ABSTRACT
The aim was to examine the impact of pulmonary metastasectomy in patients with recurrent gynecologic cancers. Thirty-seven patients with isolated lung metastases (< 3 nodules) in recurrent epithelial gynecologic cancers were treated at Nagoya University Hospital between 1985 and 2013. The clinicopathological data for the 23 patients who underwent surgical resection were retrospectively analyzed, and their survival was compared with patients who received chemotherapy only. The median age at the time of surgery was 56 years (range 28–77). The studied population comprised 7 patients with 2 or 3 nodules and 8 patients with chemoresistant tumors, including fourteen cervical, 4 endometrial, and 5 ovarian primary tumors, with 5-year overall survivals (OSs) after surgery of 61, 100, and 100%, respectively. The survival of recurrence-free interval after initial treatment (>2 years) was significantly favorable (5-year OS 100% vs. 41.7%, p=0.006). Among the 6 patients with re-recurrence of lung metastases, 5 patients underwent a second pulmonary metastasectomy, and all of the patients are currently alive without disease. None of the 29 operations yielded severe complications. Although the survival rate showed a tendency to be higher in the surgery group than in the chemotherapy-only group, no significant difference was observed (5-year OS 81.7% vs. 49.5%, p=0.072). Our results indicate that pulmonary metastasectomy contributed to long-term survival with a low-risk of complications. Surgery to remove isolated lung metastases might provide a favorable prognosis for patients with long recurrence-free intervals and for patients with chemoresistant or re-recurrent tumors.
Key Words: pulmonary metastasectomy, lung metastasis, prognosis, uterine cancer, ovarian cancer
INTRODUCTION
Metastatic lung cancers, with the exception of sarcomas, have been reported to primarily originate in the colon, rectum, kidney, and breast, whereas the rate of metastasis of gynecologic cancers is relatively low.1, 2) In gynecologic cancers, the rate of lung metastasis is higher in patients with choriocarcinoma3) or sarcoma4, 5) than in patients with epithelial gynecologic cancers, such as cervical, endometrial or ovarian carcinoma.6-10) In addition, the frequency of cases with isolated lung metastasis is low in patients with recurrent epithelial tumors because most tumors in these patients metastasize to the pelvis, vagina, peritoneum, or lymph nodes. Therefore, the first line of treatment for these patients is often chemotherapy.11) As a surgical treatment for patients with recurrent endometrial carcinoma, it has been reported that reductive surgery of the central pelvis-vagina area, when it is the only site of recurrence other than the lungs, is significantly associated with survival.12) However, some authors have shown a favorable prognosis after surgery for lung metastases in uterine malignancies, including sarcomas and epithelial tumors.2, 13, 14) Therefore, the prognosis for patients with recurrent gynecologic epithelial cancers and isolated lung metastasis is unclear, especially regarding whether they would benefit from pulmonary resection.
The aim of our study was to analyze the long-term outcomes of pulmonary metastasectomy at a single institution with respect to clinicopathological factors in patients with recurrent gynecologic epithelial cancers.
METHODS
Between January 1985 and December 2013, 3,110 patients with epithelial gynecologic cancers were registered and treated at Nagoya University Hospital. We analyzed all patients with epithelial gynecologic cancers, including cervical, endometrial, and ovarian cancer, except germ cell tumors, choriocarcinomas, and sarcomas. Approval from the Ethics Committee of our hospital was obtained before the study was registered. A total of 166 (5.3%) of the 3,110 patients were diagnosed with lung metastasis. In this study, a lung nodule that measured more than 1 cm according to computed tomography (CT) images that were obtained over time was defined as a lung metastasis. Of the 166 patients, those who had multiple lung metastases (i.e., more than 3 nodules), those who had metastasis in multiple organs, and those who had synchronous primary disease were excluded. Thirty-seven patients with lung metastases (<3 nodules) during recurrence remained; of these patients, 23 (62.2%) underwent surgical treatment, 12 (32.4%) received chemotherapy only, and 2 received radiotherapy (5.4%). One patient who had already been treated for other metastases was included in each treatment group. The prognoses for the 23 patients were retrospectively evaluated according to their clinicopathological factors. Pathological staging was assessed according to the International Federation of Gynecology and Obstetrics (FIGO) criteria, and all of the tumors were histologically classified according to the World Health Organization classification.
