Abstract
The unprecedented global efforts for malaria elimination in the past decade have resulted in altered vectorial systems, vector behaviors, and bionomics. These changes combined with increasingly evident heterogeneities in malaria transmission require innovative vector control strategies in addition to the established practices of long-lasting insecticidal nets and indoor residual spraying. Integrated vector management will require focal and tailored vector control to achieve malaria elimination. This switch of emphasis from universal coverage to universal coverage plus additional interventions will be reliant on improved entomological monitoring and evaluation. In 2010, the National Institutes for Allergies and Infectious Diseases (NIAID) established a network of malaria research centers termed ICEMRs (International Centers for Excellence in Malaria Research) expressly to develop this evidence base in diverse malaria endemic settings. In this article, we contrast the differing ecology and transmission settings across the ICEMR study locations. In South America, Africa, and Asia, vector biologists are already dealing with many of the issues of pushing to elimination such as highly focal transmission, proportionate increase in the importance of outdoor and crepuscular biting, vector species complexity, and “sub patent” vector transmission.
Introduction
The unprecedented global efforts for malaria elimination in the past decade have resulted in the reduction of malaria cases in several settings,1 but also in dramatic increases in resistance to pyrethroids and other insecticides,2 changes in the relative importance of outdoor (residual) malaria transmission, and major shifts in biting time, for example, Anopheles farauti in the Solomon Islands3 and Anopheles funestus in Benin and Senegal.4,5 Together these new trends have already resulted in quantifiable changes in human–vector interactions in several endemic areas, and threaten to jeopardize future gains. Long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) and have been the mainstays of malaria control and have had a major impact on reducing global malaria, particularly where vectors are primarily endophagic (indoor biting), endophilic (indoor resting), and anthropophilic.6
As such, the goal of global malaria elimination will require additional interventions and improvements in both the application of current control measures and entomological monitoring.7 The single biggest threat to sustainable malaria control is insecticide resistance, which has reached alarmingly high levels in some vector populations of Africa, India, and China (M. L. Quiñones and others, unpublished data).2 Second, there are indications of local adaptation in vector biting behavior, possibly in response to reliance on LLINs and IRS.3,5,8,9 Whether this reflects a lack of vector ingress because of physical barriers, that is, mosquito-proof houses, adaptation of endophagic vectors to exophagy (outdoor feeding), or selection on phenotypic plasticity, is unknown.10,11 It has been hypothesized that in some areas endophagic populations may have been eliminated, leaving the inadequately controlled exophagic population.12 Also, in the Solomon Islands during the 1970s malaria eradication campaign, late night biting of Anopheles koliensis and Anopheles punctulatus, which had been common, virtually disappeared.13 Similarly, a switch from endophily to exophily (outdoor resting) has been documented in areas under intense IRS (R. Sloof, unpublished data).14,15 The third major issue is the recognition that transmission is both focal and heterogeneous and that we urgently need to incorporate, for example, ecological context of mosquito foraging behavior and vector diversity into our transmission models to improve predictive accuracy.16,17 Fourth, the use of LLINs at high coverage, although extremely effective overall, can alter species composition, which could change transmission patterns and possibly the entomological inoculation rate (EIR) because of different vectorial capacities, biting times, and behaviors, for example, a decrease in Anopheles gambiae and a concurrent increase in the relative proportion of Anopheles arabiensis.6,18,19
Vectorial systems vary dramatically across regions and countries,20–22 and this variation will be reflected in how well malaria transmission responds to control. A suggested benchmark for adequate vector control is the decrease and maintenance of EIR below 1, together with epidemiological measures of malaria in humans.23 Achieving EIRs of < 1 is especially challenging in those endemic areas where the current approach of IRS and/or LLINS may not be adequate to cover changing transmission scenarios.
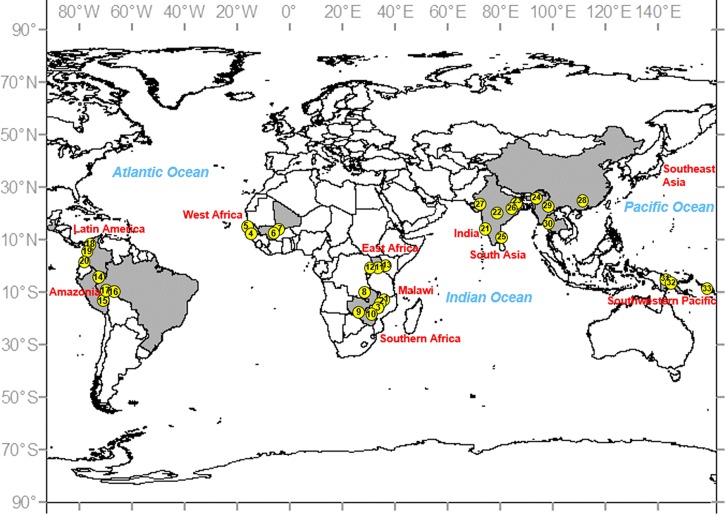
The United States' National Institutes of Health funded 10 International Centers of Excellence in Malaria Research (ICEMR) in 2010 with a series of common aims including a concerted effort to closely link epidemiology and transmission metrics with vector biology. One of the unique features of the ICEMR program is the focus on longitudinal surveillance sites in diverse epidemiological settings across the globe (Figure 1 ). Each ICEMR uses similar approaches and metrics to quantify transmission. The strengths of this approach are our ability to incorporate seasonal and multiyear variation in routine entomological monitoring that can quantify temporal changes in insecticide susceptibility, EIR, vector species composition, and the effects of epidemiology interventions. Such data, when incorporated into malaria transmission models, should increase accuracy and predictive power.
Figure 1.
Location of the 33 field sites across 10 International Centers of Excellence in Malaria Research (ICEMR) (in red). The field sites (yellow circles) are numbered consecutively and are described in Table 1.
The objective of this article is to provide an introduction to the broad-sense ecology of vectors in the 10 major geographic regions covered by the ICEMR projects and to discuss how similarities and contrasts between the areas will build to a comprehensive view of malaria transmission globally. We are not intending to provide a detailed historical review of transmission ecology in each setting, and as a consequence the extant literature has been sampled broadly but with only limited depth. Vector biologists from each ICEMR selected references that they believed to be the most pertinent to the objective of this article.
Entomological Metrics at ICEMR Sites
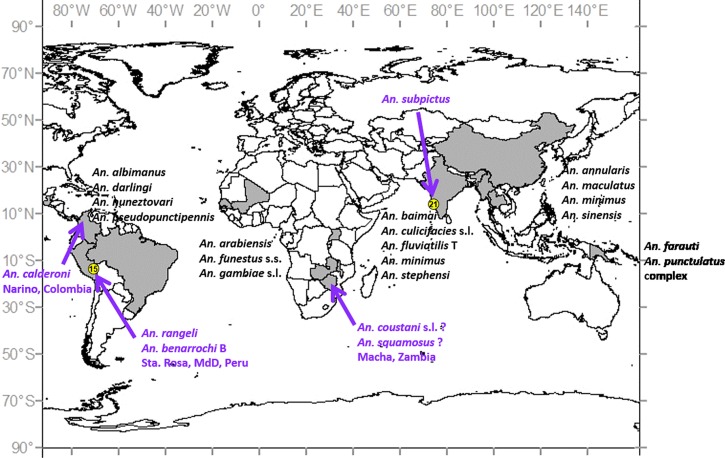
Across the ICEMR sites, there is diversity in vector species and their contributions to malaria transmission of, principally, Plasmodium falciparum and Plasmodium vivax are variable. Although challenging, this is also an extraordinary opportunity to identify commonalities that may lead to new integrated approaches to control and eliminate malaria. Investigations in the 10 major regions are described in brief below, with summary vector biology data (Table 1) together with a corresponding figure of the 33 individual sites geographically located (Figure 1). The wide range of primary vector species, and putative new vectors that several ICEMR studies have detected, is illustrated in Figure 2 .
Table 1.
