Health, Universidad del Valle, Cali, Colombia, E-mail: marevalo@inmuno.org. Laura Chery, Department of Chemistry, University of Washington, Seattle, WA, E-mail: chery@chem.washington.edu. Marcelo U. Ferreira, Department of Parasitology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil, E-mail: muferrei@gmail.com. Daouda Ndiaye, Department of Parasitology, Faculty of Medicine, University Cheikh Anta Diop, Dakar, Senegal, E-mail: daouda.ndiaye@ucad.edu.sn. Don P. Mathanga, Malaria Alert Centre, College of Medicine, University of Malawi, Blantyre, Malawi, E-mail: dmathang@mac.medcol.mw. Alex Eapen, National Institute of Malaria Research (Indian Council of Medical Research), National Institute of Epidemiology Campus, Tamil Nadu, India, E-mail: alexeapen@yahoo.com.
References
- 1.Tatem AJ, Gething PW, Smith DL, Hay SI. Urbanization and the global malaria recession. Malar J. 2013;12:1–10. doi: 10.1186/1475-2875-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne N. Urban malaria risk in sub-Saharan Africa: where is the evidence? Travel Med Infect Dis. 2007;5:135–137. doi: 10.1016/j.tmaid.2006.04.003. http://www.ncbi.nlm.nih.gov/pubmed/17298922 Available at. Accessed May 10, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3130901&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omumbo JA, Guerra CA, Hay SI, Snow RW. The influence of urbanisation on measures of Plasmodium falciparum infection prevalence in east Africa. Acta Trop. 2005;93:11–21. doi: 10.1016/j.actatropica.2004.08.010. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3191363&tool=pmcentrez&rendertype=abstract Available at. Accessed June 11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMichael AJ. The urban environment and health in a world of increasing globalization: issues for developing countries. Bull World Health Organ. 2000;78:1117–1126. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2560839&tool=pmcentrez&rendertype=abstract Available at. [PMC free article] [PubMed] [Google Scholar]
- 6.Utzinger J, Keiser J. Urbanization and tropical health—then and now. Ann Trop Med Parasitol. 2006;80:517–533. doi: 10.1179/136485906X97372. http://www.ncbi.nlm.nih.gov/pubmed/16899152 Available at. Accessed December 8, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Tatem AJ, Hay SI. Measuring urbanization pattern and extent for malaria research: a review of remote sensing approaches. J Urban Health. 2004;81:363–376. doi: 10.1093/jurban/jth124. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3173841&tool=pmcentrez&rendertype=abstract Available at. Accessed November 30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatem AJ, Guerra CA, Kabaria CW, Noor AM, Hay SI. Human population, urban settlement patterns and their impact on Plasmodium falciparum malaria endemicity. Malar J. 2008;7:1–17. doi: 10.1186/1475-2875-7-218. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2586635&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11:131–141. doi: 10.1016/S1473-3099(10)70223-1. http://www.ncbi.nlm.nih.gov/pubmed/21272793 Available at. Accessed May 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cyril S, Oldroyd JC, Renzaho A. Urbanisation, urbanicity, and health: a systematic review of the reliability and validity of urbanicity scales. BMC Public Health. 2013;13:513. doi: 10.1186/1471-2458-13-513. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3671972&tool=pmcentrez&rendertype=abstract Available at. Accessed May 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser J, Utzinger J, Caldas de Castro M, Smith TA, Tanner M, Singer BH. Urbanization in sub-Saharan Africa and implication for malaria control. Am J Trop Med Hyg. 2004;71(Suppl):118–127. http://www.ncbi.nlm.nih.gov/pubmed/15331827 Available at. [PubMed] [Google Scholar]
