Abstract
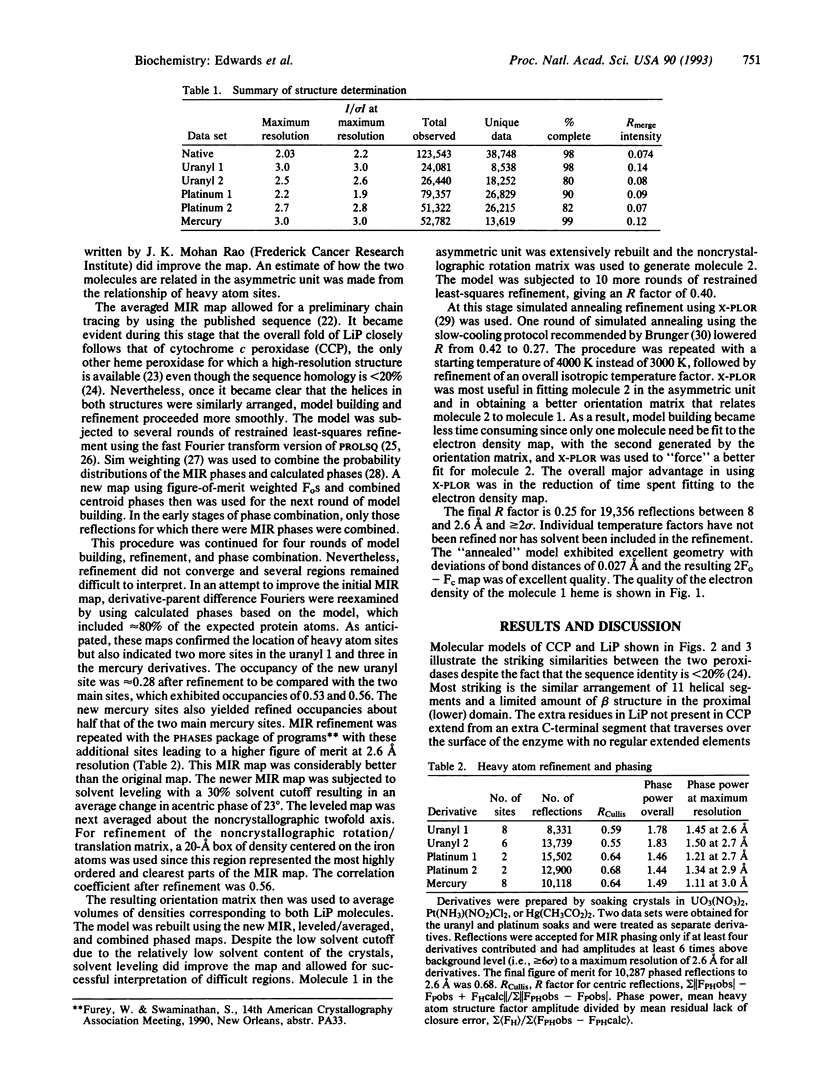
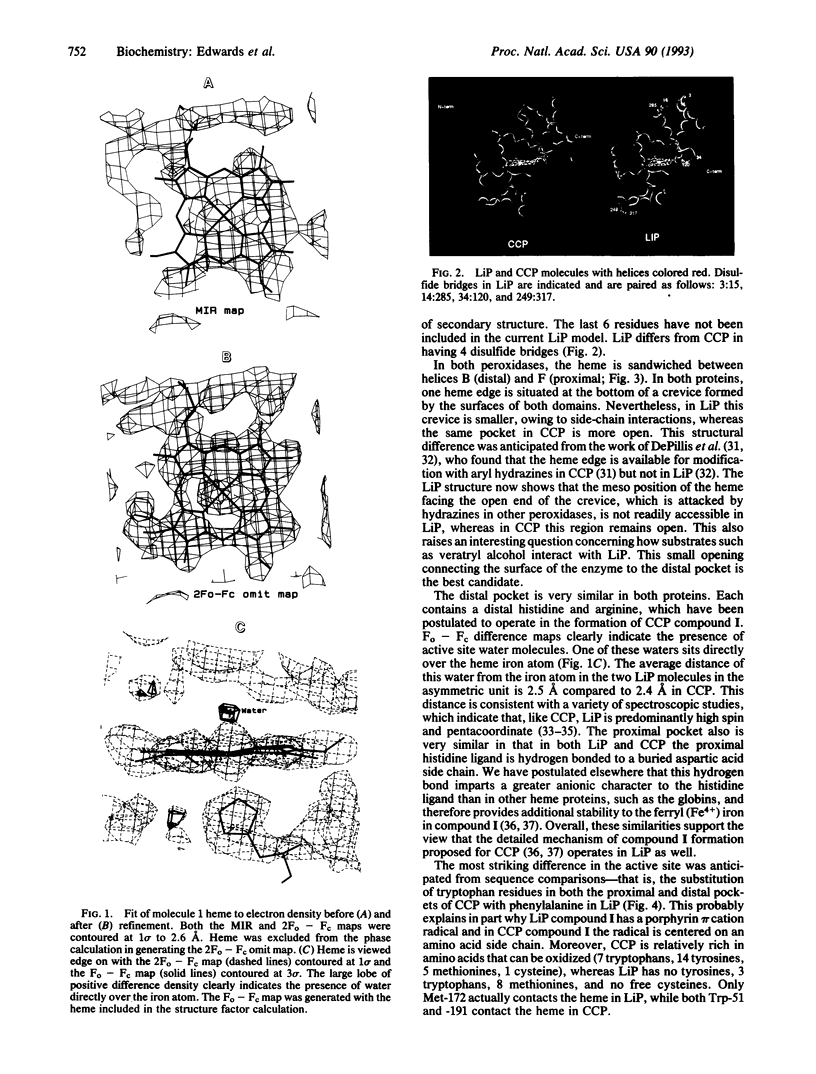
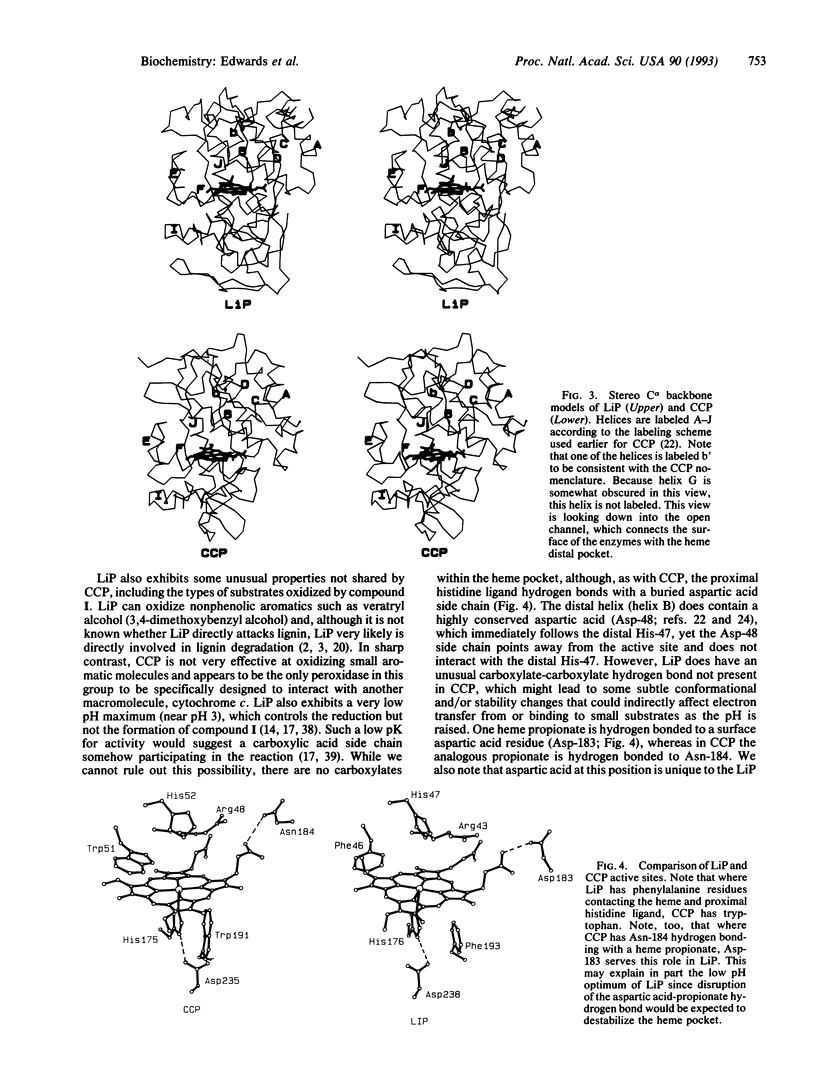
The crystal structure of lignin peroxidase (LiP) from the basidiomycete Phanerochaete chrysosporium has been determined to 2.6 A resolution by usine multiple isomorphous replacement methods and simulated annealing refinement. Of the 343 residues, residues 3-335 have been accounted for in the electron density map, including four disulfide bonds. The overall three-dimensional structure is very similar to the only other peroxidase in this group for which a high-resolution crystal structure is available, cytochrome c peroxidase, despite the fact that the sequence identity is only approximately 20%, LiP has four disulfide bonds, while cytochrome c peroxidase has none, and LiP is larger (343 vs. 294 residues). The basic helical fold and connectivity defined by 11 helical segments with the heme sandwiched between the distal and proximal helices found in cytochrome c peroxidase is maintained in LiP. Both enzymes have a histidine as a proximal heme ligand, which is hydrogen bonded to a buried aspartic acid side chain. The distal or peroxide binding pocket also is similar, including the distal arginine and histidine. The most striking difference is that, whereas cytochrome c peroxidase has tryptophans contacting the distal and proximal heme surfaces, LiP has phenylalanines. This in part explains why, in the reaction with peroxides, cytochrome c peroxidase forms an amino acid-centered free radical, whereas LiP forms a porphyrin pi cation radical.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. A., Renganathan V., Chiu A. A., Loehr T. M., Gold M. H. Spectral characterization of diarylpropane oxygenase, a novel peroxide-dependent, lignin-degrading heme enzyme. J Biol Chem. 1985 May 25;260(10):6080–6087. [PubMed] [Google Scholar]
- Andersson L. A., Renganathan V., Loehr T. M., Gold M. H. Lignin peroxidase: resonance Raman spectral evidence for compound II and for a temperature-dependent coordination-state equilibrium in the ferric enzyme. Biochemistry. 1987 Apr 21;26(8):2258–2263. doi: 10.1021/bi00382a028. [DOI] [PubMed] [Google Scholar]