Vector biology and ecology data from ICEMR field sites
| ICEMR regional center | Specific site | Plasmodium spp. | Major vector(s) | Trap type | Primary behaviors (exo/endo/phagic/philic) | Main control method | Transmission seasonality | EIR (per year) |
|---|---|---|---|---|---|---|---|---|
| Malawi | Blantyre District (urban) | Pf | Anopheles funestus s.s., Anopheles arabiensis | CDC, ASP | Endophagic, endophilic (indirect evidence) | LLIN | Rainy season (November–April), low transmission rest of year | N/A |
| Thyolo District (rural highland) | Pf | An. funestus s.s., An. arabiensis | CDC, ASP | Endophagic, endophilic (indirect evidence) | LLIN | Rainy season (November–April), low transmission rest of year | N/A | |
| Chikwawa District (rural lowland) | Pf | An. funestus s.s., An. arabiensis, Anopheles gambiae s.s. | CDC, ASP | Endophagic, endophilic (indirect evidence) | LLIN | Perennial, one annual rainy season | N/A | |
| West Africa | The Gambia | Pf | An. arabiensis, An. gambiae | HLC, CDC | Primarily endophagic and endophilic | LLIN, IRS | Rainy season (August–November) | Peak of 23/month |
| Gambissara (Upper River) | ||||||||
| Senegal | Pf | An. arabiensis | HLC | Primarily exo/ endophagic and endophilic | LLIN | Rainy season peak (August–December) | Peak of 5/month | |
| Medina Fall (Thiès) | ||||||||
| Mali | Pf | An. arabiensis, An. gambiae | HLC | Primarily endophagic and endophilic; more recently comparable frequency indoors and outdoors | LLIN | Rainy season peak (July–December) | Peak of 51/month | |
| Dangassa | ||||||||
| Koila Bamana (Dioro) | Pf | An. arabiensis, An. gambiae | HLC | Same as Dangassa | LLIN | Virtually perennial; rainy season peak plus irrigation (August–May) | Rainy season peak = 5/month | |
| South Africa | Zambia | Pf | An. funestus s.s., An. gambiae s.s. | CDC, PSC, backpack aspirator | Not evaluated | LLIN, IRS | All year with seasonal fluctuations | 8–108 for An. funestus; 0–8 for An. gambiae |
| Nchelenge District | ||||||||
| Choma District | Pf | An. arabiensis | CDC, HLC, PSC, cattle-baited trap | Exophagic, exophilic | LLIN | Single rainy season | 0 | |
| Zimbabwe | Pf | An. funestus s.s., An. gambiae s.l. | CDC, PSC | Not evaluated | LLIN, IRS | Single rainy season | 0–7 for An. funestus; N/A for An. gambiae | |
| Mutasa District | ||||||||
| East Africa | Uganda | Pf | An. arabiensis, An. gambiae s.s. | HLC, PSC/exit trap, CDC | Primarily endophagic, endophilic | ITN | Perennial, two annual rainy seasons | 4 |
| Jinja District | ||||||||
| Kanungu District | Pf | An. gambiae s.s. | HLC, PSC/exit trap, CDC | Primarily endophagic, endophilic | ITN | Perennial, two annual rainy seasons | 27 | |
| Tororo District | Pf | An. gambiae s.s. | HLC, PSC/exit trap, CDC | Primarily endophagic, endophilic | ITN | Perennial, two annual rainy seasons | 125 | |
| Amazonia | Peru | Pv, Pf | Anopheles darlingi | HLC, CDC, Shannon trap | Exophagic, exophilic | LLIN, IRS, local bed nets | Seasonal, peaks w/rainy season (March–May) | 0–86.7 |
| Loreto Department | ||||||||
| Madre de Dios Department* | Pv | Anopheles rangeli, Anopheles benarrochi B | HLC, CDC, Shannon trap | Not evaluated | ITN | Perennial, peaks w/rainy season (December–February) | Too few mosquitoes to calculate | |
| Brazil | Pv | An. darlingi | Shannon trap | Primarily exophagic, exophilic | ITN, IRS | Seasonal; minor peaks during dry season (May–September) | N/A | |
| Granada, ∼25-year-old rural settlement, Acrelandia | ||||||||
| Remansinho, ∼8-year-old settlement, Acrelandia | Pv | An. darlingi | Shannon trap | Exo/endophagic, primarily exophilic | ITN | Perennial† | N/A | |
| Latin America | Colombia | Pv, Pf | Anopheles nuneztovari, An. darlingi | HLC | Exophagic, exophilic | LLIN | Perennial | Three localities for An. nuneztovari: 3.5, 3.2, 1.9 |
| Tierralta | ||||||||
| Buenaventura | Pv, Pf | An. nuneztovari, Anopheles pseudo punctipennis, Anopheles albimanus | HLC | Exophagic, exophilic | LLIN | Perennial, modest peaks March–April, July–September | Too few mosquitoes to calculate | |
| Tumaco | Pv, Pf | An. albimanus, Anopheles calderoni | HLC | Exophagic, exophilic | LLIN, larvicide | Perennial, main peak March–April; minor peak July | 2.85 for An. calderoni | |
| South Asia | India | Pv, Pf | Anopheles stephensi, Anopheles subpictus | Mosquito magnet, CDC | Endophagic, exophilic; endophagic, endophilic | Larvicide (temephos), BTI, larvivorous fish (guppy) | All year, peaks during rainy season | 2.35 overall for An. stephensi in multiple localities in Goa; 18.1 for Panaji (within Goa) alone |
| Goa | ||||||||
| Wardha | Pv, Pf | Anopheles culicifacies | Hand catch | Endophilic, endophagic | IRS | All year, peaks during rainy season | Unreported | |
| Ranchi | Pv, Pf | An. culicifacies, Anopheles fluviatilis | Hand catch | Endophilic, endophagic | LLIN, IRS | An. culicifacies all year, peaks post rainy season (southwest monsoons); An. fluviatilis peaks February–March | Unreported | |
| Assam | Pv, Pf, Po, Pm | Anopheles baimaii, Anopheles minimus | CDC | Exophilic, exo/endophagic; exo/endophagic | LLIN, ITN, IRS | All year, peaks during rainy season | Unreported | |
| Chennai | Pv, Pf | An. stephensi | IRC, PSC | Endo/exophilic (variable; mainly based on microenvironmental conditions) endophagic; exophagic during summer | Larvicide (temephos) | Perennial, mesoendemic, southwest and northeast monsoon (predominantly NE) | Unreported | |
| Raurkela | Pv, Pf, Pm | An. fluviatilis, An. culicifacies | IRC | An. fluviatilis classically endophilic and endophagic w/ evidence of switch toward exophily (cattle sheds) and more exophagy (zoophagy). An. culicifacies strongly zoophilic (rests, feeds in cattle sheds); any human feeding tends to be endophagic | IRS, LLIN | Perennial, meso-hyperendemic, peak in winter | 7.3–127 seasonally dependent | |
| Nadiad | Pv, Pf | An. culicifacies A and C in rural areas | IRC, PSC | Endophilic, endophagic | Larvicide, biological control, IRS, LLIN, ITN | Seasonal, hypoendemic (unstable malaria) | 0.05–0.21 | |
| Southeast Asia | China | Pv, Pf | An. minimus, Anopheles maculatus, Anopheles sinensis | CDC aspirator | Exo/endophilic; strongly zoophilic, exophagic | ITN, IRS | Perennial, one rainy season | 0.10 |
| Yingjiang County, Yunnan Province | ||||||||
| Myanmar | Pv, Pf | An. minimus, An. maculatus, An. sinensis | CDC | Exo/endophilic; strongly zoophilic, exophagic | LLIN, IRS | Perennial, one rainy season | 0.53 | |
| Laiza, Kachin State | ||||||||
| Thailand | Pv, Pf | An. minimus, An. maculatus, Anopheles annularis | CDC aspirator | Exo/endophilic | LLIN, IRS | Perennial, one rainy season | 0.25 | |
| Tha Song Yang District, Tak Province | ||||||||
| Southwest Pacific | PNG | Pv, Pf, Po, Pm | Anopheles punctulatus complex | HLC, barrier screens | Exo/endophilic | LLIN | Perennial, one to two rainy seasons | 10.1–27.8 |
| East Sepik Province | ||||||||
| Madang Province | Pv, Pf, Po, Pm | Anopheles farauti | HLC, barrier screens | Exo/endophilic | LLIN | Perennial, peak in rainy season | 40.8 | |
| Solomon Islands | Pv, Pf, Po, Pm | An. farauti | HLC, barrier screens | Exophilic | LLIN | Perennial, peak in rainy season | 3–44 | |
| Central Province and Western Province |
ASP = battery powered aspirator of the Prokopack or Insectazooka type; CDC = Centers for Disease Control and Prevention; EIR = entomological inoculation rate; HLC = human landing catch; ICEMR = International Centers for Excellence in Malaria Research; IR = infection rate (in vector); IRS = indoor residual spray; IRC = indoor resting collections; LLIN = long-lasting insecticide-impregnated net; PNG = Papua New Guinea; PSC = pyrethroid spray catch; Pf = Plasmodium falciparum; Pm = Plasmodium malariae; Po = Plasmodium ovale; Pv = Plasmodium vivax; N/A = Not applicable.
Locality numbers in Column 2 correspond to numbers in Figure 1.
Malaria cases in Madre de Dios Department have steadily declined since 2011. In 2013, there were 251 cases (MINSA, Peru, 2013).
Malaria (P. vivax) is disappearing in Remansinho (2010–2013).24
Figure 2.
Anopheline malaria vector species across the 10 ICEMRs. New potential vector species, detected in these or affiliated studies, are depicted in purple. In Macha, Zambia, there is not yet conclusive evidence implicating Anopheles coustani s.l. or Anopheles squamosus as vectors.
Africa.
The four ICEMRs in Africa (Malawi, west Africa, southern Africa, and east Africa; Figure 1) are near exclusively P. falciparum transmission settings where malaria is vectored by one or more of the four major African vectors (An. gambiae s.s., Anopheles coluzzii, An. arabiensis, and An. funestus s.s.) (Figure 2). Vector control is reliant on LLINs, IRS, or some combination of the two (Table 1). In west and east Africa, vectors are primarily endophagic and endophilic (but see An. arabiensis, Table 1), implying that nets should be highly effective, assuming high levels of local coverage. For two of the higher transmission sites in southern Africa, An. funestus is similarly expected to be endophagic and endophilic. Although not yet reported for all the African sites, EIRs are, with the exception of the high transmission site in Tororo, Uganda (annual PfEIR ∼125; Table 1),23 moderate relative to earlier studies.1 A major concern for these ICEMRs is how vectors will respond to the massive rollout of LLINs in sub-Saharan Africa. Already, some populations of An. gambiae s.s., such as those in Guinea, have shifted from primarily endophagic biting to primarily exophagic1,8; whereas in other situations, such as the highlands of Kenya, endophilic An. gambiae have been dramatically reduced, with low transmission maintained by An. arabiensis and novel anopheline species that are primarily exophilic and bite early in the evening when people are generally unprotected.12
East Africa ICEMR.
