Abstract
Context
Metastatic uveal melanoma recurrence after 10 years or more is not well studied in the clinical literature. This study describes the clinical characteristics and natural history of patients with delayed tumor recurrence.
Objective
To describe the characteristics of patients with delayed systemic recurrence of uveal melanoma and the natural history of the disease after recurrence.
Evidence Acquisition
This is a chart review of patients treated between 1994 and 2008 at The University of Texas MD Anderson Cancer Center for uveal melanoma whose disease recurred 10 or more years after treatment of the primary tumor.
Results
Of 463 patients treated for metastatic uveal melanoma, 305 developed systemic recurrence within 5 years from the time of diagnosis of primary melanoma, 97 developed systemic recurrences between 5 and 10 years, while 61 patients developed metastasis 10 years or more. The interval between primary to first systemic metastasis was a significant independent predictor of survival time from first systemic metastasis. The median survival time for patients with delayed metastatic recurrence after ten years or more was significantly longer than for patients who had intermediate or early systemic recurrence. Levels of lactate dehydrogenase, serum alkaline phosphatase, serum albumin, age, M-Stage and performance status at time of recurrence, as well as gender were also independent predictors of survival time from systemic recurrence.
Conclusion
Longer time interval between primary and first systemic metastasis is significantly correlated with prolonged survival. Patients who survive 10 years or more without tumor metastasis after treatment for primary uveal melanoma cannot be considered cured. Prognosis remains poor for patients with metastatic uveal melanoma.
INTRODUCTION
Uveal melanoma is the most common primary intraocular malignant tumor in adults. It is the second most common type of primary malignant melanoma, representing 5-6% of all melanoma diagnoses. The incidence of ocular melanoma in the USA is reported to be about 4,800 new cases per year. Metastasis is via vascular spread, and approximately 40-50% of patients with primary uveal melanoma ultimately develop metastases. Over 95% of patients have disease limited to the eye at diagnosis. Many patients today undergo eye-preserving therapies such as radiation brachytherapy based on clinical findings or preoperative biopsy and enjoy the same survival as patients who undergo enucleation of the eye. About 30% of patients die of systemic metastases within 5 years of diagnosis and 45% within 15 years.1 Of the patients who die of uveal melanoma, 62% and 90% do so within 5 and 15 years, respectively.
The survival of patients with metastatic uveal melanoma is directly related to the presence of liver metastasis. The liver is involved in as many as 95% of individuals who develop metastatic disease.2,3 Liver is the first site of systemic metastasis for most patients and the exclusive site of systemic metastasis in about 40% of patients. The clinical course of uveal melanoma is determined by progression of the disease in the liver. The median survival of patients who develop liver metastasis is reported to be 5 to 6 months, regardless of treatment, and the one-year survival rate is estimated to be 10-15%.4 In contrast, patients with metastatic melanoma confined to extrahepatic sites have a median survival of about 19-28 months, and 76% of the patients survive over a year.1,5,6
Despite advances in diagnosis and management of primary uveal melanoma, systemic metastases from uveal melanoma are usually difficult to treat.6 The management of metastatic uveal melanoma is dependent on location(s) of metastases. Regional therapies such as surgical resection, isolated hepatic perfusion, intra-arterial hepatic chemotherapy, and chemoembolization of liver metastasis are used for the treatment of metastatic disease confined to the liver; with limited success.7 These therapies have not had an impact on duration of patient survival. Systemic approaches involving chemotherapy, immunotherapy, or a combination of both have been used to treat extrahepatic metastatic disease, most often with little significant benefit.7
This study is a chart review of patients with metastatic uveal melanoma whose tumor recurred more than 10 years after treatment of their primary tumor. We describe the characteristics of these patients and the natural history of their disease after tumor recurrence.
PATIENTS AND METHODS
Review Methods
We reviewed our database of 463 patients with metastatic uveal melanoma and selected the patients who had systemic recurrence 10 years or more after diagnosis of the treatment of primary tumor. Patients were divided into those with disease recurrence within 5 years, between 5 and 10 years, and after 10 years or more. Patients were treated for systemic metastasis by the medical oncologists in our department over a nearly 14-year period starting in 1994. The eligibility criteria of patients for this study included established diagnosis of primary uveal melanoma that was treated either with localized plaque radiation therapy or by enucleation of the affected eye, presence of radiologically confirmed systemic tumor metastasis, availability of information about the evolution of and therapy prescribed for the recurrent disease over time, and follow-up information about current status. Upon arrival at The University of Texas MD Anderson Cancer Center, each patient underwent baseline evaluation that included medical history, physical examination, and laboratory studies including complete blood cell counts, serum chemistry levels, and renal and hepatic function profiles. Radiologic staging workup included computed tomographic (CT) scans of chest, abdomen, and pelvis. The brain was evaluated by CT scan or magnetic resonance imaging, if the patient participated in a clinical trial or had neurologic symptoms.