- Andrawis A., Johnson K. A., Tien M. Studies on compound I formation of the lignin peroxidase from Phanerochaete chrysosporium. J Biol Chem. 1988 Jan 25;263(3):1195–1198. [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Krukowski A., Erickson J. W. Slow-cooling protocols for crystallographic refinement by simulated annealing. Acta Crystallogr A. 1990 Jul 1;46(Pt 7):585–593. doi: 10.1107/s0108767390002355. [DOI] [PubMed] [Google Scholar]
- Cai D. Y., Tien M. Lignin peroxidase of Phanerochaete chrysosporium. Evidence for an acidic ionization controlling activity. J Biol Chem. 1991 Aug 5;266(22):14464–14469. [PubMed] [Google Scholar]
- DePillis G. D., Sishta B. P., Mauk A. G., Ortiz de Montellano P. R. Small substrates and cytochrome c are oxidized at different sites of cytochrome c peroxidase. J Biol Chem. 1991 Oct 15;266(29):19334–19341. [PubMed] [Google Scholar]
- DePillis G. D., Wariishi H., Gold M. H., Ortiz de Montellano P. R. Inactivation of lignin peroxidase by phenylhydrazine and sodium azide. Arch Biochem Biophys. 1990 Jul;280(1):217–223. doi: 10.1016/0003-9861(90)90539-b. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Poulos T. L., Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J Biol Chem. 1984 Nov 10;259(21):13027–13036. [PubMed] [Google Scholar]
- Hayashi Y., Yamazaki I. The oxidation-reduction potentials of compound I/compound II and compound II/ferric couples of horseradish peroxidases A2 and C. J Biol Chem. 1979 Sep 25;254(18):9101–9106. [PubMed] [Google Scholar]
- Henrissat B., Saloheimo M., Lavaitte S., Knowles J. K. Structural homology among the peroxidase enzyme family revealed by hydrophobic cluster analysis. Proteins. 1990;8(3):251–257. doi: 10.1002/prot.340080307. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Marquez L., Wariishi H., Dunford H. B., Gold M. H. Spectroscopic and kinetic properties of the oxidized intermediates of lignin peroxidase from Phanerochaete chrysosporium. J Biol Chem. 1988 Aug 5;263(22):10549–10552. [PubMed] [Google Scholar]