Comparing three transmission sites (low, moderate, and extremely high; Table 1) from this Uganda-based ICEMR, only at the low site, Jinja, there was a suggestion of a reduction in An. gambiae s.s. and a concurrent increase in the abundance of An. arabiensis compared with previous findings.25,26 The study site in Jinja is peri-urban, and it is possible that the predominance of An. arabiensis reflects its adaptation to this disrupted environment as has been observed in west Africa.27–29
Malawi ICEMR.
This ICEMR is undertaking studies of Plasmodium transmission and malaria risk and prevention in various environments of southern Malawi, particularly in the districts of Blantyre, Chikwawa, and Thyolo (Figure 1).30,31 Study sites differ considerably, ranging from predominantly rural lowlands in Chikwawa where transmission is intense and essentially year-round, to the rural highlands of Thyolo with moderate seasonal transmission, to sites in and around urban Blantyre City, with apparently lower-level, heterogeneous infection (Table 1). Until the ICEMR-supported research began, little had been published about Anopheles vectors in Malawi. One of the first studies by Spiers and others32 undertaken in Chikwawa reported that the predominant vectors were An. arabiensis and An. funestus s.s., although An. gambiae s.s. was also present in this Shire River valley area. Other work on filariasis vectors by Merelo-Lobo and others33 also found these three species, with An. funestus being the most abundant. However, little is known about the relative abundance and role of Anopheles species in relation to malaria patterns from Malawi.
Vector ecology and infection studies are now underway as part of two ICEMR-supported projects involving a) health facility-identified, case–control comparisons of urban/peri-urban households in/around Blantyre City and b) cross-sectional, household visit-based sampling across districts of Chikwawa, Thyolo, and Blantyre. In all of these settings, Prokopack-style aspirators and Centers for Disease Control and Prevention (CDC) light traps are being used to test for the presence of indoor adult mosquitoes that are identified by microscopy and confirmed by polymerase chain reaction (PCR) (Table 1). In rural settings, An. funestus s.s. is most abundant, with fewer An. arabiensis and rare Anopheles quadriannulatus. In urban/peri-urban Blantyre, year-round aspiration of 511 households during April 2012 through March 2014 showed that 64% of houses had mosquitoes, with Culex spp. representing 98.7% of the sample (M. Wilson, personal communication). Very few Anopheles spp. (12 males, 29 females) were found. Nevertheless, more Anopheles were captured in households of cases (4.2%) than of controls (1.9%). In this urban setting, it remains very difficult to find many Anopheles using aspiration and light traps.
Public health efforts to reduce vector–human contact have used widespread distribution of LLINs and focused use of IRS.34 Nationwide distribution of free LLINs has been enhanced since 2012 when ownership was at only 58% of households, and now all children born in health facilities receive an LLIN, as do pregnant women when they first visit an antenatal care clinic. Similarly, a free LLIN is now given to each child at her/his first Expanded Program on Immunization (EPI) visit. From 2008 through 2012, more than 6 million LLINs were distributed in Malawi.34 However, Plasmodium infection measured by PCR among children under 5 years of age was still 43% overall, and 60.5% in the lowest wealth quintile. The scale-up plan for 2012–2015 aims to achieve one LLIN for every two people in each household.34
Other control efforts using IRS are coordinated by the Malawi government, but this program is not nationwide, instead focusing on seven districts of particularly high disease burden: Karonga and Nkhata Bay (northern region), Nkhotakota and Salima (central region), and Mangochi, Chikwawa, and Nsanje (southern region). The 2012 MIS survey indicated that less than 10% of Malawi households had received IRS within the preceding 12 months, suggesting that this form of vector control is relatively less important.34
Effectiveness of interventions to reduce vector–human contact depends on where, when, and on whom competent Anopheles are feeding, but again little is known about this in Malawi. Recent work in northern Malawi (Karonga) has shown that An. funestus and Anopheles rivulorum were mostly found indoors, but none were infected with either P. falciparum or P. vivax.35 A new An. funestus-like species was also mostly collected indoors, but mainly had fed on animals and also was uninfected. Other studies from countries that border Malawi support the general pattern that both An. funestus and An. arabiensis predominantly feed indoors and on people. Investigations in southeastern Zambia (∼500 km west of southern Malawi) have shown that An. funestus and An. quadriannulatus were captured both indoors and outdoors, but nearly all were found to have fed indoors, reinforcing the importance of LLIN use.36 More generally, this pattern of An. gambiae complex and An. funestus group predominantly biting humans indoors at night seems to be common in eastern Africa.37
West African ICEMR.
Three malaria endemic countries: Mali, Senegal, and The Gambia, comprise the focus of this ICEMR (Figure 1). Across this broad geographical area vector populations and malaria transmission differ in their complexity. The site types (riverine: Gambissara, Dangassa; urban: Medina Fall, and rice irrigation: Dioro) were chosen in part to explore differences in length of transmission season, EIR (from ∼5 to 51 bites/month; Table 1), and status of malaria control.38 Anopheles gambiae and An. arabiensis are the major vectors in all the three countries (Figure 2). However, other anopheline vectors are encountered such as An. funestus and Anopheles pharoensis inland and Anopheles melas on the coast.39–43 In The Gambia and Mali sites, as in the other African ICEMR localities (Figure 1), An. gambiae is mainly endophagic, although since the inception of this study, it has been collected feeding outdoors 45–50% of the time (M. Coulibaly, personal communication), similar to the change in behavior documented on Bioko Island, Equatorial Guinea.8 Anopheles arabiensis is primarily exophagic except in the urban site of Medina Fall where it feeds both indoors and outdoors (Table 1).
Overall malaria transmission is seasonal and coincident with the rainy season. The peaks of transmission occur toward the end of the rains when mosquito densities are waning.38 Nevertheless, transmission is perennial in some areas where irrigated rice cultivation maintains anopheline breeding during the dry season.44 The urban site of Medina Fall (Senegal) showed the lowest transmission level. The current large-scale vector control strategies in use in Mali, The Gambia, and Senegal are LLINs and IRS. Although the LLINs distribution is country wide at all the three sites through campaigns and routine antenatal consultation and EPI, IRS has been implemented only in targeted areas in the respective countries. None of the west African ICEMR study sites has received IRS to date, but insecticide resistance is widespread.
Southern Africa ICEMR.
In Macha, Choma District, southern Zambia (Figure 1), there is marked spatial and temporal heterogeneity in the foraging behavior of the main vector An. arabiensis and previously undocumented high anthropophily in secondary vectors Anopheles coustani s.l. and Anopheles squamosus (Figure 2).45–47 Choma District has the potential to become a malaria-free zone, in part because the formerly primary vector, An. funestus s.s., was locally eradicated in 2004, possibly by a drought.48 Even though the Macha population of An. arabiensis is highly anthropophilic with foraging times that extend from dusk until dawn with a greater tendency for exophagy (Table 1), no specimens have been detected positive for Plasmodium since 2006 and transmission in the district remains very low (D. Norris, personal communication).46,49 Despite the low overall risk, it should be recognized that individual and household risk is very unevenly distributed and spatially clustered, and most importantly, this heterogeneity may be further exacerbated by anti-vector interventions and multiple host feeding by the vector.16
In contrast, both endophagic An. funestus s.s. and An. gambiae s.s. are responsible for very high levels of transmission despite reported coverage of 1.73 LLINs per person (2012) and greater than 90% coverage with IRS (2011) in Nchelenge District, northern Zambia (S. Das, unpublished data).50 The inability to achieve control here is likely due to high levels of insecticide resistance to dichlorodiphenyltrichloroethane and deltamethrin and an inability to apply effective control measures to the vector populations that are physically difficult to access or may reside in transient households (D. Norris, unpublished data), a condition further limited by resources.50 In Nchelenge District, the two vector species exhibit enormous temporal and spatial heterogeneity, which is hypothesized to exacerbate the observed perennial year-round transmission (S. Das and D. E. Norris, unpublished data) (Table 1). High rates of feeding on multiple human hosts in a single gonotrophic cycle (S. Das and D. E. Norris, unpublished data) and human movement into malaria risk zones are seen as added challenges to control in this area (K. M. Searle and W. J. Moss, unpublished data).
The third site for this ICEMR is Mutasa, eastern Zimbabwe (Figure 1), where resurgent malaria occurs seasonally and An. funestus s.s. appears to be the only significant vector. Although current loss of vector control here is likely due largely to insecticide resistance of An. funestus s.s. (M. Coetzee, and others, unpublished data), historically An. gambiae s.s. was the primary vector in this region.51 This change in primary vectors may be attributed to gaps in malaria control because of economic constraints that allowed mainly endophagic An. funestus s.s. to invade from nearby Mozambique or emerge from unknown refugia. Insecticides used subsequent to this event, to which the An. funestus s.s. population were likely already resistant, would have helped this invasive vector population to fully establish and thrive.