All of the surgical patients received standard treatment for each primary cancer as an initial treatment, including 18 patients (78.2%) who underwent radical surgery with or without adjuvant therapy and 5 patients (21.7%) who received primary chemoradiation. With regard to chemotherapy, a platinum-based regimen was used for all patients as first-line chemotherapy. All of the patients achieved remission after the initial treatments.
Each patient underwent surgery with a conventional thoracotomy or a video-assisted thoracic surgery (VATS) approach for pulmonary metastasectomy. Conventional thoracotomy consisted of a wedge resection, lobectomy, or segmentectomy via open thoracotomy. After the diagnosis of lung metastases, whether the patients received preoperative chemotherapy was determined individually for each case. With regard to the chemotherapy regimen, patients with a recurrence-free interval longer than 6 months received platinum-based chemotherapy, whereas patients with a recurrence-free interval of less than 6 months received non-platinum chemotherapy. Complications and side effects were estimated with the Common Terminology Criteria for Adverse Events (CTCAE, version 4).
All of the responses to treatment were evaluated according to the guidelines of the Response Evolution Criteria in Solid Tumors (RECIST, version 1.1). To evaluate the re-recurrence rate after pulmonary metastasectomy, a CT scan was performed every six months for the first year and then once a year for five years. The patients who received chemotherapy only underwent a CT scan every 3–6 months during treatment, then every year for five years thereafter to confirm whether a complete response (CR) had been achieved.
Survival was measured from the date of diagnosis until the date of death or the date of the final follow-up visit, whichever occurred first. Disease free-survival (DFS) and overall survival (OS) curves were calculated using the Kaplan-Meier method, and the significance was determined using log-rank tests. Categorical data were analyzed using the chi-squared test or Fisher’s exact test for comparisons of each group; p-values less than 0.05 were considered statistically significant. All of the statistical analyses were performed using the Statistical Package for Social Science (SPSS) software package for Windows®, version 21.
RESULTS
Patient characteristics and survival
The characteristics and survival of the 23 patients who underwent pulmonary metastasectomy are summarized in Table 1. The interval between the initial treatment and recurrence as lung metastases ranged from 7 to 117 months (median 28.5 months). The median age at the time of surgery was 56 years old (range 28–77). Fifteen patients, including 11 with only one pulmonary nodule and 4 patients with 2 nodules, first underwent pulmonary metastasectomy after confirmation that no other recurrent tumors were present. Eight patients received first-line chemotherapy, 2 patients with advanced primary cancers, 2 patients with re-recurrent tumors, and 3 patients with 2 pulmonary nodules each. The mean number of chemotherapy cycles was 5.1 (range 3–8). The disease control rate was 62.5% (1 with a CR, 1 with a partial response (PR), and 3 with stable disease (SD)); of the remaining 37.5%, all 3 patients had progressive disease (PD). Therefore, pulmonary metastasectomy was selected for these patients as the next mode of treatment. The 1 patient with a CR underwent this surgery due to a re-recurrence at the same site within 1 year. A total of 5 patients (21.7%) underwent 3 cycles of post-surgery chemotherapy, whereas the remaining 18 patients received no treatments after surgery.
Table 1.
Patient characteristics and survival after pulmonary metastasectomy
| Variables | Number | 5-year OS | P | ||
|---|---|---|---|---|---|
| Age(years) | |||||
| <60 | 13 | 65.6% | 0.109 | ||
| >60 | 10 | 100.0% | |||
| Primary disease | |||||
| Cervical cancer | 14 | 61.0% | 0.145 | ||
| Endometrial cancer | 4 | 100.0% | |||
| Ovarian cancer | 5 | 100.0% | |||
| Histological type | |||||
| Squamous carcinoma | 9 | 75.0% | 0.178 | ||
| Mucinous adenocarcinoma | 5 | 50.0% |
|
||
| Endometrioid adenocarcinoma | 8 | 100.0% | |||
| Serous adenocarcinoma | 1 | 100.0% | |||
| FIGO Stage | |||||
| I | 13 | 80.0% | 0.640 | ||
| II | 5 | 100.0% | |||
| III, IV | 5 | 66.7% | |||
| Number of pulmonary metastases | |||||
| 1 | 16 | 77.1% | 0.366 | ||
| 2 or 3 | 7 | 100.0% | |||
| Recurrence-free interval | |||||
| <2 years | 8 | 41.7% | 0.006 | ||
| >2 years | 15 | 100.0% | |||
| Chemotherapy before pulmonary metastasectomy | |||||
| Yes | 9 | 68.6% | 0.271 | ||
| No | 14 | 90.9% | |||
| Pulmonary metastasectomy | |||||
| VATS | 13 | 90.0% | 0.446 | ||
| Conventional thoracotomy | 10 | 71.4% | |||
OS: Overall survival, Recurrence-free interval: the interval between initial treatment and recurrence in the lung, VATS: video-assisted thoracic surgery. Conventional thoracotomy included 5 lobe, 4 segment, and 1 wedge resection.
