ABSTRACT
We aimed to assess the influence of background parenchymal enhancement (BPE) on surgical planning performed using preoperative MRI for breast cancer evaluation. Between January 2009 and December 2010, 91 newly diagnosed breast cancer patients (mean age, 55.5 years; range, 30−88 years) who underwent preoperative bilateral breast MRI followed by planned breast conservation therapy were retrospectively enrolled. MRI was performed to assess the tumor extent in addition to mammography and breast ultrasonography. BPE in the contralateral normal breast MRI at the early dynamic phase was visually classified as follows: minimal (n=49), mild (n=27), moderate (n=7), and marked (n=8). The correlations between the BPE grade and age, menopausal status, index tumor size, changes in surgical management based on MRI results, positive predictive value (PPV) of MRI, and surgical margins were assessed. Patients in the strong BPE groups were significantly younger (p=0.002) and generally premenopausal (p<0.001). Surgical treatment was not changed in 67 cases (73.6%), while extended excision and mastectomy were performed in 12 cases (13.2%), each based on additional lesions on MRI. Six of 79 (7.6%) patients who underwent breast conservation therapy had tumor-positive resection margins. In cases where surgical management was changed, the PPV for MRI-detected foci was high in the minimal (91.7%) and mild groups (66.7%), and 0% in the moderate and marked groups (p=0.002). Strong BPE causes false-positive MRI findings and may lead to overly extensive surgery, whereas MRI may be beneficial in select patients with weak BPE.
Key Words: breast cancer, background parenchymal enhancement, preoperative MRI
INTRODUCTION
Magnetic resonance imaging (MRI) is used to locally stage breast cancer because it is the most sensitive breast imaging modality, especially for detecting multifocal and multicentric breast cancer.1) Preoperative MRI is thought to improve surgical planning and reduce the need for repeat surgeries. Complete excision of breast carcinoma lowers the risk of local recurrence; however, there is no consensus on the role of breast MRI in the local staging of breast cancer. Emerging data show that local staging of the breast using preoperative MRI may not reduce reoperation rates,2, 3) and false-positive findings may lead to more women undergoing mastectomy unnecessarily without any improvement in surgical outcomes or prognosis.3)
Detecting malignancy by MRI relies on the differential enhancement between neoplastic tissue and normal breast parenchyma. Background parenchymal enhancement (BPE) on breast MRI refers to the normal enhancement of the fibroglandular tissue and is categorized by the Breast Imaging Reporting and Data System (BIRADS) as minimal, mild, moderate, and marked.4) Moderate and marked BPE can possibly be misinterpreted as suspicious or may mask actual malignant lesions.5, 6) Although it is important to understand limitations of the ability of MRI on surgical planning, to date, it is unclear if and how the degree of BPE influences MRI interpretation. Nevertheless, most studies of preoperative breast MRI do not consider the degree of BPE.
The purpose of this study was to assess whether the degree of BPE affected surgical planning for primary breast cancer and to determine whether patients were more likely to undergo appropriate and complete surgical excision based on preoperative MRI findings. The rate of change in surgical management based on the MRI results was evaluated. We also examined the histological incidence of additional lesions that were detected by pre-operative breast MRI but missed on conventional diagnostic methods.
MATERIALS AND METHODS
Study Patients
The institutional review board approved the retrospective data collection and analysis for this study, and waived the requirement for informed patient consent.
The institutional breast image database was searched to recruit patients who underwent mammography (MMG), ultrasonography (US), and MRI of both breasts from January 2009 to December 2010 at our hospital. During this period, 248 consecutive female patients underwent bilateral breast MRI to assess the extent of a known primary tumor. Women who were not treated at our hospital (n = 45), received chemotherapy or hormonal therapy (n = 38), had bilateral breast cancer (n = 12), had poor quality images due to artifacts (n = 2), or underwent MMG/US and MRI more than 2 months apart (n = 2) were excluded. We also excluded 58 women who underwent an initial mastectomy due to MMG/US findings or patient discretion. The remaining 91 female patients (mean age, 55.5 years; range, 30–88 years) planning breast conservation therapy due to MMG/US findings were enrolled in this study.