Latin America.
In marked contrast to the African ICEMR sites, most malaria in Latin America is caused by P. vivax (∼70%), except for relatively uncommon hot spots such as Haiti, Guyana, and gold-mining areas across the Amazon, where P. falciparum case numbers are higher than the average ∼30%.1 In the vast area of the basin drained by the Amazon and its tributaries, Anopheles darlingi is the main vector,52,53 but, as is evident in the Latin American study sites, several other vector species contribute to transmission, and much less is known about their ecologies and entomological metrics (Table 1). Many vector species in the neotropics are exophagic and exophilic (Table 1), with the notable exceptions of An. darlingi, Anopheles albimanus, and Anopheles nuneztovari (see the summary below for Latin American ICEMR), which display endo/exophagy depending on host availability and environmental characteristics.65 Therefore, control by IRS has been a mainstay for many years, and, partly for reasons of logistics and distribution, the use of LLINs has spread more slowly in Latin America than Africa, Asia, or the southwest Pacific.52,55,56 An unresolved issue is the relatively high use of IRS combined with very low levels of insecticide resistance (M. Quinones, personal communication).
Amazonian ICEMR.
There are very few reports in Latin America where An. darlingi is no longer the dominant malaria vector, for example, Suriname.53 Infrequent, extensive flooding that coincided with the beginning of the interventions in Suriname likely contributed to the local collapse of An. darlingi.53 Anopheles darlingi is the predominant vector in study sites near Iquitos, Peru, and near Acre, western Brazil, in this ICEMR. In these localities An. darlingi is the main vector, the most abundant, highly seasonal, exo- and endophagic, and nearly exclusively exophilic (M. Moreno and others, unpublished data) (Table 1).
Despite Ministry of Health and international (e.g., Control de la Malaria en las Zonas Fronterizas de la Región Andina: Un Enfoque Comunitario [PAMAFRO]) efforts to distribute LLINs in Brazil and Peru from 2006 to 2011, unimpregnated net use remains common in some localities, although an integrated approach of LLINs combined with IRS has been recommended.56–59 Many populations of Amazonian An. darlingi, including those in our study sites (M. Moreno and others, unpublished data) display multimodal biting times.60 A crepuscular peak (∼19–21 hours) is common, well before most people retire for the evening reducing the potential impact of LLINs.61 Major issues in Peru are the correlation of deforestation with significantly high human biting rates along highways62 and in riverine settlements (W. Lainhart, unpublished data), and hyperendemic malaria hot spots related to occupational travel.62,63 In western Amazonian Brazil, deforestation linked to agricultural settlements and gold mining is of primary concern.56 In study sites in Madre de Dios region, southern Peru, An. darlingi was not common, and both Anopheles rangeli and Anopheles benarrochi B were detected infected with P. vivax for the first time in this region (Table 1, Figure 2), but sample size was so small that the actual role of these species in transmission could not be evaluated (M. Moreno and J. E. Conn, unpublished data). These data suggest that elimination efforts might be concentrated more usefully on the detection and rapid treatment of occupational malaria transmission hot spots. Initial blood meal data (M. Moreno and J. E. Conn, unpublished data) from barrier screens support previous findings from eastern Amazonian Brazil that An. darlingi feeds opportunistically, and strongly suggest that host availability is the prime driver of blood meal preference.64 It remains to be seen how best to exploit these new findings to improve vector control in this region.
Latin American ICEMR.
Latin American countries in the Centro Latino Americano de Investigación en Malaria (CLAIM) include Guatemala, Panama, Colombia, and northwestern Peru.52 In this broad area, the most important regional vectors are An. darlingi, An. nuneztovari s.l., and An. albimanus (Table 1, Figure 1).21 Nevertheless, in one field site in the Pacific region, Tumaco (Figures 1 and 2, Table 1), the species Anopheles calderoni was found infected with P. vivax (M. L. Quiñones, unpublished data). It was also infected with P. falciparum in specimens from a palm-oil plantation in the same region.55 These data infer that An. calderoni may be relatively important in local transmission, and ecological and biological investigation in addition to control efforts should be increased. There are many critical information gaps for these species, such as lack of data on vector ecology, vector competence, and effects of environmental change on vectors.54 The most common malaria control methods have been IRS, LLINs, and early detection, diagnosis, and treatment.23 Regrettably, for LLINs, there have been basically no evaluations of the potential suppression of vector populations, vector behavioral changes, transmission level, or location.54 Behavioral changes toward increased exo- and endophagy in the northern part of the geographical range of An. albimanus (Mexico and Panama) may have been induced by early adoption of IRS.3 Overall, the predominant behavior among these species is exophagy and exophily (Table 1); however, in several localities in the Amazon, An. darlingi is mainly endophagic and An. albimanus displays considerable plasticity, exhibiting both behaviors, depending on host availability and locality.21,65 Broadly distributed across northern South America, An. nuneztovari is more exophagic in the Amazon (where it may be Anopheles goeldii) and more endophagic in Colombia and western Venezuela.66 As such, decisions on the most appropriate intervention(s) differs across its geographic range necessitating more locally tailored control than that seen in Africa.65
New findings of a cross-sectional study using human-landing catch (HLC) in 70 localities in western Colombia (Cordoba, Narino, and Valle), where most transmission occurs, found that An. albimanus and An. nuneztovari together constituted approximately 80% of the 12,052 adult mosquitoes collected and identified, and these were the only two species positive for Plasmodium by enzyme-linked immunosorbent assay (ELISA). Furthermore, 35% of these adults were endophagic. Of all An. albimanus collected, ∼22% were endophagic, compared with ∼45% endophagic An. nuneztovari (M. L. Quiñones, unpublished data). A survey of breeding sites found that most positive water bodies were either fish ponds or small reservoirs for domestic use; 70% of all larvae were An. nuneztovari. At least where An. nuneztovari is most abundant and endophagic, continued use of LLINs combined with focal application of larvicides might be the most effective tools, even though malaria elimination in the near term may be difficult to achieve.
South Asia ICEMR.
Entomological results from this ICEMR have implicated Anopheles stephensi, collected during 85 nights from multiple urban and rural localities in Goa, western coastal India, as a vector of P. falciparum (Figures 1 and 2). Panaji City, within Goa, had an EIR of 18.1 compared with an overall EIR of 2.35 for all of Goa (Table 1).67 In this city, An. stephensi is endophagic, but rests outdoors. Most An. stephensi (N = 55) were actively biting between 03:00 and 06:00, although there were seasonal differences. Both Anopheles fluviatilis (N = 75) and Anopheles culicifacies (N = 32) were collected biting humans but neither species was positive for Plasmodium. Mosquito control in and around Goa relies on larval suppression using fish and larvicides (Table 1).
In Wardha (central India) and Ranchi (eastern India) (Figure 1), An. culicifacies and An. fluviatilis are exophagic and exophilic. Here, IRS is the only vector control, and transmission is perennial with peaks during the rainy season. In Ranchi, An. culicifacies transmits year long, with peaks during the rainy season, and An. fluviatilis transmits primarily during February and March. In Assam state, northeastern India, the vectors are Anopheles baimai and Anopheles minimus. This is an atypical part of India in which four Plasmodium species circulate and are transmitted (Table 1). Malaria is also perennial here. To date, EIRs have not been determined for the localities in Wardha, Ranchi, or Assam.
India ICEMR.
Urban and rural sites with contrasting transmission dynamics are the main focus of this ICEMR. In urban Chennai, India, a consistent hot and humid climate supports stable, low level transmission of predominantly P. vivax malaria by a single vector species, An. stephensi (Figure 1, Table 1). Malaria control in Chennai follows strategies adopted by the Urban Malaria Scheme of the national program of India wherein vector control is based on antilarval measures such as the use of abate (temephos), application of Bacillus thuringiensis israelensis formulations, and, to a certain extent, larvivorous fish, Gambusia affinis. Despite a reduction in malaria prevalence68 the disease persists, possibly due to rapid urbanization, regular reintroduction, large numbers of breeding sites, a submicroscopic and/or asymptomatic parasite load, and the difficulty of targeting dormant stages of P. vivax. Mosquito control tends to be restricted to application of larval insecticides, targeting known breeding habitats of An. stephensi such as wells, overhead tanks, and other water storage containers. Use of interventions against the adult vectors within domestic dwellings, such as LLINs or IRS, is minimal. The reasons for the lack of adult mosquito control are varied but include the extremely dense and complex nature of the housing within urban slum settings (it is logistically challenging to access every house), discomfort in using nets in the hot and humid conditions, low transmission rates (there are many challenges at the household level above and beyond occasional infection with generally nonlethal P. vivax), and very low density of adult populations of An. stephensi. Indeed, determining where and when local transmission occurs is very difficult. The adult vectors are highly zoophagic (human blood index [HBI] = 0.028) and almost exclusively found in cattle sheds (Table 1). However, these biting and resting behaviors create the potential for novel control strategies targeting the more limited focal sites (i.e., cattle sheds) with tools such as toxic sugar baits, or possibly treating cattle and other livestock directly with insecticides or antihelminthics. Nonetheless, given that the majority of adult malaria vectors are not feeding on humans or resting in domestic dwellings, such focal interventions targeting zoophagic and exophilic behavior could have a dramatic impact on local transmission, which appears to be almost a secondary foraging “spillover” phenomenon.