Trials and Regimens
The choice of therapy for recurrent disease depended on whether liver metastasis was present and the extent of extrahepatic disease. Patients with metastatic disease located mostly in the liver were treated with regional therapy directed to the liver metastases, such as chemoembolization of liver metastases with starch particles or polyvinyl alcohol together with cisplatin, while those with significant extrahepatic disease were encouraged to participate in clinical trials ongoing at the time. Single-agent Phase II clinical trials included drugs such as paclitaxel, bryostatin, RFS 2000 (9-nitrocamptothecin), bexarotene, liposomal vincristine, and docosahexaenoic acid–conjugated paclitaxel. Patients who did not want to participate in clinical trials were offered therapy with the bleomycin, vincristine, lomustine, and dacarbazine (BOLD)-interferon regimen8. Response to treatment was assessed according to the standard World Health Organization response criteria9 initially and, during the last 5 years of the study period, to RECIST criteria. The Institutional Review Board at MD Anderson Cancer Center granted approval for all clinical trials. All patients gave their informed written consent to participate in the studies in accordance with institutional and federal guidelines.
Analysis
Patient or tumor characteristics were summarized descriptively using summary statistics (median, range) for continuous variables or frequency and percentage for categorical variables, as appropriate. Demographic and clinical characteristics include age; sex; performance status; site of primary; treatment of primary; disease stage before treatment; number and sites of metastatic lesions; baseline (at the time of systemic metastasis) levels of albumin, lactate dehydrogenase (LDH), and alkaline phosphatase; and response to treatments. Baseline serum albumin level was categorized as normal or abnormal (< 3. 5 g/dL) (to convert to g/L, multiply by 10), as were levels of LDH (abnormal > 618 IU/L) and alkaline phosphatase (abnormal > 126 IU/L).
Time to systemic tumor recurrence was defined as the time in months from diagnosis of primary to the time when first systemic metastasis was diagnosed. Survival from metastasis was defined as the time from diagnosis of first systemic metastasis to date of death for the patients who died or the date of last contact for patients still alive. Patients still alive at the last contact date were censored. The Kaplan-Meier product limit method was used to construct survival curves and estimate median survival with corresponding 95% confidence intervals.10 Multivariable Cox proportional hazard models11 were utilized to examine the predicted values of patients characteristics for time from primary melanoma to systemic metastasis and for overall survival time from systemic metastasis. We used the backward selection method to select independent predictors. All statistical analyses were performed by using SAS12 and the R statistical project (http://www.r-project.org/). The retrospective study was conducted based on IRB approved protocol.
RESULTS
Between April 1994 and March 2008, 463 patients with systemic metastasis from uveal melanoma were evaluated in our institution. The clinical characteristics of patients are summarized in Tables 1. Patients who had systemic tumor recurrence 10 years or more from time of diagnosis of the primary tumor are the subjects of interest in this study. The median age at time of systemic recurrence for patients with delayed recurrence was 61 years, with a range from 32-82 years. Fifty-six percent were female. Seventy-one percent of the patients had ECOG performance status 0-1. The right eye was the site of primary tumor in 59% of the patients. About half of the patients underwent enucleation of the eye as the first treatment of the primary. Thirty-three percent of the patients underwent plaque radiotherapy and 12% underwent both, usually radiotherapy followed by enucleation of the eye for locally progressive tumor. One patient underwent local resection of the tumor, while the modality of therapy for the primary was not mentioned for three patients. Ninety-three percent of the patients had stage M1C disease, and 98% had visceral metastasis, 64% with only one organ involved. Eleven (18%) of the patients had two visceral metastatic sites, and nine (15%) had three visceral sites involved. The liver was the most commonly involved site of recurrent systemic metastasis and was the first site of recurrent disease in 82% of the patients. Twenty-six percent of the patients had pulmonary metastasis upon first recurrence and 10% cutaneous metastasis. Eight percent had lymph node metastasis (mostly in the abdomen). Four patients had bone metastasis, while three patients each (5%) had brain, adrenal gland, and kidney metastasis. Metastasis to the breast and peritoneum was present in two patients each. Sites of metastasis upon last follow up for delayed uveal melanoma patients remained similar. The liver remained the most frequent site of metastasis, with 87% of patients eventually developing liver disease. The lung remained the second most common site, with 26% of people developing lung metastasis. Eight patients (13%) developed cutaneous metastasis, and 8 (13%) developed brain metastasis. Seven patients (11%) developed bone metastasis while four patients each (7%) developed adrenal and kidney metastasis. Two patients each developed metastasis to the breast and peritoneum and 10 patients had disease spread to other sites. At the time of diagnosis of first systemic metastasis, serum LDH and alkaline phosphatase levels were elevated in 31% and 30% of the patients, while level of serum albumin was low in 15% and total bilirubin elevated in 5% of the patients.
Table 1.