* Mucinous vs. endometrioid adenocarcinoma; p=0.044
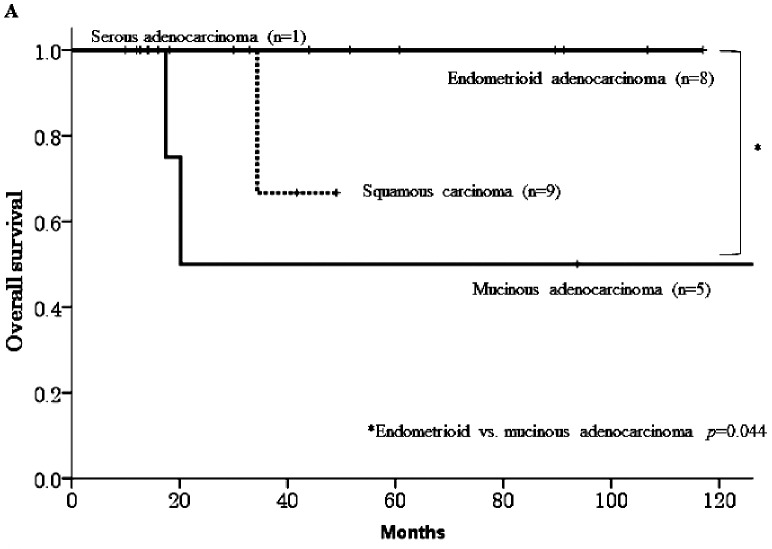
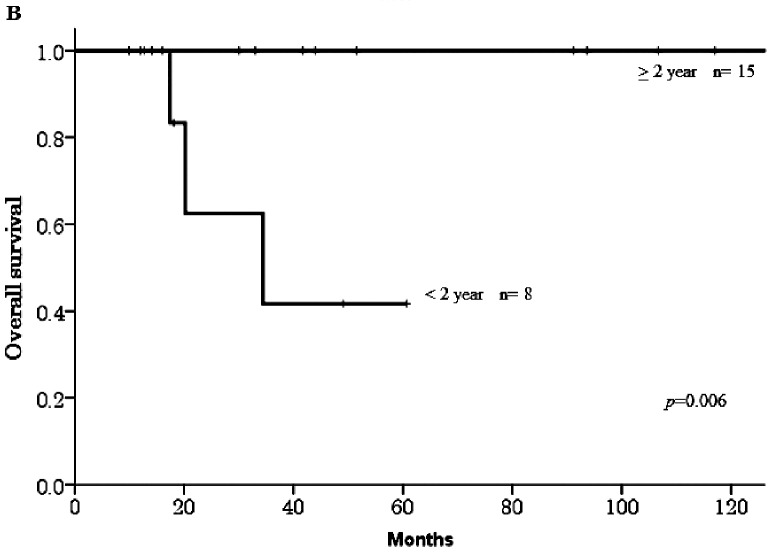
The median follow-up duration was 47 months (range 9.9–180) after pulmonary metastasectomy. In the 23 patients who underwent the surgery, 10 patients (43.4%) experienced re-recurrence, three of the 10 patients died from their disease, and the 5-year OS rate was 81.7% among all 23 patients. The primary disease in the 3 patients who died was cervical cancer, and all of these patients experienced recurrence within six months of surgery. Table 1 shows that no significant differences were observed in OS with respect to age, primary disease, stage, or surgical procedure. However, a significant difference was observed between the histological types mucinous adenocarcinoma and endometrioid adenocarcinoma (5-year OS 50% vs. 100%, respectively, p=0.044, Figure 1-A). The survival rate in patients who were diagnosed with a recurrence more than 2 years after their initial treatment was significantly higher than in patients who were diagnosed within 2 years (100% vs. 41.7%, respectively, p=0.006, Figure 1-B).