Vector control practices in forested tribal areas such as Raurkela, follow more established approaches with intensive IRS and LLIN programs. The perennial vector in these rural settings is An. culicifacies, which tends to be zoophagic and exophilic. The most common sibling species, B, is refractory to P. vivax.69,70 The primary vector responsible for peak P. falciparum transmission is An. fluviatilis. This species is restricted to breeding in slow-moving fresh water that occurs post-monsoon, and so exhibits highly seasonal dynamics. The predominant sibling species, S, is an efficient vector, with previous studies showing it to be highly anthropophagic and endophagic, with a HBI up to 0.90.71,72 Recent results may suggest a shift in An. fluviatilis (M. B. Thomas, unpublished data) feeding behavior, mirroring changes observed elsewhere in response to wide-scale use of IRS and LLINs.8,73,74
Studies suggest that An. fluviatilis has shifted from resting within human dwellings to semi-enclosed animal sheds.75 This apparent behavioral change is actually species replacement; the S type now comprises only 20% of the 2013–2014 population and the zoophagic and exophilic sibling species T, now comprises the majority. This replacement might be due to increased comparative fitness of T during control measures (i.e., some form of competitive replacement), or it could be that S is simply disproportionately affected by interventions with T, feeding in cattle sheds, unaffected by IRS and LLINs, and remaining at similar absolute levels but showing a relative increase. These patterns (including the challenges in interpretation) again mirror those reported elsewhere.8,73,74
Southeast Asia ICEMR.
The China–Myanmar and Thai–Myanmar border regions have been the geographical emphasis of this project (Figure 1). The Government of China has set a goal of malaria elimination by 2020, and Thailand is pursuing spatially progressive elimination and has a national goal to eliminate malaria from 80% of the country by 2020.76 However, high malaria incidence in neighboring Myanmar and cross-border human movement present major challenges for malaria elimination in China and Thailand.1 Therefore, understanding vectorial systems and developing site-appropriate transmission control methods in the border regions are crucial.
The study sites on either side of the China–Myanmar border area are separated by less than 10 km but have significantly different vectorial systems. In the Chinese sites (around Nabang town in Yingjiang County, Yunnan Province), the major malaria vectors in the 2010–2012 survey by CDC light traps were An. minimus, Anopheles sinensis, and Anopheles maculatus (Figure 2). Anopheles minimus and An. maculatus are endophagic and anthropophilic whereas An. sinensis is generally exophagic (Table 1).77 Anopheline density was highly variable among sites and between seasons. Peak mosquito density was in May, during the rainy malaria transmission season. Molecular taxonomy of a subsample of An. minimus s.l. found that the only member of this complex present was An. minimus s.s. Moreover, of the anophelines examined, only An. minimus was detected infected with P. vivax, albeit only one positive individual out of 1,500 tested (0.07%). Blood meal analysis of a modest sample size (N = 104) revealed that humans were the main host (82.6%), followed by cattle, pigs, and dogs.77 Mixed blood meals of human/pig and human/cow were detected at low frequency (0.9% each). In the Myanmar study sites near Laza Town, Kachin Special Zone, CDC light trap collections in 2013–2014 found An. minimus was predominant, constituting 89% of anophelines collected despite high anopheline species diversity (20 species collected). The sporozoite rate of An. minimus (1.8%) in Laza Town, Myanmar, was considerably higher than in Nabang Town on the Chinese side.
It is important to note that malaria vector species composition varied significantly by mosquito collection methods. For example, larval mosquito collection in the same study sites in Myanmar found that the four most abundant vectors were An. sinensis, Anopheles barbirostris, An. minimus, and Anopheles splendidus (G. Yan, unpublished data) rather than An. minimus. Larval and adult survivorship in life table studies found differential survival because of local climate conditions as well as land use and land cover variation (G. Yan, unpublished data). In any predictive model for malaria, the environmental variables that effect anopheline survivorship need to be measured and incorporated.17 On the Thai–Myanmar border area, the main vectors are An. minimus, An. maculatus, and Anopheles annularis. All three species rest indoors and outdoors, so LLINs should exert some control on the former portion of their populations.
Southwest Pacific ICEMR.
The geographic foci of the southwest Pacific ICEMR are the Madang and East Sepik Provinces in Papua New Guinea as well as Central and Western Provinces in the Solomon Islands. Current vector control in Papua New Guinea and the Solomon Islands relies on LLINs with limited use of IRS in the Solomon Islands.78 The main malaria vectors across the region are members of the An. punctulatus group: An. punctulatus s.s., An. koliensis, and the An. farauti complex, which consists of eight cryptic species (Figure 1), and the diversity of these species varies across sites.79–81 Two of the primary vectors in the southwest Pacific, An. koliensis and An. punctulatus, which were endophagic and more anthropophic than An. farauti, were dramatically reduced by IRS, and An. koliensis may have disappeared from the Solomon Islands.13 In Papua New Guinea, two other species in the An. farauti group are important vectors: Anopheles hinesorum and An. farauti 4, with An. farauti 6 and An. farauti 8 considered to be minor vectors with circumsporozoite antigen detected by ELISA.82 Other minor malaria vectors in Papua New Guinea are, either uncommon or with a limited geographical distribution, Anopheles longirostris, Anopheles bancroftii, Anopheles subpictus, and Anopheles karwari.81,82 Collections made by HLC in Papua New Guinea found An. farauti s.s. was the predominant species in Mirap village whereas An. punctulatus was the most common species found in the inland villages of Yauatong and Wasab in the East Sepik Province. The minor vector, An. longirostris, had a relatively high population density in Wasab village. In the Solomon Islands, the only species caught biting humans at significant densities in Central Province was An. farauti s.s.
In Papua New Guinea, the populations of An. punctulatus and An. farauti were mostly zoophilic, late night biting, and exophilic but exhibited both exophagic and endophagic biting habits.83 Recent data from the Central Province of the Solomon Islands reported An. farauti as highly anthropophagic with more than 90% of blood meals on humans (Table 1).84 Both the timing of night biting behavior (from late to early) and location (from indoors/outdoors to predominantly outdoors) of An. farauti in the Solomon Islands shifted in response to selective pressure to avoid insecticides following IRS decades ago and these behavioral changes have persisted.3 Physiological resistance to insecticides has not been found yet in Papua New Guinea or the Solomon Islands, with the temporal biting shift to earlier possibly providing a behavioral resistance mechanism to minimize exposure of this vector to insecticides.85 This change in blood-feeding behavior has appeared independently on multiple islands in this archipelago, suggesting that LLINS and IRS will have a limited impact on malaria transmission for this important regional vector.86 Elimination may require the use of supplemental and complementary interventions to be implemented with LLINs.
Lessons Learned from Vector Biology Across ICEMRS
Collection methods.
The wide range of trap types and/or control methods favored by each ICEMR is a result of project-specific research questions, trap type collector bias, and controversy surrounding the HLC method because of a perceived infectivity risk to collectors (see Gimnig and others87). Furthermore, HLC is expensive and labor intensive. In several of the ICEMR sites, CDC traps combined with either pyrethroid spray catch or aspiration have replaced or supplemented HLC (Table 1). A recent study in the three east Africa ICEMR sites revealed statistically comparable EIRs for CDC compared with HLC such that the former can safely and effectively replace the latter.25 On the other hand, the Amazonian ICEMR found that HLC resulted in significantly higher numbers of An. darlingi compared with Shannon traps and CDC traps, although the number of infected mosquitoes was so low that EIRs could not be compared (M. Moreno and others, unpublished data).
An important outcome of the ICEMR vector biology studies is a new push to standardize monitoring across sites by the use of barrier screens, recently developed in Indonesia and the south Pacific.84 Advantages of this method include simplicity of construction and use of local material, collection of high numbers of both nulliparous and parous anophelines, collection of infected anophelines (to date only evidence from Iquitos; M. Moreno and others, unpublished data), no bias related to feeding preference (humans and animals are not involved as attractants), and physical integrity of specimens collected. The Amazonian, Latin American, southeast Asia, southern Africa, and southwest Pacific ICEMRs are currently testing these traps for effectiveness in collecting resting and host-seeking anophelines across the diverse settings, habitat types, and vector species.
New putative malaria vectors.
Using standard incrimination criteria (presence of infected, correctly identified anthropophilic vectors concurrent with malaria transmission), evidence of new or potential vector species has been collected in four of the 10 ICEMRs (Figure 2). Although EIRs have been determined only for An. calderoni in Colombia, they are in progress for An. rangeli and An. benarrochi B in southern Peru, and will be calculated, pending vector confirmation, for the additional species and sites (i.e., An. subpictus in Goa, possibly An. coustani s.l. and An. squamosus in Macha).45–47 In Western Province, Solomon Islands, Anopheles lungae is the most common anopheline collected by HLC in several villages. However, incrimination as a potential vector of human malaria awaits confirmation of the presence of sporozoites by PCR or ELISA.81,82 How these and other putative new vectors will respond to changes in LLIN use or climate change is being actively addressed in the Amazonian ICEMR.88
Value of longitudinal surveillance sites to malaria elimination.
Highlights of the value of these sites thus far include the following:
-
1.