Demographic and clinical characteristics for primary melanoma.
| Covariates | levels | Developed systemic met within 5 yrs | Developed systemic met within 5-10 yrs | Developed systemic met after 10 yrs | Total | p-value |
|---|---|---|---|---|---|---|
| Gender | Male | 160(52.5%) | 41(42.3%) | 27(44.3%) | 228(49.2%) | 0.153 |
| Female | 145(47.5%) | 56(57.7%) | 34(55.7%) | 235(50.8%) | ||
| Side of eyes at primary | Left | 138(45.5%) | 48(49.5%) | 25(41%) | 211(45.8%) | 0.594a |
| Right | 165(54.5%) | 49(50.5%) | 36(59%) | 250(54.2%) | ||
| Therapy for primary melanoma | Both | 14(4.6%) | 9(9.3%) | 7(11.5%) | 30(6.5%) | 0.033a |
| Enucleation | 136(44.6%) | 29(29.9%) | 29(47.5%) | 194(41.9%) | ||
| Radiotherapy | 109(35.7%) | 46(47.4%) | 20(32.8%) | 175(37.8%) | ||
| Unknown | 35(11.5%) | 11(11.3%) | 3(4.9%) | 49(10.6%) | ||
| none | 8(2.6%) | 0 (%) | 0 (%) | 8(1.7%) | ||
| other | 3(1%) | 2(2.1%) | 2(3.3%) | 7(1.5%) | ||
| Involved sites at systemic recurrence | Liver | 272 (89.2%) | 84 (86.6%) | 50 (82.0%) | 406 (87.7%) | n.a |
| Lung | 60 (19.7%) | 25 (25.8%) | 16 (26.2%) | 101 (21.8%) | ||
| Skin/soft tissue | 36 (11.8%) | 12 (12.4%) | 6 (9.8%) | 54 (11.7%) | ||
| Lymph nodes | 19 (6.2%) | 14 (14.4%) | 5 (8.2%) | 38 (8.2%) | ||
| Bone | 36 (11.8%) | 13 (13.4%) | 4 (6.6%) | 53 (11.4%) | ||
| Adrenal | 7 (2.3%) | 4 (4.1%) | 3 (4.9%) | 14 (3.0%) | ||
| Brain | 5 (1.6%) | 1 (1.0%) | 3 (4.9%) | 9 (1.9%) | ||
| Kidney | 3 (1.0%) | 2 (2.1%) | 3 (4.9%) | 8 (1.7%) | ||
| Breast | 6 (2.0%) | 3 (3.1%) | 2 (3.3%) | 11 (2.4%) | ||
| Spleen | 8 (2.6%) | 2 (2.1%) | 0 (0.0%) | 10 (2.2%) | ||
| Other | 15 (4.9%) | 5 (5.2%) | 13 (21.3%) | 33 (7.1%) | ||
| M Stage | M1a | 4(1.3%) | 3(3.1%) | 1(1.6%) | 8(1.7%) | 0.444 |
| M1b | 9(3%) | 6(6.2%) | 3(4.9%) | 18(3.9%) | ||
| M1c | 292(95.7%) | 88(90.7%) | 57(93.4%) | 437(94.4%) | ||
| ECOG performance status at systemic recurrence | 0 | 93(30.9%) | 22(22.9%) | 9(14.8%) | 124(27.1%) | 0.035 |
| 1 | 166(55.1%) | 53(55.2%) | 43(70.5%) | 262(57.2%) | ||
| 2 | 35(11.6%) | 16(16.7%) | 9(14.8%) | 60(13.1%) | ||
| 3 | 7(2.3%) | 5(5.2%) | 12(2.6%) | |||
| No. of visceral sites | 0 | 5(1.6%) | 2(2.1%) | 1(1.6%) | 8(1.7%) | n.a |
| 1 | 217(71.1%) | 66(68.8%) | 39(63.9%) | 322(69.7%) | ||
| 2 | 66(21.6%) | 17(17.7%) | 11(18%) | 94(20.3%) | ||
| 3 | 9(3%) | 5(5.2%) | 9(14.8%) | 23(5%) | ||
| 4 | 8(2.6%) | 6(6.3%) | 1(1.6%) | 15(3.2%) | ||
| Albumin | Normal | 161(52.8%) | 52(53.6%) | 33(54.1%) | 246(53.1%) | 0.839 |
| Abnormal | 38(12.5%) | 9(9.3%) | 9(14.8%) | 56(12.1%) | ||
| Unknown | 106(34.8%) | 36(37.1%) | 19(31.1%) | 161(34.8%) | ||
| Alkaline phosphatase | Normal | 129(42.3%) | 39(40.2%) | 23(37.7%) | 191(41.3%) | 0.904 |
| Abnormal | 82(26.9%) | 24(24.7%) | 18(29.5%) | 124(26.8%) | ||
| Unknown | 94(30.8%) | 34(35.1%) | 20(32.8%) | 148(32%) | ||
| Bilirubin | Normal | 207(67.9%) | 65(67%) | 45(73.8%) | 317(68.5%) | 0.884a |
| Abnormal | 13(4.3%) | 3(3.1%) | 3(4.9%) | 19(4.1%) | ||
| Unknown | 85(27.9%) | 29(29.9%) | 13(21.3%) | 127(27.4%) | ||
| Lactate dehydrogenase | Normal | 68(22.3%) | 22(22.7%) | 21(34.4%) | 111(24%) | 0.279 |
| Abnormal | 126(41.3%) | 36(37.1%) | 19(31.1%) | 181(39.1%) | ||
| Unknown | 111(36.4%) | 39(40.2%) | 21(34.4%) | 171(36.9%) | ||
| Age at time of primary (years) Median (range) | 58.6 (16, 85.1) | 50.8 (20.3, 78.4) | 45.2 (21, 65.8) | <0.001b | ||
| Age at time of recurrence (years) Median (range) |
60.9 (18.1, 87.3) | 59.6 (27.4, 85.2) | 61.2 (31.7, 81.5) | 0.313b |
based on Fisher’s exact test;
based on Kruskal-Wallis test. n.a. no test was performed due to large categories and small sample size per cell.