Fig. 1A.
Overall survival curves according to histological type.
Fig. 1B.
Overall survival curves according to the disease-free interval after initial treatment.
Re-recurrence after pulmonary metastasectomy
The medical data for the 10 cases of re-recurrence are summarized in Table 2. The interval between surgery and re-recurrence ranged from 2.2 to 41.6 (median 18.8) months, and the 5-year DFS rate was 44.7%. Of the 10 patients, 6 (60%) tumors appeared in the lung, 2 (20%) in a pelvic organ, 1 (10%) in distant lymph nodes, and 1 (10%) in the muscles. Five of 6 patients with lung metastasis underwent pulmonary metastasectomy again, and the remaining 1 patient with multiple bilateral lung metastases received chemotherapy. Of these 5 patients, 1 patient with 3 nodules on both sides underwent pulmonary surgery in two steps. All 5 of the patients were alive at the time of this writing, although 1 patient had experienced a third recurrence in the lung.
Table 2.
Re-recurrence after pulmonary metastasectomy
| Variables | Number | % | ||
|---|---|---|---|---|
| Re-recurrence | ||||
| Yes | 10 | 43.5% | ||
| No | 13 | 56.5% | ||
| Time to diagnosis of re-recurrence | ||||
| Median time (range) | 18.8 (2.2–41.6) months | |||
| Re-recurrence site | ||||
| Lung | 6 | 60.0% | ||
| Ipsilateral side | 3 | |||
| Contralateral side | 1 | |||
| Both sides | 2 | |||
| Others | 4 | 40.0% | ||
| Treatment for re-recurrence | ||||
| Pulmonary surgery with/without chemotherapy | 5 | 50.0% | ||
| Chemotherapy only | 5 | 50.0% | ||
Complications of pulmonary metastasectomy
Of the 23 patients who underwent a total of 29 surgeries, 24.1% of the patients were older than 70 years of age. Among them, no serious complications or extended hospitalizations after surgery were observed except in 1 patient who underwent 3 pulmonary metastasectomies. This particular patient, who was 79 years old, required temporary home oxygen therapy postoperatively, but her condition improved within 6 months.
Chemotherapy for lung metastasis
The survival rates were compared between the pulmonary metastasectomy group and the chemotherapy-only group. The chemotherapy-only group comprised patients with 3 or fewer lung metastases who were extracted from the same population. Table 3 shows the characteristics of the patients in the pulmonary metastasectomy versus the chemotherapy-only groups. No differences were observed between the 2 groups after performing an analysis of the categorical data using the chi-squared test. Of the 12 patients who received chemotherapy, only 6 (50%) had a CR, 1 (8.3%) had a PR, 3 (25%) had SD, and 2 (16.7%) had PD. One of the 6 patients with a CR experienced recurrence in 1 lung. Although the survival rate in the patients in the pulmonary metastasectomy group tended to be higher than in the patients in the chemotherapy-only group, no significant differences were observed (5-year OS 81.7% vs. 49.5%, respectively, p=0.072, Figure 2).
Table 3.
Characteristics of the patients in the pulmonary metastasectomy versus chemotherapy only groups
| Variables | Total | Number of patients | P | |||
|---|---|---|---|---|---|---|
| Surgery* (n=23) | Chemotherapy (n=12) | |||||
| Age (years) | ||||||
| Median age (range) in years | 56 (28–77) | 58.5 (44–76) | ||||
| <60 | 20 | 13 | 60.9% | 7 | 58% | 0.884 |
| >60 | 15 | 10 | 41.7% | 5 | 42% | |
| Primary disease | ||||||
| Cervical cancer | 21 | 14 | 60.9% | 7 | 58% | 0.607 |
| Endometrial cancer | 8 | 4 | 17.4% | 4 | 33% | |
| Ovarian cancer | 6 | 5 | 21.7% | 1 | 8% | |
| Histological type | ||||||
| Squamous carcinoma | 15 | 9 | 39.1% | 6 | 50% | 0.797 |
| Mucinous adenocarcinoma | 8 | 5 | 21.7% | 3 | 25% | |
| Endometrioid adenocarcinoma | 11 | 8 | 34.8% | 3 | 25% | |
| Serous adenocarcinoma | 1 | 1 | 4.3% | 0 | 0% | |
| Number of lung metastases | ||||||
| 1 | 22 | 16 | 69.6% | 6 | 50% | 0.256 |
| 2 or 3 | 13 | 7 | 30.4% | 6 | 50% | |
| Diagnosis time of pulmonary metastasis post initial treatment | ||||||
| <2 years | 13 | 7 | 30.4% | 6 | 50% | 0.256 |
| >2 years | 22 | 16 | 69.6% | 6 | 50% | |
Surgery*: pulmonary metastasectomy
Fig. 2.