No detectable or very reduced transmission in Choma and Macha, Zambia46,49; Villa de Buen Pastor, near Iquitos, Peru; Madre de Dios, Peru; and Granada and Remansinho,24 western Brazil.
-
2.
Change in the proportion of endophagic to exophagic An. gambiae in The Gambia and Mali sites.
-
3.
Species replacement of An. gambiae s.s. by An. funestus s.s. in Mutasa, Zimbabwe and species replacement of An. fluviatilis S by An. fluviatilis T in Raurkela, India.
-
4.
Temporal biting shift in An. farauti from multiple islands in southwest Pacific archipelago.85,86
-
5.
Several new putative vector species (see above); evidence for the role of An. stephensi in transmission in Goa, India (first EIRs), and An. minimus in Yingjiang, China, and Laza, Myanmar; An. hinesorum, An. farauti 4, An. farauti 6, and An. farauti 8 in Papua New Guinea.82
-
6.
Confirmation of the effectiveness of the barrier trap from studies in the southwest Pacific to collect unbiased samples of outdoor resting mosquitoes (see above).84
-
7.
Significant contribution to malaria transmission by An. albimanus and An. nuneztovari s.s. in 70 localities in western Colombia.
-
8.
Evidence from blood meal analysis near Iquitos, Peru, that An. darlingi is more locally opportunistic than anthropophilic.
Each of these discoveries contributes to more accurate EIR values and provides feedback to parallel epidemiological and parasitological studies ongoing in the ICEMR sites.
Management of outdoor (residual) transmission.
Overall, malaria vector control in the ICEMR study sites is reliant on the two insecticide-based interventions for which there exists a strong, primarily Africa-derived evidence-base: LLINs and IRS.89,90 However, the ICEMR sites reveal a variety of transmission scenarios that will require a more tailored approach that can be monitored and modified rapidly as the need arises. Where endophagy remains dominant and vectors are resistant to pyrethroids, the deployment of attractive toxic sugar baits (ATSB) indoors in combination with LLINs, is one possibility91,92 although indoor ATSBs may be temporally unsustainable (S. Lindsay, personal communication). Reduction of crepuscular human–vector contact outside houses might be accomplished by the use of ATSB outdoors, as this intervention was predicted to be especially effective against An. arabiensis, which is primarily exophilic.91 Furthermore, if these exophilic populations are also mainly zoophilic, treating nearby animal hosts as suggested for An. fluviatilis, in India, as mentioned above, could be an effective part of an integrated vector control plan.93,94 Additional important options to prevent outdoor transmission in the context of integrated control include larviciding, as used in the ICEMR urban sites of Tumaco, Goa, Chennai, and Nadiad (Table 1), and environmental management.95,96 More broadly, new interventions could include transgenic mosquitoes, sterile male releases, or cost-effective consumer products.
Conclusions
Data from the ICEMRs clearly illustrate that malaria transmission and vectors are highly spatially and temporally heterogeneous. In addition, behaviors exhibited by many vector species involved are diverse, and although they can be broadly categorized as endophagic, exophagic, endophilic, and exophilic, most vectors exhibit a mix of behaviors (e.g., some “outdoor feeding” vectors will occasionally blood feed indoors). Local behavioral adaptations will require new combinations of sampling, surveillance, and control tools. For example, at one location a program may have to address the control of multiple species, but also the control of a single species that can present multiple behaviors.
Footnotes
Financial support: This study was supported by grants from the National Institute of Allergy and Infectious Diseases to each of the ICEMR sites whose research is represented in this report.
Authors' addresses: Jan E. Conn, The Wadsworth Center, New York State Department of Health, Albany, NY, E-mail: jan.conn@health.ny.gov. Douglas E. Norris, The W. Harry Feinstone Department of Molecular Microbiology and Immunology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: douglas.norris@jhu.edu. Martin J. Donnelly, Department of Vector Biology, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mail: m.j.donnelly@liverpool.ac.uk. Nigel W. Beebe, School of Biological Sciences, The University of Queensland, Brisbane, Australia, E-mail: n.beebe@uq.edu.au. Thomas R. Burkot, Queensland Tropical Health Alliance, James Cook University, Cairns, Australia, E-mail: tom.burkot@jcu.edu.au. Mamadou B. Coulibaly, Malaria Research and Training Centre, University of Sciences, Techniques and Technologies of Bamako, Bamako, Mali, E-mail: doudou@icermali.org. Laura Chery, Department of Chemistry, University of Washington, Seattle, WA, E-mail: chery@chem.washington.edu. Alex Eapen, National Institute of Malaria Research, National Institute of Epidemiology Campus Chennai, India, E-mail: alexeapen@yahoo.com. John B. Keven, Papua New Guinea Institute of Medical Research, Madang, Papua New Guinea, E-mail: jbkeven@gmail.com. Maxwell Kilama, Infectious Diseases Research Collaboration, Kampala, Uganda, E-mail: kilamam@gmail.com. Ashwani Kumar, National Institute of Malaria Research, Goa, India, E-mail: ashwani07@gmail.com. Steve W. Lindsay, School of Biological and Biomedical Sciences, Durham University, Durham, United Kingdom, E-mail: s.w.lindsay@durham.ac.uk. Marta Moreno, Division of Infectious Diseases, University of California at San Diego, La Jolla, CA, E-mail: mmorenoleirana@ucsd.edu. Martha Quinones, Public Health Department, National University of Colombia, Bogotá, Colombia, E-mail: marthalquinones@gmail.com. Lisa J. Reimer, Papua New Guinea Institute of Medical Research, Goroka and Madang, Papua New Guinea, E-mail: lisa.reimer@liverpool.ac.uk. Tanya L. Russell, Australian Centre for Tropical and International Health, University of Queensland, Herston, Australia, E-mail: tanya.russell@jcu.edu.au. David L. Smith, Fogarty International Center, National Institutes of Health, Bethesda, MD, E-mail: smitdave@gmail.com. Matthew B. Thomas, Department of Entomology, Pennsylvania State University, University Park, PA, E-mail: mbt13@psu.edu. Edward D. Walker, Department of Entomology, Michigan State University, East Lansing, MI, E-mail: walker@msu.edu. Mark L. Wilson, Department of Epidemiology, University of Michigan, Ann Arbor, MI, E-mail: wilsonml@umich.edu. Guiyun Yan, Program in Public Health, University of California at Irvine, Irvine, CA, E-mail: guiyuny@uci.edu.
References
- 1.WHO . World Malaria Report 2013. Geneva, Switzerland: World Health Organization; 2013. p. 284. [Google Scholar]
- 2.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. doi: 10.1186/1475-2875-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moiroux N, Gomez MB, Pennetier C, Elanga E, Djenontin A, Chandre F, Djegbe I, Guis H, Corbel V. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- 5.Sougoufara S, Diedhiou SM, Doucoure S, Diagne N, Sembene PM, Harry M, Trape JF, Sokhna C, Ndiath MO. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles Mosquitoes—New Insights into Malaria Vectors. Rijeka, Croatia: InTech Open; 2013. [Google Scholar]
- 7.Mnzava AP, Macdonald MB, Knox TB, Temu EA, Shiff CJ. Malaria vector control at a crossroads: public health entomology and the drive to elimination. Trans R Soc Trop Med Hyg. 2014;108:550–554. doi: 10.1093/trstmh/tru101. [DOI] [PubMed] [Google Scholar]
- 8.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, Slotman MA. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, Gould F, Hastings I, Marshall J, Ranson H, Rowland M, Shaman J, Lindsay SW. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geissbuhler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, Mtasiwa D, Mshinda H, Fillinger U, Lindsay SW, Kannady K, de Castro MC, Tanner M, Killeen GF. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killeen GF, Chitnis N. Potential causes and consequences of behavioural resilience and resistance in malaria vector populations: a mathematical modelling analysis. Malar J. 2014;13:97. doi: 10.1186/1475-2875-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson J, St Laurent B, Lobo NF, Cooke MK, Kahindi SC, Oriango RM, Harbach RE, Cox J, Drakeley C. Novel vectors of malaria parasites in the western highlands of Kenya. Emerg Infect Dis. 2012;18:1547–1549. doi: 10.3201/eid1809.120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor B. Observations on malaria vectors of the Anopheles punctulatus complex in the British Solomon Islands Protectorate. J Med Entomol. 1975;11:677–687. doi: 10.1093/jmedent/11.6.677. [DOI] [PubMed] [Google Scholar]
- 14.Thevasagayam ES. Malaria Control Strategies in the Southwest Pacific Countries—Reappraisal. Kuala Lumpur, Malaysia: World Health Organization; 1983. [Google Scholar]
- 15.Gil LH, Alves FP, Zieler H, Salcedo JM, Durlacher RR, Cunha RP, Tada MS, Camargo LM, Camargo EP, Pereira-da-Silva LH. Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol. 2003;40:636–641. doi: 10.1603/0022-2585-40.5.636. [DOI] [PubMed] [Google Scholar]