Abbreviations: ECOG= Eastern Cooperative Group, M Stage=Metastasis Stage
Treatment regimens used to treat these patients depended on whether the metastatic tumor was primarily limited to the liver. Hepatic arterial chemoembolization (HACE), the most frequent treatment of patients with tumor metastasis confined to the liver was associated with a median survival of 11 months compared with median survival of 8 months observed with other systemic treatment regimens. It is not clear as to how much of the survival improvement with HACE is related to treatment and how much to patient selection. Systemic therapies, as described in earlier reviews2,3 included single agent therapies being investigated at the time and were of limited benefit because they rarely resulted in partial or complete response.
From time of diagnosis of the primary melanoma, median survival for the 463 patients was 53 months and median time for systemic metastasis was 39 months (Figure 1). The median overall survival from tumor recurrence was significantly longer for patients with delayed recurrence than for patient who had recurrence between 5 to 10 years or within 5 years of diagnosis (17.7 months (95%CI: 9.5-24.8 months)) vs. 10.7 months (95%CI: 8-15.5 months) v. 8.4 months (95%CI: 7.3-10.2 months). The overall test for the distribution of overall survival time from recurrence was significant (p=0.0003, Figure 2).
Figure 1.
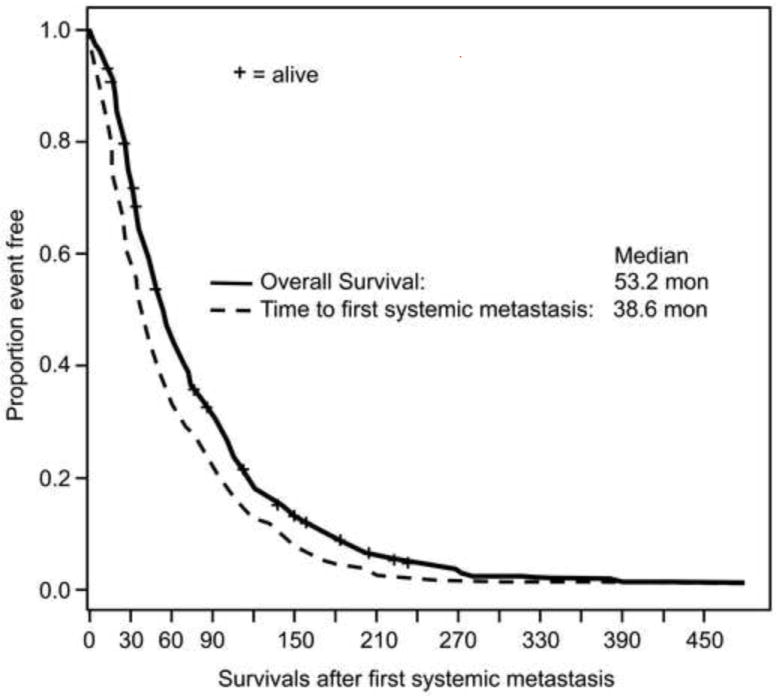
Survivals after treatment of primary uveal melanoma
Figure 2.
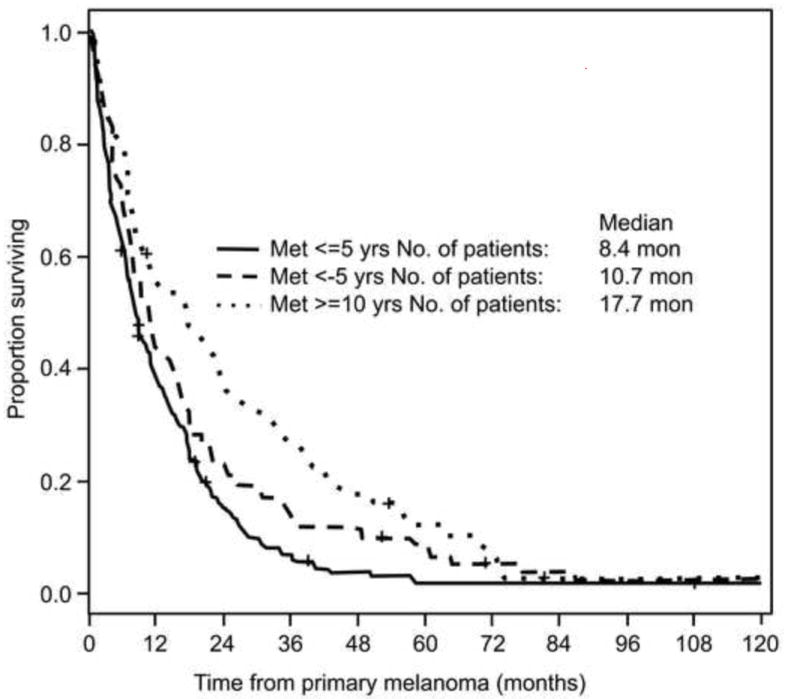
Survivals after development of first systemic metastasis
Older age at the time of primary melanoma and no treatment for the primary tumor were independent predictors for shorter time from primary melanoma to first systemic metastasis (Table 2). The differences between all other treatment groups were not statistically significant. Gender and side of eye of primary melanoma were not significant predictor for systemic metastasis free interval.