Overall survival curves according to treatment for recurrent lung metastasis.
DISCUSSION
Few reports have been published regarding pulmonary metastasectomy for patients with recurrent gynecologic epithelial carcinoma. In an analysis of 5,206 patients who underwent the surgery, Pastorino et al.1) reported that the number of uterine cancer cases was 83 (3.6%) out of the 2,273 female patients, but the number of breast cancer cases was 396 (17.4%). In patients with cervical cancer, which has the highest rate of lung metastasis among cervical, endometrial and ovarian cancer,13, 14) the rate of isolated lung metastasis was founded to be 1.5 to 6%.11, 15, 16) According to our data, the rate of isolated lung metastasis among 35 epithelial carcinomas was 1.1% among the 3,110 patients with gynecologic cancers.
In general, pulmonary metastasectomy is accepted for patients with the following conditions: (1) the patient must have adequate pulmonary reserve and be at low risk for surgical intervention; (2) the primary malignancy must be either controlled or controllable; (3) there must be no evidence of extra-thoracic metastatic disease; and (4) a complete resection of the pulmonary metastases must be possible.1, 2, 17) In our study, all of the patients also underwent pulmonary resection according to these criteria after an evaluation for extra-thoracic metastatic disease. In most of these patients, a pathological diagnosis of lung tumor was made after a CT-guided biopsy or a biopsy with bronchoscopy prior to surgery, but the histologic diagnosis was made by VATS in some patients.
Various results have been reported for survival after pulmonary metastasectomy due to the different populations studied, including patients with epithelial tumors and patients with non-epithelial tumors. The 5-year OS rate was 10 to 46.8% in patients with mixed epithelial carcinomas and sarcomas.14, 16) In addition, this report also showed a significant difference between adenocarcinoma and sarcoma in 17 patients with uterine cancer (median survival; 46 months vs. 25 months, respectively, p=0.02). With respect to the survival of patients with cervical cancer, the 5-years OS rate ranged from 0 to 45.7%.13, 14, 16) An analysis of the histological types of this cancer showed that the 5-year OS rate of patients with squamous cell carcinoma (SCC) showed a tendency to be higher than that in patients with adenocarcinoma13, 18). Yamamoto et al.19). reported data from a multicenter study and found a significant difference in the DFS between the 20 patients with SCC and the 9 patients with adenosquamous cell carcinoma or adenocarcinoma in both a univariate analysis (5-year DFS: 47.4% vs. 0%, respectively, p=0.0141) and a multivariate analysis (hazard ratio: 3.775, 95% confidence interval 1.271–11.212, p=0.0168). In contrast, in Anraku’s report13) on a large number of patients enrolled from more than 20 institutions, no statistically significant difference was found between the 5-year OS rates of the 2 groups (58 SCCs vs. 13 adenocarcinomas: 46.8% vs. 40.3%, respectively, p=0.92). Our single-institution study indicated a favorable prognosis for patients with SCC in comparison with adenocarcinoma, but no significant difference was observed. Regarding endometrial cancer, the 5-year OS rate has been reported to be 27.8% to 75.5% in previous studies1, 2, 14, 16) and was 100% in our study. Furthermore, the endometrioid adenocarcinoma subtype of endometrial or ovarian cancer had a significantly better prognosis than the mucinous adenocarcinoma subtype of cervical cancer (p=0.044). Our data featured higher survival rates than other studies, and there are two possible reasons for this result: first, most patients had 1 or 2 lung nodules, and no patient had more than 4 nodules; second, most patients underwent a complete pulmonary metastasectomy. Many authors have demonstrated that complete resection is a significant prognostic factor.1, 2, 14, 16)
Prognostic factors are also important indicators for the selection of pulmonary resection or chemotherapy for patients. Some authors have reported that the disease-free interval (DFI) from the initial treatment does not significantly influence survival.18, 19) In contrast, it has also been reported that a longer DFI is a strong prognostic factor for the survival of patients after pulmonary resection (>12 vs. <12 months, 5-year OS 59.8 vs. 17.1%, p<0.001,13) >24 vs. <24 months: approximately 65 vs. 25%, p=0.00414)). Our data were similar to these results in that we also found that a favorable prognosis was associated with a longer recurrence-free interval.