- 12.Donnelly MJ, McCall PJ, Lengeler C, Bates I, D'Alessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape J-F, Hastings IM, Mutero C. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=552321&tool=pmcentrez&rendertype=abstract Available at. Accessed May 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatinelli G, Bosman A, Lamizana L, Rossi P. Prevalence du paludism a Ouagadougou et dans le milieu rural limitrophe en periode de transmission maximale. Parassitologia. 1986;28:17–31. [PubMed] [Google Scholar]
- 14.Trape JF, Zoulani A. Malaria and urbanization in central Africa: the example of Brazzaville. Part II. Trans R Soc Trop Med Hyg. 1987;81(Suppl 2):10–18. doi: 10.1016/0035-9203(87)90472-x. [DOI] [PubMed] [Google Scholar]
- 15.Vlahov D, Galea S. Urbanization, urbanicity, and health. J Urban Health. 2002;79(Suppl 1):S1–S12. doi: 10.1093/jurban/79.suppl_1.S1. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3456615&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.N. Centre for Human Settlements . Cities in a Globalizing World. London, United Kingdom: Earthscan; 2001. [Google Scholar]
- 17.Phillips DR. Urbanization and human health. Parassitologia. 1993;106(Suppl):S93–S107. doi: 10.1017/s0031182000086145. [DOI] [PubMed] [Google Scholar]
- 18.Dahly DL, Adair LS. Quantifying the urban environment: a scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;64:1407–1419. doi: 10.1016/j.socscimed.2006.11.019. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2001275&tool=pmcentrez&rendertype=abstract Available at. Accessed May 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDade TW, Adair LS. Defining the “urban” in urbanization and health: a factor analysis approach. Soc Sci Med. 2001;53:55–70. doi: 10.1016/s0277-9536(00)00313-0. http://www.ncbi.nlm.nih.gov/pubmed/11380161 Available at. [DOI] [PubMed] [Google Scholar]
- 20.Phillips DR. Urbanization and human health. Parasitology. 1993;106(Suppl):S93–S107. doi: 10.1017/s0031182000086145. [DOI] [PubMed] [Google Scholar]
- 21.Machault V, Vignolles C, Borchi F, Vounatsou P, Pages F, Briolant S, Lacaux J-P, Rogier C. The use of remotely sensed environmental data in the study of malaria. Geospat Health. 2011;5:151–168. doi: 10.4081/gh.2011.167. http://www.ncbi.nlm.nih.gov/pubmed/21590665 Available at. [DOI] [PubMed] [Google Scholar]
- 22.Machault V, Vignolles C, Pagès F, Gadiaga L, Tourre YM, Gaye A, Sokhna C, Trape J-F, Lacaux J-P, Rogier C. Risk mapping of Anopheles gambiae s.l. densities using remotely-sensed environmental and meteorological data in an urban area: Dakar, Senegal. PLoS One. 2012;7:e50674. doi: 10.1371/journal.pone.0050674. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3511318&tool=pmcentrez&rendertype=abstract Available at. Accessed November 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cator LJ, Thomas S, Paaijmans KP, Ravishankaran S, Justin JA, Mathai MT, Read AF, Thomas MB, Eapen A. Characterizing microclimate in urban malaria transmission settings : a case study from Chennai, India. Malar J. 2013;12:1–10. doi: 10.1186/1475-2875-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med. 2012;2012:1–10. doi: 10.1155/2012/819563. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3483782&tool=pmcentrez&rendertype=abstract Available at. Accessed May 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belizario V, Saul A, Bustos M, Lansang M, Pasay C, Gatton M, Salazar N. Field epidemiological studies on malaria in a low endemic area in the Philippines. Acta Trop. 1997;63:241–256. doi: 10.1016/s0001-706x(96)00624-9. http://linkinghub.elsevier.com/retrieve/pii/S0001706X96006249 Available at. [DOI] [PubMed] [Google Scholar]