- 16.Norris LC, Norris DE. Heterogeneity and changes in inequality of malaria risk after introduction of insecticide-treated bed nets in Macha, Zambia. Am J Trop Med Hyg. 2013;88:710–717. doi: 10.4269/ajtmh.11-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith DL, Perkins TA, Reiner RC, Jr, Barker CM, Niu T, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam JR, Bisanzio D, Buckee C, Chiyaka C, Cummings DA, Garcia AJ, Gatton ML, Gething PW, Hartley DM, Johnston G, Klein EY, Michael E, Lloyd AL, Pigott DM, Reisen WK, Ruktanonchai N, Singh BK, Stoller J, Tatem AJ, Kitron U, Godfray HC, Cohen JM, Hay SI, Scott TW. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg. 2014;108:185–197. doi: 10.1093/trstmh/tru026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bugoro H, Iro'ofa C, Mackenzie DO, Apairamo A, Hevalao W, Corcoran S, Bobogare A, Beebe NW, Russell TL, Chen CC, Cooper RD. Changes in vector species composition and current vector biology and behaviour will favour malaria elimination in Santa Isabel Province, Solomon Islands. Malar J. 2011;10:287. doi: 10.1186/1475-2875-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Okara RM, Van Boeckel T, Godfray HC, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrich JN, Naranjo DP, Alimi TO, Muller GC, Beier JC. How much vector control is needed to achieve malaria elimination? Trends Parasitol. 2013;29:104–109. doi: 10.1016/j.pt.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos Mda S, Nicolete VC, Fontoura PS, Goncalves RM, Viana SA, Menezes MJ, Scopel KK, Cavasini CE, Malafronte Rdos S, da Silva-Nunes M, Vinetz JM, Castro MC, Ferreira MU. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, Kamya MR, Staedke SG, Donnelly MJ, Drakeley C, Greenhouse B, Dorsey G, Lindsay SW. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 27.Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013;27:276–283. doi: 10.1111/j.1365-2915.2012.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 29.Manoukis NC, Powell JR, Toure MB, Sacko A, Edillo FE, Coulibaly MB, Traore SF, Taylor CE, Besansky NJ. A test of the chromosomal theory of ecotypic speciation in Anopheles gambiae. Proc Natl Acad Sci USA. 2008;105:2940–2945. doi: 10.1073/pnas.0709806105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathanga DP, Walker ED, Wilson ML, Ali D, Taylor TE, Laufer MK. Malaria control in Malawi: current status and directions for the future. Acta Trop. 2012;121:212–217. doi: 10.1016/j.actatropica.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson ML, Walker ED, Mzilahowa T, Mathanga DP, Taylor TE. Malaria elimination in Malawi: research needs in highly endemic, poverty-stricken contexts. Acta Trop. 2012;121:218–226. doi: 10.1016/j.actatropica.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiers AA, Mzilahowa T, Atkinson D, McCall PJ. The malaria vectors of the Lower Shire valley, Malawi. Malawi Med J. 2002;14:4–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Merelo-Lobo AR, McCall PJ, Perez MA, Spiers AA, Mzilahowa T, Ngwira B, Molyneux DH, Donnelly MJ. Identification of the vectors of lymphatic filariasis in the lower Shire Valley, southern Malawi. Trans R Soc Trop Med Hyg. 2003;97:299–301. doi: 10.1016/s0035-9203(03)90149-0. [DOI] [PubMed] [Google Scholar]
- 34.National Malaria Control Programme (NMCP, Malawi) ICF International . Malawi Malaria Indicator Survey (MIS) 2012. Lilongwe, Malawi, and Calverton, MD: NMCP and ICF International; 2012. pp. 1–103. [Google Scholar]
- 35.Vezenegho SB, Chiphwanya J, Hunt RH, Coetzee M, Bass C, Koekemoer LL. Characterization of the Anopheles funestus group, including Anopheles funestus-like, from northern Malawi. Trans R Soc Trop Med Hyg. 2013;107:753–762. doi: 10.1093/trstmh/trt089. [DOI] [PubMed] [Google Scholar]
- 36.Seyoum A, Sikaala CH, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, Miller JM, Russell TL, Briet OJ, Killeen GF. Human exposure to anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa Valley, south-east Zambia. Parasit Vectors. 2012;5:101. doi: 10.1186/1756-3305-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huho B, Briet O, Seyoum A, Sikaala C, Bayoh N, Gimnig J, Okumu F, Diallo D, Abdulla S, Smith T, Killeen G. Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol. 2013;42:235–247. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceesay SJ, Bojang KA, Nwakanma D, Conway DJ, Koita OA, Doumbia SO, Ndiaye D, Coulibaly TF, Diakite M, Traore SF, Coulibaly M, Ndiaye JL, Sarr O, Gaye O, Konate L, Sy N, Faye B, Faye O, Sogoba N, Jawara M, Dao A, Poudiougou B, Diawara S, Okebe J, Sangare L, Abubakar I, Sissako A, Diarra A, Keita M, Kandeh B, Long CA, Fairhurst RM, Duraisingh M, Perry R, Muskavitch MA, Valim C, Volkman SK, Wirth DF, Krogstad DJ. Sahel, savana, riverine and urban malaria in west Africa: similar control policies with different outcomes. Acta Trop. 2012;121:166–174. doi: 10.1016/j.actatropica.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaria Atlas Project. http://www.map.ox.ac.uk/ Available at.
- 40.Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, Mazenot C, Richard V, Badiane A, Dieye-Ba F, Faye J, Ndiaye G, Diene Sarr F, Roucher C, Bouganali C, Bassene H, Toure-Balde A, Roussilhon C, Perraut R, Spiegel A, Sarthou JL, da Silva LP, Mercereau-Puijalon O, Druilhe P, Rogier C. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 41.Toure YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, Sow M, Di Deco MA, Coluzzi M. The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, west Africa. Parassitologia. 1998;40:477–511. [PubMed] [Google Scholar]
- 42.Fillinger U, Sombroek H, Majambere S, van Loon E, Takken W, Lindsay SW. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar J. 2009;8:62. doi: 10.1186/1475-2875-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinka ME, Michael JB, Sylvie M, Yasmin R-P, Theeraphap C, Maureen C, Charles MM, Janet H, Anand PP, William HT, Peter WG, Caroline WK, Thomas RB, Ralph EH, Simon IH. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolo G, Briet OJ, Dao A, Traore SF, Bouare M, Sogoba N, Niare O, Bagayogo M, Sangare D, Teuscher T, Toure YT. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Zambia and identification of digested blood meals. Am J Trop Med Hyg. 2008;79:876–880. [PMC free article] [PubMed] [Google Scholar]
- 46.Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg. 2010;83:848–853. doi: 10.4269/ajtmh.2010.10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 2011;11:1173–1179. doi: 10.1089/vbz.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss WJ, Norris DE, Mharakurwa S, Scott A, Mulenga M, Mason PR, Chipeta J, Thuma PE. Challenges and prospects for malaria elimination in the southern Africa region. Acta Trop. 2012;121:207–211. doi: 10.1016/j.actatropica.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent RJ, Mharakurwa S, Norris DE. Spatial and temporal genetic structure of Anopheles arabiensis in southern Zambia over consecutive wet and drought years. Am J Trop Med Hyg. 2007;77:316–323. [PMC free article] [PubMed] [Google Scholar]
- 50.Mukonka VM, Chanda E, Haque U, Kamuliwo M, Mushinge G, Chileshe J, Chibwe KA, Norris DE, Mulenga M, Chaponda M, Muleba M, Glass GE, Moss WJ. High burden of malaria following scale-up of control interventions in Nchelenge District, Luapula Province, Zambia. Malar J. 2014;13:153. doi: 10.1186/1475-2875-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mharakurwa S, Mutambu SL, Mberikunashe J, Thuma PE, Moss WJ, Mason PR. Changes in the burden of malaria following scale up of malaria control interventions in Mutasa District, Zimbabwe. Malar J. 2013;12:223. doi: 10.1186/1475-2875-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrera S, Quinones ML, Quintero JP, Corredor V, Fuller DO, Mateus JC, Calzada JE, Gutierrez JB, Llanos A, Soto E, Menendez C, Wu Y, Alonso P, Carrasquilla G, Galinski M, Beier JC, Arevalo-Herrera M. Prospects for malaria elimination in non-Amazonian regions of Latin America. Acta Trop. 2012;121:315–323. doi: 10.1016/j.actatropica.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiwat H, Mitro S, Samjhawan A, Sardjoe P, Soekhoe T, Takken W. Collapse of Anopheles darlingi populations in Suriname after introduction of insecticide-treated nets (ITNs); malaria down to near elimination level. Am J Trop Med Hyg. 2012;86:649–655. doi: 10.4269/ajtmh.2012.11-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arevalo-Herrera M, Quinones ML, Guerra C, Cespedes N, Giron S, Ahumada M, Pineros JG, Padilla N, Terrientes Z, Rosas A, Padilla JC, Escalante AA, Beier JC, Herrera S. Malaria in selected non-Amazonian countries of Latin America. Acta Trop. 2012;121:303–314. doi: 10.1016/j.actatropica.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naranjo-Diaz N, Altamiranda M, Luckhart S, Conn JE, Correa MM. Malaria vectors in ecologically heterogeneous localities of the Colombian Pacific region. PLoS One. 2014;9:e103769. doi: 10.1371/journal.pone.0103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.da Silva-Nunes M, Moreno M, Conn JE, Gamboa D, Abeles S, Vinetz JM, Ferreira MU. Amazonian malaria: asymptomatic human reservoirs, diagnostic challenges, environmentally driven changes in mosquito vector populations, and the mandate for sustainable control strategies. Acta Trop. 2012;12:281–291. doi: 10.1016/j.actatropica.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grietens KP, Muela Ribera J, Soto V, Tenorio A, Hoibak S, Aguirre AR, Toomer E, Rodriguez H, Llanos Cuentas A, D'Alessandro U, Gamboa D, Erhart A. Traditional nets interfere with the uptake of long-lasting insecticidal nets in the Peruvian Amazon: the relevance of net preference for achieving high coverage and use. PLoS One. 2013;8:e50294. doi: 10.1371/journal.pone.0050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conn JE, Quiñones ML, Póvoa MM. Phylogeography, vectors, and transmission in Latin America. In: Manguin S, editor. Anopheles Mosquitoes—New Insights into Malaria Vectors. Rijeka, Croatia: InTech Open; 2013. [Google Scholar]