In cervical cancer, the response rates (CR plus PR) for a single chemotherapy agent for patients with recurrent tumors have been reported to be 14 to 40% for cisplatin or ifosfamide,6) and 46% for paclitaxel-cisplatin regimens for patients with recurrence after radiotherapy.20) In endometrial cancer, the response rates have been reported to be 20 to 42% for cisplatin or doxorubicin21) and to be 60% for paclitaxel-carboplatin regimens in recurrent and advanced cancers.22) In our study, a total of 20 patients received chemotherapy for recurrent lung tumors, including patients who underwent surgery after chemotherapy because of tumor chemoresistance. The response rate for cervical cancer (33.3%) was lower than that for endometrial cancer (83.3%), similarly to previous reports. Although there was no significant difference between chemotherapy only and surgery, patients who underwent surgery were more likely to have a favorable long-term outcome (8-year OS rate 81.7% vs. 24.8%, p=0.072). There may have been some bias because the chemotherapy-only group included patients who were not able to undergo surgery to remove even just one nodule, such as patients who refused treatment or who had 5 cm tumors in the hilar region. However, our data suggested that even patients who did not achieve remission after chemotherapy were helped by surgery. Anderson’s study16) also indicated that pulmonary resection could provide a survival advantage even for patients with isolated lung metastases with chemoresistance.
Furthermore, we examined re-recurrence rates after pulmonary metastasectomy. Few reports have been published on this topic. Of the 23 total patients who underwent surgery, 6 patients (26%) had re-recurrent lung tumors, 5 of which (83%) underwent lung surgery again.
Most authors have reported that pulmonary metastasectomy is safe and effective and that it is associated with very low perioperative morbidity and mortality.1, 2, 13, 16) In our study, approximately 25% of the patients at the time of surgery were older than 70 years of age. No severe complications higher than grade 3 were observed, except in one patient. Many authors have suggested that pulmonary metastasectomy provides a survival advantage for selected patients with isolated lung metastases.16, 18, 23) With respect to the therapeutic management of recurrence, it is necessary to consider various conditions when choosing among surgery, chemotherapy, and radiation therapy.
In conclusion, our results indicated that pulmonary metastasectomy contributed to the long-term survival and was a low-risk treatment. Surgery for isolated lung metastasis might provide a favorable prognosis not only for patients with a long RFI but also for patients with chemoresistant tumors or re-recurrent tumors. Our study has several limitations due to the small number of patients and the retrospective nature of the study. It will be necessary to evaluate the benefits and complications of the surgery in a larger sample in the future.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- 1).Pastorino U BM, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, Johnston M, Putnam JB, . Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg, 1997; 113: 37–49. [DOI] [PubMed]
- 2).Monteiro A, Arce N, Bernardo J, Eugenio L, Antunes MJ. Surgical resection of lung metastases from epithelial tumors. Ann Thorac Surg, 2004; 77: 431–437. [DOI] [PubMed]
- 3).Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, Savage PM, Seckl MJ. EMA/CO for high-risk gestational trophoblastic neoplasia: good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol, 2013; 31: 280–286. [DOI] [PubMed]
- 4).Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas: emphasis on impact of lymphadenectomy and oophorectomy. Cancer, 2008; 112: 820–830. [DOI] [PubMed]