- Poulos T. L. Heme enzyme crystal structures. Adv Inorg Biochem. 1988;7:1–36. [PubMed] [Google Scholar]
- Purcell W. L., Erman J. E. Cytochrome c peroxidase catalyzed oxidations of substitution inert iron(II) complexes. J Am Chem Soc. 1976 Oct 27;98(22):7033–7037. doi: 10.1021/ja00438a049. [DOI] [PubMed] [Google Scholar]
- Renganathan V., Miki K., Gold M. H. Multiple molecular forms of diarylpropane oxygenase, an H2O2-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Aug 15;241(1):304–314. doi: 10.1016/0003-9861(85)90387-x. [DOI] [PubMed] [Google Scholar]
- Ritch T. G., Jr, Nipper V. J., Akileswaran L., Smith A. J., Pribnow D. G., Gold M. H. Lignin peroxidase from the basidiomycete Phanerochaete chrysosporium is synthesized as a preproenzyme. Gene. 1991 Oct 30;107(1):119–126. doi: 10.1016/0378-1119(91)90304-t. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Valli K., Gold M. H. Degradation of 2,4-dichlorophenol by the lignin-degrading fungus Phanerochaete chrysosporium. J Bacteriol. 1991 Jan;173(1):345–352. doi: 10.1128/jb.173.1.345-352.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Degradation of 2,7-dichlorodibenzo-p-dioxin by the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1992 Apr;174(7):2131–2137. doi: 10.1128/jb.174.7.2131-2137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Oxidation of monomethoxylated aromatic compounds by lignin peroxidase: role of veratryl alcohol in lignin biodegradation. Biochemistry. 1990 Sep 18;29(37):8535–8539. doi: 10.1021/bi00489a005. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Gold M. H. Lignin peroxidase compound III. Mechanism of formation and decomposition. J Biol Chem. 1990 Feb 5;265(4):2070–2077. [PubMed] [Google Scholar]
- Wariishi H., Huang J., Dunford H. B., Gold M. H. Reactions of lignin peroxidase compounds I and II with veratryl alcohol. Transient-state kinetic characterization. J Biol Chem. 1991 Nov 5;266(31):20694–20699. [PubMed] [Google Scholar]