- 26.Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med. 2005;60:563–573. doi: 10.1016/j.socscimed.2004.05.025. http://www.ncbi.nlm.nih.gov/pubmed/15550304 Available at. Accessed November 19, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Utarini A, Chandramohan D, Nystrom L. Comparison of active and passive case detection systems in Jepara District, Indonesia. Asia-Pacific J Public Heal. 2007;19:14–17. doi: 10.1177/10105395070190010401. http://aph.sagepub.com/cgi/doi/10.1177/10105395070190010401 Available at. Accessed November 19, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Siri JG, Lindblade KA, Rosen DH, Onyango B, Vulule JM, Slutsker L, Wilson ML. A census-weighted, spatially-stratified household sampling strategy for urban malaria epidemiology. Malar J. 2008;7:1–10. doi: 10.1186/1475-2875-7-39. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2292736&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siri JG, Lindblade KA, Rosen DH, Onyango B, Vulule J, Slutsker L, Wilson ML. Quantitative urban classification for malaria epidemiology in sub-Saharan Africa. Malar J. 2008;7:1–9. doi: 10.1186/1475-2875-7-34. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2277427&tool=pmcentrez&rendertype=abstract Available at. Accessed December 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez F, Carrasquilla G, Muñoz A. Risk factors associated with malaria infection in an urban setting. Trans R Soc Trop Med Hyg. 2000;94:367–371. doi: 10.1016/s0035-9203(00)90106-8. http://www.ncbi.nlm.nih.gov/pubmed/11127234 Available at. [DOI] [PubMed] [Google Scholar]
- 31.Watts TE, Wray JR, Ng NH, Drapes CC. Malaria in an urban and a rural area of Zambia. Trans R Soc Trop Med Hyg. 1990;84:196–200. doi: 10.1016/0035-9203(90)90251-9. [DOI] [PubMed] [Google Scholar]
- 32.Osorio L, Todd J, Bradley DJ. Travel histories as risk factors in the analysis of urban malaria in Colombia. Am J Trop Med Hyg. 2004;71:380–386. [PubMed] [Google Scholar]
- 33.Pindolia DK, Garcia AJ, Huang Z, Smith DL, Alegana VA, Noor AM, Snow RW, Tatem AJ. The demographics of human and malaria movement and migration patterns in east Africa. Malar J. 2013;12:397. doi: 10.1186/1475-2875-12-397. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3829999&tool=pmcentrez&rendertype=abstract Available at. Accessed May 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yukich JO, Taylor C, Eisele TP, Reithinger R, Nauhassenay H, Berhane Y, Keating J. Travel history and malaria infection risk in a low-transmission setting in Ethiopia: a case control study. Malar J. 2013;12:33. doi: 10.1186/1475-2875-12-33. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3570338&tool=pmcentrez&rendertype=abstract Available at. Accessed November 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi Q, Guerra CA, Moyes CL, Elyazar IRF, Gething PW, Hay SI, Tatem AJ. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar J. 2012;11:403. doi: 10.1186/1475-2875-11-403. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3528462&tool=pmcentrez&rendertype=abstract Available at. Accessed May 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beier JC. Vector incrimination and entomological inoculation rates. In: Doolan DL, editor. Malaria Methods and Protocols. Totowa, NJ: Humana Press; 2002. pp. 3–11. [DOI] [PubMed] [Google Scholar]
- 37.Robert V, Macintyre K, Keating J, Trape J-F, Duchemin J-B, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. http://www.ncbi.nlm.nih.gov/pubmed/12641407 Available at. [PubMed] [Google Scholar]
- 38.Pagès F, Texier G, Pradines B, Gadiaga L, Machault V, Jarjaval F, Penhoat K, Berger F, Trape J-F, Rogier C, Sokhna C. Malaria transmission in Dakar: a two-year survey. Malar J. 2008;7:178. doi: 10.1186/1475-2875-7-178. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2556698&tool=pmcentrez&rendertype=abstract Available at. Accessed June 11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Govoetchan R, Gnanguenon V, Azondékon R, Agossa RF, Sovi A, Oké-Agbo F, Ossè R, Akogbéto M. Evidence for perennial malaria in rural and urban areas under the Sudanian climate of Kandi, northeastern Benin. Parasit Vectors. 2014;7:79. doi: 10.1186/1756-3305-7-79. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3938969&tool=pmcentrez&rendertype=abstract Available at. Accessed June 14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonio-Nkondjio C, Simard F, Awono-Ambene P, Ngassam P, Toto J-C, Tchuinkam T, Fontenille D. Malaria vectors and urbanization in the equatorial forest region of south Cameroon. Trans R Soc Trop Med Hyg. 2005;99:347–354. doi: 10.1016/j.trstmh.2004.07.003. http://www.ncbi.nlm.nih.gov/pubmed/15780341 Available at. Accessed August 17, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg Infect Dis. 2010;16:1108–1115. doi: 10.3201/eid1607.091785. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3321904&tool=pmcentrez&rendertype=abstract Available at. Accessed December 3, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefani A, Dusfour I, Corrêa APS, Cruz MCB, Dessay N, Galardo AKR, Galardo CD, Girod R, Gomes MSM, Gurgel H, Lima ACF, Moreno ES, Musset L, Nacher M, Soares ACS, Carme B, Roux E. Land cover, land use and malaria in the Amazon: a systematic literature review of studies using remotely sensed data. Malar J. 2013;12:192. doi: 10.1186/1475-2875-12-192. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3684522&tool=pmcentrez&rendertype=abstract Available at. Accessed December 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, Ferreira MU. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012;121:281–291. doi: 10.1016/j.actatropica.2011.10.001. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3308722&tool=pmcentrez&rendertype=abstract Available at. Accessed November 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis JC, Clark TD, Kemble SK, Talemwa N, Njama-Meya D, Staedke SG, Dorsey G. Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment. Malar J. 2006;5:18. doi: 10.1186/1475-2875-5-18. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1434757&tool=pmcentrez&rendertype=abstract Available at. Accessed May 19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3156969&tool=pmcentrez&rendertype=abstract Available at. Accessed November 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, Kamya MR, Staedke SG, Donnelly MJ, Drakeley C, Greenhouse B, Dorsey G, Lindsay SW. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. doi: 10.1186/1475-2875-13-111. http://www.ncbi.nlm.nih.gov/pubmed/24656206 Available at. Accessed April 24, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar DS, Andimuthu R, Rajan R, Venkatesan MS. Spatial trend, environmental and socioeconomic factors associated with malaria prevalence in Chennai. Malar J. 2014;13:14. doi: 10.1186/1475-2875-13-14. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3893554&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das A, Anvikar AR, Cator LJ, Dhiman RC, Eapen A, Mishra N, Nagpal BN, Nanda N, Raghavendra K, Read AF, Sharma SK, Singh OP, Singh V, Sinnis P, Srivastava HC, Sullivan SA, Sutton PL, Thomas MB, Carlton JM, Valecha N. Malaria in India: the center for the study of complex malaria in India. Acta Trop. 2012;121:267–273. doi: 10.1016/j.actatropica.2011.11.008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3294179&tool=pmcentrez&rendertype=abstract Available at. Accessed May 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shalini S, Chaudhuri S, Sutton PL, Mishra N, Srivastava N, David JK, Ravindran KJ, Carlton JM, Eapen A. Chloroquine efficacy studies confirm drug susceptibility of Plasmodium vivax in Chennai, India. Malar J. 2014;13:129. doi: 10.1186/1475-2875-13-129. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4021252&tool=pmcentrez&rendertype=abstract Available at. Accessed December 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva-do-nascimento TF, Lourenço-de-oliveira R. Diverse population dynamics of three Anopheles species belonging to the Triannulatus Complex (Diptera : Culicidae) Mem Inst Oswaldo Cruz. 2007;102:975–982. doi: 10.1590/s0074-02762007000800013. [DOI] [PubMed] [Google Scholar]