Table 2.
Analyses of systemic metastasis free interval from time of primary melanoma.
| Variable | Levels | Univariable Cox model | Multivariable Cox model | ||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
|
| |||||
| Age at time of primary | Increment of 5 yrs | 1.195 (1.149,1.242) | <.0001 | 1.036 (1.028,1.044) | <.0001 |
|
| |||||
| Treatment for primary | Both vs. Rad | 0.64 0(0.429,0.954) | 0.0286 | 0.717 (0.483,1.065) | 0.0991 |
| Enuc vs. Rad | 0.913 (0.736,1.132) | 0.4048 | 1.041 (0.843,1.285) | 0.7102 | |
| Unknown vs. Rad | 1.254 (0.911,1.726) | 0.1656 | 1.286 (0.935,1.768) | 0.1216 | |
| None vs. Rad | 7.083 (3.301,15.199) | <.0001 | 6.856 (3.183,14.771) | <.0001 | |
| Other vs. Rad | 0.709 (0.332,1.513) | 0.374 | 0.785 (0.367,1.677) | 0.5313 | |
|
| |||||
| Side of eye | Right vs. left | 0.993 (0.822,1.200) | 0.9448 | Not included | |
|
| |||||
| Gender | Male vs. female | 1.157 (0.958,1.397) | 0.1289 | Not included | |
Abbreviations: rad=radiotherapy, enuc= enucleation, yrs=years
After adjusted for other variables, factors that were associated with significantly poorer survival after the development of systemic recurrence included shorter systemic recurrence free interval (5 vs. >=10 yrs, HR=2.28 (95%CI: 1.67-3.12), p<0.0001), abnormal serum LDH level (abnormal vs. normal, hazards ratio=2.37 (95%CI: 1.77-3.17), p<0.0001), abnormal serum alkaline phosphatase level (abnormal vs. normal HR=1.79 (95%CI: 1.35-2.36), p<0.0001), and abnormal serum albumin level (abnormal vs. normal HR=1.83 (95%CI: 1.24-2.69), p=0.002), abnormal total serum bilirubin level (abnormal vs. normal HR=3.48 (95%CI: 1.95-6.20), p<0.001), Male gender (male vs. female, HR=1.51 (95%CI: 1.24-1.84), p<0.0001), M1c stage vs. M1a/M1b, HR=1.66 (95%CI: 1.15-2.39), p=0.0065), worse ECOG performance status (1 vs. 0, HR=1.34 (95%CI: 1.06-1.70), p=0.014; 2 vs. 0 HR=2.50 (95%CI: 1.77-3.52), p<0.0001; 3 vs. 0 HR=2.47 (95%CI: 1.18-5.15), p=0.0065). Older age at recurrence was marginally significant predictor of overall survival after adjusted for other variables. (HR=1.04 (95%CI: 0.995-1.08) for each 5 years, p=0.09). The number of visceral at recurrence and site of primary melanoma were not associated with overall survival from systemic recurrence (Table 3).
Table 3.
Analyses of overall survival time from first systemic metastasis disease.