- 59.Martins-Campos KM, Pinheiro WD, Vitor-Silva S, Siqueira AM, Melo GC, Rodrigues IC, Fe NF, Barbosa M, Tadei WP, Guinovart C, Bassat Q, Alonso PL, Lacerda MV, Monteiro WM. Integrated vector management targeting Anopheles darlingi populations decreases malaria incidence in an unstable transmission area, in the rural Brazilian Amazon. Malar J. 2012;11:351. doi: 10.1186/1475-2875-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiwat H, Bretas G. Ecology of Anopheles darlingi root with respect to vector importance: a review. Parasit Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174. doi: 10.1186/1475-2875-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Sanches Lozano W, Pinedo Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 63.Parker BS, Paredes Olortegui M, Penataro Yori P, Escobedo K, Florin D, Rengifo Pinedo S, Cardenas Greffa R, Capcha Vega L, Rodriguez Ferrucci H, Pan WK, Banda Chavez C, Vinetz JM, Kosek M. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar J. 2013;12:178. doi: 10.1186/1475-2875-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmerman RH, Galardo AK, Lounibos LP, Arruda M, Wirtz R. Bloodmeal hosts of Anopheles species (Diptera: Culicidae) in a malaria-endemic area of the Brazilian Amazon. J Med Entomol. 2006;43:947–956. doi: 10.1603/0022-2585(2006)43[947:bhoasd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Montoya-Lerma J, Solarte YA, Giraldo-Calderon GI, Quinones ML, Ruiz-Lopez F, Wilkerson RC, Gonzalez R. Malaria vector species in Colombia: a review. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):223–238. doi: 10.1590/s0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foster PG, Bergo ES, Bourke BP, Oliveira TM, Nagaki SS, Sant'Ana DC, Sallum MA. Phylogenetic analysis and DNA-based species confirmation in Anopheles (Nyssorhynchus) PLoS One. 2013;8:e54063. doi: 10.1371/journal.pone.0054063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Mosquito biting activity on humans and detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res. 2012;135:120–126. doi: 10.4103/0971-5916.93434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Directorate General of Health Services MoHFW Trend of Malaria (2001–2013) http://nvbdcp.gov.in/malaria9.html Available at. Accessed May 13, 2015.
- 69.Adak T, Singh OP, Nanda N, Sharma VP, Subbarao SK. Isolation of a Plasmodium vivax refractory Anopheles culicifacies strain from India. Trop Med Int Health. 2006;11:197–203. doi: 10.1111/j.1365-3156.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- 70.Sahu NK, Sahu S, Kohli DV. Novel molecular targets for antimalarial drug development. Chem Biol Drug Des. 2008;71:287–297. doi: 10.1111/j.1747-0285.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 71.Sharma SK, Tyagi PK, Padhan K, Upadhyay AK, Haque MA, Nanda N, Joshi H, Biswas S, Adak T, Das BS, Chauhan VS, Chitnis CE, Subbarao SK. Epidemiology of malaria transmission in forest and plain ecotype villages in Sundargarh District, Orissa, India. Trans R Soc Trop Med Hyg. 2006;100:917–925. doi: 10.1016/j.trstmh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Tripathy A, Samanta L, Das S, Parida SK, Marai N, Hazra RK, Kar SK, Mahapatra N. Distribution of sibling species of Anopheles culicifacies s.l. and Anopheles fluviatilis s.l. and their vectorial capacity in eight different malaria endemic districts of Orissa, India. Mem Inst Oswaldo Cruz. 2010;105:981–987. doi: 10.1590/s0074-02762010000800006. [DOI] [PubMed] [Google Scholar]
- 73.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, Gatakaa H, Githure J, Borgemeister C, Keating J, Beier JC. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sahu SS, Gunasekaran K, Vanamail P, Jambulingam P. Seasonal prevalence and resting behaviour of Anopheles minimus Theobald and An. fluviatilis James (Diptera: Culicidae) in east-central India. Indian J Med Res. 2011;133:655–661. [PMC free article] [PubMed] [Google Scholar]
- 76.WHO . The Asia Pacific Malaria Elimination Network (APMEN) Supporting the Common Goal of a Malaria-Free Asia Pacific. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 77.Yu G, Yan G, Zhang N, Zhong D, Wang Y, He Z, Yan Z, Fu W, Yang F, Chen B. Parasit Vectors. 2013;6:264. doi: 10.1186/1756-3305-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hetzel MW. An integrated approach to malaria control in Papua New Guinea. P N G Med J. 2009;52:1–7. [PubMed] [Google Scholar]
- 79.Kazura JW, Siba PM, Betuela I, Mueller I. Research challenges and gaps in malaria knowledge in Papua New Guinea. Acta Trop. 2012;121:274–280. doi: 10.1016/j.actatropica.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henry-Halldin CN, Nadesakumaran K, Keven JB, Zimmerman AM, Siba P, Mueller I, Hetzel MW, Kazura JW, Thomsen E, Reimer LJ, Zimmerman PA. Multiplex assay for species identification and monitoring of insecticide resistance in Anopheles punctulatus group populations of Papua New Guinea. Am J Trop Med Hyg. 2012;86:140–151. doi: 10.4269/ajtmh.2012.11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beebe NW, Russell TL, Burkot TR, Lobo NF, Cooper RD. The systematics and bionomics of malaria vectors in the southwest Pacific. In: Manguin S, editor. Anopheles Mosquitoes—New Insights into Malaria Vectors. Rijeka, Croatia: InTech Open; 2013. [Google Scholar]
- 82.Cooper RD, Waterson DG, Frances SP, Beebe NW, Pluess B, Sweeney AW. Malaria vectors of Papua New Guinea. Int J Parasitol. 2009;39:1495–1501. doi: 10.1016/j.ijpara.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Burkot TR, Narara A, Paru R, Graves PM, Garner P. Human host selection by anophelines: no evidence for preferential selection of malaria or microfilariae-infected individuals in a hyperendemic area. Parasitology. 1989;98:337–342. doi: 10.1017/s0031182000061400. [DOI] [PubMed] [Google Scholar]
- 84.Burkot TR, Russell TL, Reimer LJ, Bugoro H, Beebe NW, Cooper RD, Sukawati S, Collins FH, Lobo NF. Barrier screens: a method to sample blood-fed and host-seeking exophilic mosquitoes. Malar J. 2013;12:49. doi: 10.1186/1475-2875-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keven JB, Henry-Halldin CN, Thomsen EK, Mueller I, Siba PM, Zimmerman PA, Reimer LJ. Pyrethroid susceptibility in natural populations of the Anopheles punctulatus group (Diptera: Culicidae) in Papua New Guinea. Am J Trop Med Hyg. 2010;83:1259–1261. doi: 10.4269/ajtmh.2010.10-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ambrose L, Cooper RD, Russell TL, Burkot TR, Lobo NF, Collins FH, Hii J, Beebe NW. Microsatellite and mitochondrial markers reveal strong gene flow barriers for Anopheles farauti in the Solomon Archipelago: implications for malaria vector control. Int J Parasitol. 2014;44:225–233. doi: 10.1016/j.ijpara.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Ombok M, Williamson J, Marwanga D, Abong'o D, Desai M, Kariuki S, Hamel MJ, Lobo NF, Vulule J, Bayoh MN. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tonnang HE, Kangalawe RY, Yanda PZ. Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malar J. 2010;9:111. doi: 10.1186/1475-2875-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004:Cd000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 90.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010:Cd006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marshall JM, White MT, Ghani AC, Schlein Y, Muller GC, Beier JC. Quantifying the mosquito's sweet tooth: modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar J. 2013;12:291. doi: 10.1186/1475-2875-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS One. 2013;8:e84168. doi: 10.1371/journal.pone.0084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rowland M, Durrani N, Kenward M, Mohammed N, Urahman H, Hewitt S. Control of malaria in Pakistan by applying deltamethrin insecticide to cattle: a community-randomised trial. Lancet. 2001;357:1837–1841. doi: 10.1016/S0140-6736(00)04955-2. [DOI] [PubMed] [Google Scholar]
- 94.Habtewold T, Prior A, Torr SJ, Gibson G. Could insecticide-treated cattle reduce Afrotropical malaria transmission? Effects of deltamethrin-treated Zebu on Anopheles arabiensis behaviour and survival in Ethiopia. Med Vet Entomol. 2004;18:408–417. doi: 10.1111/j.0269-283X.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 95.Fillinger U, Lindsay SW. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop Med Int Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- 96.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infect Dis. 2005;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]