- 51.Arevalo-Herrera M, Quiñones ML, Guerra C, Céspedes N, Giron S, Ahumada M, Piñeros JG, Padilla N, Terrientes Z, Rosas A, Padilla JC, Escalante AA, Beier JC, Herrera S. Malaria in selected non-Amazonian countries of Latin America. Acta Trop. 2012;121:303–314. doi: 10.1016/j.actatropica.2011.06.008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3237935&tool=pmcentrez&rendertype=abstract Available at. Accessed May 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segula D, Frosch AP, SanJoaquin M, Taulo D, Skarbinski J, Mathanga DP, Allain TJ, Molyneux M, Laufer MK, Heyderman RS. Prevalence and spectrum of illness among hospitalized adults with malaria in Blantyre, Malawi. Malar J. 2014;13:391. doi: 10.1186/1475-2875-13-391. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4190438&tool=pmcentrez&rendertype=abstract Available at. Accessed December 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roca-Feltrer A, Kwizombe CJ, Sanjoaquin MA, Sesay SSS, Faragher B, Harrison J, Geukers K, Kabuluzi S, Mathanga DP, Molyneux E, Chagomera M, Taylor T, Molyneux M, Heyderman RS. Lack of decline in childhood malaria, Malawi, 2001–2010. Emerg Infect Dis. 2012;18:272–278. doi: 10.3201/eid1802.111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett A, Kazembe L, Mathanga DP, Kinyoki D, Ali D, Snow RW, Noor AM. Mapping malaria transmission intensity in Malawi, 2000–2010. Am J Trop Med Hyg. 2013;89:840–849. doi: 10.4269/ajtmh.13-0028. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3820324&tool=pmcentrez&rendertype=abstract Available at. Accessed December 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathanga DP, Walker ED, Wilson ML, Ali D, Taylor TE, Laufer MK. Malaria control in Malawi: current status and directions for the future. Acta Trop. 2012;121:212–217. doi: 10.1016/j.actatropica.2011.06.017. http://dx.doi.org/10.1016/j.actatropica.2011.06.017 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson ML, Walker ED, Mzilahowa T, Mathanga DP, Taylor TE. Malaria elimination in Malawi: research needs in highly endemic, poverty-stricken contexts. Acta Trop. 2012;121:218–226. doi: 10.1016/j.actatropica.2011.11.002. http://dx.doi.org/10.1016/j.actatropica.2011.11.002 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar A, Sharma V, Thavaselvam D. Malaria related to constructions in Panaji, Goa. Indian J Malariol. 1991;28:219–225. [PubMed] [Google Scholar]
- 58.Sumodan PK, Ashwani K, Yadav RS. Resting behavior and malaria vector incrimination of Anopheles stephensi in Goa, India. J Am Mosq Control Assoc. 2004;20:317–318.. [PubMed] [Google Scholar]
- 59.Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Sampling of adult mosquito vectors with mosquito MagnetTM Pro in Panaji, Goa, India. J Am Mosq Control Assoc. 2008;24:604–607. doi: 10.2987/5756.1. http://www.bioone.org/doi/abs/10.2987/5756.1 Available at. [DOI] [PubMed] [Google Scholar]
- 60.Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Mosquito biting activity on humans and detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. 2012;135:120–126. doi: 10.4103/0971-5916.93434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanasamy K, Chery L, Basu A, Duraisingh MT, Escalante A, Fowble J, Guler JL, Herricks T, Kumar A, Majumder P, Maki J, Mascarenhas A, Rodrigues J, Roy B, Sen S, Shastri J, Smith J, Valecha N, White J, Rathod PK. Malaria evolution in south Asia: knowledge for control and elimination. Acta Trop. 2012;121:256–266. doi: 10.1016/j.actatropica.2012.01.008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3894252&tool=pmcentrez&rendertype=abstract Available at. Accessed May 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ceesay SJ, Bojang KA, Nwakanma D, Conway DJ, Koita OA, Doumbia SO, Ndiaye D, Coulibaly TF, Diakité M, Traoré SF, Coulibaly M, Ndiaye J-L, Sarr O, Gaye O, Konaté L, Sy N, Faye B, Faye O, Sogoba N, Jawara M, Dao A, Poudiougou B, Diawara S, Okebe J, Sangaré L, Abubakar I, Sissako A, Diarra A, Kéita M, Kandeh B, Long CA, Fairhurst RM, Duraisingh M, Perry R, Muskavitch MAT, Valim C, Volkman SK, Wirth DF, Krogstad DJ. Sahel, Savana, riverine and urban malaria in west Africa: similar control policies with different outcomes. Acta Trop. 2012;121:166–174. doi: 10.1016/j.actatropica.2011.11.005. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3294051&tool=pmcentrez&rendertype=abstract Available at. Accessed November 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doumbia SO, Ndiaye D, Koita OA, Diakité M, Nwakanma D, Coulibaly M, Traoré SF, Keating J, Milner DA, Ndiaye J-L, Sene PD, Ahouidi A, Dieye TN, Gaye O, Okebe J, Ceesay SJ, Ngwa A, Oriero EC, Konaté L, Sy N, Jawara M, Faye O, Kéita M, Cissé M, Sogoba N, Poudiougou B, Diawara S, Sangaré L, Coulibaly T, Seck I, Abubakar I, Gomis J, Mather FJ, Sissako A, Diarra A, Kandeh B, Whalen C, Moyer B, Nnedu O, Thiero O, Bei AK, Daniels R, Miura K, Long CA, Fairhurst RM, Duraisingh M, Muskavitch MAT, D'Alessandro U, Conway DJ, Volkman SK, Valim C, Wirth DF, Krogstad DJ. Improving malaria control in west Africa: interruption of transmission as a paradigm shift. Acta Trop. 2012;121:175–183. doi: 10.1016/j.actatropica.2011.11.009. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3294075&tool=pmcentrez&rendertype=abstract Available at. Accessed May 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Planning Commission Government of India . Goa Development Report. 2011th edition. New Delhi, India: Academic Foundation; 2011. [Google Scholar]
- 65.The Economist Comparing Indian States and Territories with Countries. An Indian Summary. 2014. http://www.economist.com/content/indian-summary Available at.
- 66.Smoke P. Report of the Thirteenth Finance Commission: 2010–2015. Intergovernmental, State and Local Finances. Washington, DC: World Bank; 2011. http://www-wds.worldbank.org/external/default/WDSContentServer/WDSP/IB/2012/06/30/000426104_20120630120654/Rendered/PDF/703460ESW0P1240overnmental0finances.pdf Available at. [Google Scholar]
- 67.TNN Inflow of Migrants Affects Malaria Control Programme, 2013. The Times of India. http://timesofindia.indiatimes.com/city/goa/Inflow-of-migrants-affects-malaria-control-programme/articleshow/20281671.cms Available at.
- 68.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India : retrospective and prospective view. Am J Trop Med Hyg. 2007;77(Suppl 6):69–78. [PubMed] [Google Scholar]
- 69.Ministry of Tourism . Annual Report 2013–14, Incredible India. New Delhi, India: Ministry of Tourism, Government of India; 2014. http://tourism.gov.in/writereaddata/Uploaded/Tender/081220141131463.pdf Available at. [Google Scholar]
- 70.Dash A, Valecha N, Anvikar A, Kumar A. Malaria in India: challenges and opportunities. J Biosci. 2008;33:583–592. doi: 10.1007/s12038-008-0076-x. http://link.springer.com/article/10.1007/s12038-008-0076-x Available at. Accessed December 22, 2014. [DOI] [PubMed] [Google Scholar]
- 71.Sharma J, Dutta P, Khan S, Soni M, Dey D, Mahanta J. Genetic polymorphisms associated with sulphadoxine-pyrimethamine drug resistance among Plasmodium falciparum field isolates in malaria endemic areas of Assam. J Postgrad Med. 2015;611:9–14. doi: 10.4103/0022-3859.147019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohapatra PK, Sarma DK, Prakash A, Bora K, Ahmed MA, Sarma B, Goswami BK, Bhattacharyya DR, Mahanta J. Molecular evidence of increased resistance to anti-folate drugs in Plasmodium falciparum in north-east India: a signal for potential failure of artemisinin plus sulphadoxine-pyrimethamine combination therapy. PLoS One. 2014;9:e105562. doi: 10.1371/journal.pone.0105562. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4153584&tool=pmcentrez&rendertype=abstract Available at. Accessed December 22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah NK, Dhillon GPS, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3068018&tool=pmcentrez&rendertype=abstract Available at. Accessed December 22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Beaudrap P, Nabasumba C, Grandesso F, Turyakira E, Schramm B, Ii YB, Etard J. Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda. Malar J. 2011;10:132. doi: 10.1186/1475-2875-10-132. http://www.malariajournal.com/content/10/1/132 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J. 2013;12:1–22. doi: 10.1186/1475-2875-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3269430&tool=pmcentrez&rendertype=abstract Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]