| Univariable Cox model | Multivariable Cox model | ||||
|---|---|---|---|---|---|
|
| |||||
| Variables | Level | HR (95%CI) | p-value | HR (95%CI) | p-value |
|
| |||||
| Age at time of recurrence | increment of 5 yrs | 1.06 (1.02,1.101) | 0.003 | 1.037 (0.995,1.081) | 0.0888 |
|
| |||||
| When developed systemic metastasis | <5 vs. >=10 yrs | 1.727 (1.297,2.3) | 0.0002 | 2.282 (1.671,3.117) | <.0001 |
| 5-10 yrs vs. >=10 yrs | 1.312 (0.945,1.823) | 0.1051 | 1.626 (1.135,2.33) | 0.0081 | |
|
| |||||
| ECOG performance status at first metastasis | 1 vs. 0 | 1.481 (1.186,1.848) | 0.0005 | 1.343 (1.063,1.696) | 0.0135 |
| 2 vs. 0 | 2.269 (1.648,3.124) | <.0001 | 2.499 (1.772,3.524) | <.0001 | |
| 3 vs. 0 | 3.67 (2.013,6.692) | <.0001 | 2.465 (1.18,5.147) | 0.0164 | |
|
| |||||
| M-stage | M1c vs. M1a/b | 2.705 (1.74,4.206) | <.0001 | 1.658 (1.152,2.387) | 0.0065 |
|
| |||||
| Gender | Male vs. female | 1.327 (1.101,1.601) | 0.003 | 1.508 (1.236,1.838) | <.0001 |
|
| |||||
| Bilirubin | Abnormal vs. normal | 4.559 (2.852,7.29) | <.0001 | 3.475 (1.946,6.204) | <.0001 |
| Unknown vs. normal | 0.908 (0.732,1.126) | 0.3789 | 0.761 (0.507,1.141) | 0.1865 | |
|
| |||||
| Alkaline phosphatase | Abnormal vs. normal | 2.993 (2.366,3.786) | <.0001 | 1.785 (1.351,2.359) | <.0001 |
| Unknown vs. normal | 1.317 (1.054,1.645) | 0.0152 | 1.156 (0.754,1.771) | 0.5069 | |
|
| |||||
| Lactate dehydrogenase | Abnormal vs. normal | 2.881 (2.247,3.695) | <.0001 | 2.371 (1.773,3.172) | <.0001 |
| Unknown vs. normal | 1.649 (1.286,2.114) | <.0001 | 1.624 (1.095,2.408) | 0.0159 | |
|
| |||||
| Albumin | Abnormal vs. normal | 3.386 (2.518,4.551) | <.0001 | 1.828 (1.241,2.691) | 0.0022 |
| Unknown vs. normal | 1.115 (0.908,1.37) | 0.2977 | 1.43 (1.034,1.978) | 0.0308 | |
|
| |||||
| Side of eyes | Right vs. left | 0.906 (0.75,1.093) | 0.3029 | No included | na |
|
| |||||
| No. of visceral site | 0 vs. 1 | 0.615 (0.29,1.301) | 0.2034 | No included | na |
| 2 vs. 1 | 1.114 (0.881,1.407) | 0.3674 | |||
| 3 vs. 1 | 1.007 (0.653,1.553) | 0.9755 | |||
| 4+ vs. 1 | 1.345 (0.8,2.263) | 0.2639 | |||
Abbreviations: ECOG=Eastern Cooperative Group, yrs=years, M Stage=Metastasis Stage
DISCUSSION
The purpose of this study was to describe the characteristics of uveal melanoma patients with a delayed recurrence of 10 years or more and to study the natural history of the disease after recurrence. Delayed recurrence is unusual in melanoma; the majority of recurrences appear within 10 years of initial treatment. Earlier studies following melanoma patients have found rates of disease recurrence after 10 or more years of only 0.98-6.7%.13-16 In our review of 463 patients with ocular melanoma, the incidence of delayed recurrence (10 years or more) was 13.2% (n=61). The median time to recurrence in our study was 3.25 years for all patients, and 13.1 years for those with delayed recurrence. The unusually high rate of delayed recurrence in this group may be due to a referral bias, as MD Anderson is a tertiary care cancer center. Crowley and Seigler’s study found uveal melanoma patients to have longer delays to recurrence than patients with other melanomas, accounting for many of the longest intervals in their study.13 However, very few studies have been published on delayed recurrence in uveal melanoma. It has been hypothesized that delayed recurrence is due to cancer latency, which could be related to three factors: (1) angiogenic dormancy, (2) cell cycle arrest, or (3) Immune surveillance.17,18 It has also been suggested that gonadal hormones play a role in prolonging disease-free survival.13 Briele and Raderman concluded that premenopausal women might have a greater likelihood of having a prolonged disease-free interval,15,19 but Shaw et al. failed to reproduce this finding.13,14,16 In our study, 56% of patients with late recurrence were female but only 18% of female patients were premenopausal (younger than 50 years), making it unlikely that premenopausal women had an increased incidence of delayed recurrence.
Factors that have been shown to predict survival in earlier studies include site of metastasis, largest detected tumor diameter,20 abnormal liver function test results,21 patient age (younger or older than 60 years), patient sex, response to treatment, and interval until recurrence.22,23 Liver enzyme data were recorded for a majority of the patients. Normal LDH, serum alkaline phosphatase, albumin, and bilirubin levels were all significantly correlated with prolonged survival outcomes in both univariable and multivariable Cox analysis. As found in earlier studies,22,23 in addition, patient sex M-stage, ECOG performance status were independent predictors for survival
Prognosis for patients with metastasis uveal melanoma is very poor, with the median survival from date of metastasis detection ranging from less than 6 months to 12.5 months.21,22 In our study the median survival for patients with delayed recurrence was 18 months calculated from date of first metastasis to last contact date. Comparatively, the median survival for patients with recurrence between 5 and 10 years was 11 months, and only 8 months for those with recurrence within 5 years. Delayed recurrence proved to be a significant predictor for longer survival (p= 0.0003). Several factors were ruled out as explanations for the longer than average survival times of this patient population. Prognostic indicators such as LDH levels, age, the presence of liver or brain metastasis, response rates to chemotherapy, and the percentage of patients receiving localized chemoembolization versus systemic treatment were all comparable to earlier studies. This suggests that none of these factors were responsible for the high median survival time observed among this patient population. The slow-growing nature of tumors observed in patients with delayed recurrence may explain the increased survival duration. Previous studies have corroborated the correlation between interval to recurrence and survival.22
Abnormal LDH level is generally correlated with liver dysfunction and poor prognosis. A total of 48% of patients with delayed recurrence and available lab data had an elevated LDH level upon diagnosis of first metastasis, slightly lower than the figure reported in an earlier Collaborative Ocular Melanoma Study of 58%.21
Age was a significant prognostic factor for our patients in univariable (p=0.003) but was only marginally significant in multivariable Cox analysis (p=0.09). Other studies have shown a correlation between younger age and better prognosis. 22 Our patient population was not younger at time of metastasis diagnosis that those in previous studies.20 The median age of patients at time of diagnosis of first metastasis was 61 years, with a range from 32 to 81.