- 5).Mizuno M, Yatabe Y, Nawa A, Nakanishi T. Long-term medroxyprogesterone acetate therapy for low-grade endometrial stromal sarcoma. Int J Clin Oncol, 2012; 17: 348–354. [DOI] [PubMed]
- 6).Thigpen T. The role of chemotherapy in the management of carcinoma of the cervix. Cancer J, 2003; 9: 425–432. [DOI] [PubMed]
- 7).Reddoch JM, Burke TW, Morris M, Tornos C, Levenback C, Gershenson DM. Surveillance for recurrent endometrial carcinoma: development of a follow-up scheme. Gynecol Oncol, 1995; 59: 221–225. [DOI] [PubMed]
- 8).Mundt AJ, McBride R, Rotmensch J, Waggoner SE, Yamada SD, Connell PP. Significant pelvic recurrence in high-risk pathologic stage I--IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys, 2001; 50: 1145–1153. [DOI] [PubMed]
- 9).Mizuno M, Kajiyama H, Shibata K, Mizuno K, Yamamuro O, Kawai M, Nakanishi T, Nagasaka T, Kikkawa F. Adjuvant chemotherapy for stage i ovarian clear cell carcinoma: is it necessary for stage IA? Int J Gynecol Cancer, 2012; 22: 1143–1149. [DOI] [PubMed]
- 10).Kajiyama H, Mizuno M, Shibata K, Yamamoto E, Kawai M, Nagasaka T, Kikkawa F. Recurrence-predicting prognostic factors for patients with early-stage epithelial ovarian cancer undergoing fertility-sparing surgery: a multi-institutional study. Eur J Obstet Gynecol Reprod Biol, 2014; 175: 97–102. [DOI] [PubMed]
- 11).Imachi M, Tsukamoto N, Matsuyama T, Nakano H. Pulmonary metastasis from carcinoma of the uterine cervix. Gynecol Oncol, 1989; 33: 189–192. [DOI] [PubMed]
- 12).Campagnutta E, Giorda G, De Piero G, Sopracordevole F, Visentin MC, Martella L, Scarabelli C. Surgical treatment of recurrent endometrial carcinoma. Cancer, 2004; 100: 89–96. [DOI] [PubMed]
- 13).Anraku M, Yokoi K, Nakagawa K, Fujisawa T, Nakajima J, Akiyama H, Nishimura Y, Kobayashi K, Metastatic Lung Tumor Study Group of J. Pulmonary metastases from uterine malignancies: results of surgical resection in 133 patients. J Thorac Cardiovasc Surg, 2004; 127: 1107–1112. [DOI] [PubMed]
- 14).Clavero JM, Deschamps C, Cassivi SD, Allen MS, Nichols FC, 3rd, Barrette BA, Larson DR, Pairolero PC. Gynecologic cancers: factors affecting survival after pulmonary metastasectomy. Ann Thorac Surg, 2006; 81: 2004–2007. [DOI] [PubMed]
- 15).Barter JF, Soong SJ, Hatch KD, Orr JW, Shingleton HM. Diagnosis and treatment of pulmonary metastases from cervical carcinoma. Gynecol Oncol, 1990; 38: 347–351. [DOI] [PubMed]
- 16).Anderson TM, McMahon JJ, Nwogu CE, Pombo MW, Urschel JD, Driscoll DL, Lele SB. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol, 2001; 83: 472–476. [DOI] [PubMed]
- 17).Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumors. Surg Today, 2009; 39: 91–97. [DOI] [PubMed]
- 18).Seki M, Nakagawa K, Tsuchiya S, Matsubara T, Kinoshita I, Weng SY, Tsuchiya E. Surgical treatment of pulmonary metastases from uterine cervical cancer. Operation method by lung tumor size. J Thorac Cardiovasc Surg, 1992; 104: 876–881. [PubMed]
- 19).Yamamoto K, Yoshikawa H, Shiromizu K, Saito T, Kuzuya K, Tsunematsu R, Kamura T. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg, 2004; 77: 1179–1182. [DOI] [PubMed]
- 20).Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol, 1999; 17: 2676–2680. [DOI] [PubMed]
- 21).Fleming GF. Systemic chemotherapy for uterine carcinoma: metastatic and adjuvant. J Clin Oncol, 2007; 25: 2983–2990. [DOI] [PubMed]
- 22).Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, Jobo T, Hatae M, Hiura M, Yaegashi N. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Ann Oncol, 2011; 22: 636–642. [DOI] [PubMed]
- 23).Okubo K, Bando T, Miyahara R, Sakai H, Shoji T, Sonobe M, Fujinaga T, Sato K, Wada H, Tanaka T. Resection of pulmonary metastasis of non-small cell lung cancer. J Thorac Oncol, 2009; 4: 203–207. [DOI] [PubMed]