The liver was the first site of metastasis in 82% of our patient population, with 87% eventually developing liver metastasis. This figure is comparable to a published liver metastasis rate of 89% among patients who have uveal melanoma.24 The percentage of patients who developed brain metastasis was 13%, compared to 15% reported in the Collaborative Ocular Melanoma Study based on autopsy reports of patients with ocular melanoma.25 Both brain and liver metastasis are associated with poor prognosis, with an average survival of 2.8 to 4 months26, and 5 to 6 months6 after diagnosis respectively. The prolonged survival of the patients studied cannot be attributed to a decreased incidence of brain or liver metastasis.
It is also unlikely that the increased median survival among these patients was due to treatment. Patients reviewed in this study did not have a higher than average response rate to chemotherapy. Although the 61 patients in the study were given 23 different chemotherapy regimens, there was only one partial response to treatment. Patients with localized liver disease have a better prognosis than those with extra hepatic metastases, since they can be treated with chemoembolization. In earlier studies, the general response rate for chemoembolization in uveal melanoma patients was 36%, compared with less than 1% in those treated with systemic chemotherapy.5 Our patient population did not have a higher that average number of patients with localized liver metastasis. A total of 33.3% of patients with delayed recurrence had disease localized to the liver, compared to 46% of patients with uveal melanoma patients in other studies.23 The high median survival of our patient population could not be explained by response to treatment or by an unusually high number of patients with localized liver disease.
In the absence of any other explanatory factors, it is likely that the slow-growing nature of the tumors led to less aggressive disease upon metastasis and a longer than average median survival time. Molecular factors may play a role in the prolonged disease-free interval in these patients. Recently, mutations have been described in the alpha-subunit of an intracellular G-protein known as GNAQ.27,28 This, in turn, portends an aggressive phenotype. The contributions of pathways mediated by epidermal growth factor receptor (EGFR), c-Met, and the insulin-like growth factor (IGF) receptor are also being investigated in this disease.29-31 EGFR signaling initiates several downstream proteins, including those involved in the mitogen-activated protein kinase (MAPK) and Akt pathways, leading to cellular proliferation.32,33 The growth factor receptor c-Met is involved in tumor growth, motility, adhesion, and invasion.34 The IGF pathway has been shown to promote growth and inhibit apoptosis in tumor cells,35 and the ligand IGF-1 is produced in the liver, a site of frequent metastasis in uveal melanoma patients suggesting tropism for this visceral environment. The frequency of GNAQ mutation and expression of the cell surface receptors EGFR, c-Met, and IGF-1 receptor have not been evaluated in our sample. It is possible that presence of some of these molecular markers is associated with high risk for tumor recurrence.
CONCLUSION
Patients with disease-free survival of 10 years or greater following treatment for primary uveal melanoma cannot be considered cured. Patient education and awareness about possibility of delayed tumor recurrence is of paramount importance. Clinical monitoring with radiologic imaging and liver enzyme tests for tumor recurrence beyond 10 years post therapy of the primary tumor is not cost effective because of the rarity of delayed recurrence. In view of indolent natural history of recurrent melanoma in this patient population, as evidenced by prolonged survival after diagnosis of recurrent melanoma, it is possible that with heightened awareness, some of these recurrences may be diagnosed early enough to make surgical resection or radiofrequency ablation of recurrent disease possible. At present, no systemic treatment has significant impact on survival once recurrent disease is confirmed.
Acknowledgments
This research is supported in part by the National Institutes of Health through M. D. Anderson’s Cancer Center Support Grant CA016672.
References
- 1.Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 2.Bedikian AY, Kantarjian H, Young SE, Bodey GP. Prognosis in metastatic choroidal melanoma. South Med J. 1981;74(5):574–577. doi: 10.1097/00007611-198105000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Bedikian AY, Legha S, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76(9):1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98(3):383–389. doi: 10.1016/s0161-6420(91)32285-1. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh EC, Essner R, Foshag LJ, Ye X, Wang H, Morton DL. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100(1):122–129. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 6.Kath R, Hayungs J, Bornfeld N, et al. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72(7):2219–2223. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46(1):151–166. doi: 10.1097/01.iio.0000195852.08453.de. [DOI] [PubMed] [Google Scholar]
- 8.Pyrhonen S, Hahka-Kemppinen M, Muhonen T, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD) and human leukocyte interferon for metastatic uveal melanoma. Cancer. 2002;95:2366–2372. doi: 10.1002/cncr.10996. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Handbook for reporting results of cancer treatment. WHO; Geneva: 1979. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–81. [Google Scholar]
- 11.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(2):187–22. [Google Scholar]
- 12.SAS/STAT 9.2 User’s Guide. SAS Institute; Cary, NC: 2008. [Google Scholar]
- 13.Crowley NJ, Seigler HF. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann Surg. 1990;212(2):173–177. doi: 10.1097/00000658-199008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaway MP, Briggs JC. The incidence of late recurrence (greater than 10 years); an analysis of 536 consecutive cases of cutaneous melanoma. Br J Plast Surg. 1989;42(1):46–49. doi: 10.1016/s0007-1226(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 15.Briele HA, Beattie CW, Ronan SG, Chaudhuri PK, Das Gupta TK. Late recurrence of cutaneous melanoma. Arch Surg. 1983;118(7):800–803. doi: 10.1001/archsurg.1983.01390070012003. [DOI] [PubMed] [Google Scholar]
- 16.Shaw HM, Beattie CW, McCarthy WH, Milton GW. Late relapse from cutaneous stage I malignant melanoma. Arch Surg. 1985;120(10):1155–1159. doi: 10.1001/archsurg.1985.01390340053010. [DOI] [PubMed] [Google Scholar]
- 17.Hansel G, Schönlebe J, Haroske G, Wollina U. Late recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). A single-centre analysis of 1881 patients with a follow-up of 10 years or more. J Eur Acad Dermatol Venereol. 2010;24(7):833–836. doi: 10.1111/j.1468-3083.2009.03536.x. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raderman D, Giler S, Rothem A, Ben-Bassat M. Late metastases (beyond ten years) of cutaneous malignant melanoma. Literature review and case report. J Am Acad Dermatol. 1986;15(2 Pt 2):374–378. doi: 10.1016/s0190-9622(86)70182-5. [DOI] [PubMed] [Google Scholar]
- 20.Coleman K, Baak JP, Van Diest P, et al. Prognostic factors following enucleation of 111 uveal melanomas. Br J Ophthalmol. 1993;77(11):688–692. doi: 10.1136/bjo.77.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Screening for metastasis from choroidal melanoma: The Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004;22(12):2438–2444. doi: 10.1200/JCO.2004.08.194. [DOI] [PubMed] [Google Scholar]
- 22.Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23(31):8076–8080. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124(12):1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 24.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 25.Collaborative Ocular Melanoma Study Group. Assessment of metastatic disease status at death in 435 patients with large choroidal melanoma in the Collaborative Ocular Melanoma Study (COMS): COMS report no. 15. Arch Ophthalmol. 2001;119(5):670–676. doi: 10.1001/archopht.119.5.670. [DOI] [PubMed] [Google Scholar]
- 26.Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol. 1983;1(4):313–317. doi: 10.1007/BF00165714. [DOI] [PubMed] [Google Scholar]
- 27.Onken MD, Worley LA, Long MD, et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49(12):5230–5234. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topcu-Yilmaz P, Kiratli H, Saglam A, et al. Correlation of clinicopathological parameters with HGF, c-Met, EGFR, and IGF-1R expression in uveal melanoma. Melanoma Res. 2010;20(2):126–132. doi: 10.1097/CMR.0b013e328335a916. [DOI] [PubMed] [Google Scholar]
- 30.Mallikarjuna K, Pushparaj V, Biswas J, et al. Expression of insulin-like growth factor receptor (IGF-1R), c-Fos, and c-Jun in uveal melanoma: an immunohistochemical study. Curr Eye Res. 2006;31(10):875–883. doi: 10.1080/02713680600878790. [DOI] [PubMed] [Google Scholar]
- 31.Economou MA, All-Ericsson C, Bykov V, et al. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: intercorrelation and prognostic implications. Invest Ophthalmol Vis Sci. 2005;46(12):4372–4375. doi: 10.1167/iovs.05-0322. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Niederkorn JY. Role of epidermal growth factor receptor in the metastasis of intraocular melanomas. Invest Ophthalmol Vis Sci. 1998;39(7):1067–1075. [PubMed] [Google Scholar]
- 33.Hurks HM, Metzelaar-Blok JA, Barthen ER, et al. Expression of epidermal growth factor receptor: risk factor in uveal melanoma. Invest Ophthalmol Vis Sci. 2000;41(8):2023–2027. [PubMed] [Google Scholar]
- 34.Abdel-Rahman MH, Boru G, Massengill J, Salem MM, Davidorf FH. MET oncogene inhibition as a potential target of therapy for uveal melanomas. Invest Ophthalmol Vis Sci. 2010;51(7):3333–3339. doi: 10.1167/iovs.09-4801. [DOI] [PubMed] [Google Scholar]
- 35.All-Ericsson C, Girnita L, Seregard S, et al. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43(1):1–8. [PubMed] [Google Scholar]