Mammography
Mammography was performed using a full-field digital mammography unit equipped with a 24 × 29-cm amorphous selenium detector at 70-μm pixels (Lorad Selenia, Hologic, Danbury, CT, USA). The medio-lateral oblique and cranio-caudal views were acquired for each breast. All mammograms were evaluated on two monochrome 5-megapixel liquid crystal displays (MFGD5621HD, 2,048 × 2,560 pixels, 21.3 inch; BARCO, Torhout, Belgium) using image viewer software (Mammoread; TOYO Corporation, Tokyo, Japan).
Ultrasonography
Whole breast US examinations were performed independently by four breast radiologists each with more than 10 years of breast US experience. A digital electronic ultrasound unit (EUB-8500; Hitachi Medical, Tokyo, Japan) equipped with a 6–14 MHz linear array probe (EUP-L65, Hitachi Medical) was used in all patients.
MRI
Patients were examined in the prone position using a 3-T MRI unit (MAGNETOM Trio; Siemens Medical Solutions, Erlangen, Germany) with a dedicated 4-channel phased-array bilateral breast coil. Before administering the contrast media, axial bilateral fat-suppressed T2-weighted fast spin-echo images were acquired. Dynamic axial bilateral fat-suppressed, high-resolution T1-weighted three-dimensional fast gradient echo breast images (VIEWS) were sequentially acquired before and 75, 185 and 295 s after administering the contrast medium. For dynamic studies, gadopentetate dimeglumine (Magnevist; Bayer Schering, Osaka, Japan) was administered intravenously using a power injector at 0.1 mmol/kg and a flow rate of 2 ml/s, followed by a 20-mL saline bolus. The imaging parameters for VIEWS sequences were as follows: TR/TE 4.2/1.8 ms, flip angle 15°, field of view 340 × 340 mm, matrix 512 × 410, thickness 0.9 mm, acquisitions 1, and acquisition time 110 s. SPAIR for fat suppression and a parallel imaging technique using a GRAPPA acceleration factor of two were also applied. Using these parameters, the spatial voxel size was 0.7 × 0.8 × 0.9 mm. After image acquisition, the unenhanced images were subtracted from the contrast-enhanced images on a pixel-by-pixel basis.
Surgical planning
Surgical therapy was initially determined based on the MMG/ US findings. Surgery was subsequently altered to an extended excision or mastectomy based on additional suspicious MRI findings. If no additional foci were found on MRI, then the surgical management was not changed. An extended excision was performed if the lesion exhibited ductal or multifocal spread in the same quadrant as the index cancer and was at least 10 mm larger on MRI than on MMG/US. Mastectomy was performed instead of breast conservation if additional suspicious foci were found in a quadrant different from that of the index cancer, such as wide ductal spread in multiple quadrants or multicentric spread. Typically, suspicious MRI lesions that contradicted the MMG/US findings were evaluated further through additional studies such as a second-look US or an image-guided needle biopsy prior to changing the initial therapy. However, additional studies were not performed in some patients that had additional MRI findings highly suggestive of malignancy, such as a wide distribution of breast cancer, or in patients with no indication for breast-conserving surgery.
All MMG/US and MRI examinations were prospectively interpreted by experienced radiologists. The additional MRI findings were evaluated by both the radiologists and surgeons, and the final surgical plan was made considering the potential cosmetic outcome.
Background parenchymal enhancement grade
BPE in MRI was reviewed retrospectively in consensus by two radiologists (S.I. and H.S., with 10 years and 16 years of breast imaging experience, respectively), who were blinded to the patient age, menopausal status, initial MRI report, history of MMG/US, and histopathologic findings. The BPE of the entire breast parenchyma was visually assessed in the contralateral normal breast using a combination of post-contrast fat-suppressed T1-weighted and first phase subtraction images (75 s after contrast administration). The BPE was graded into four categories: minimal enhancement (≤25% enhancement of the glandular tissue); mild enhancement (26–50% enhancement); moderate enhancement (51–75%); and marked enhancement (>75% enhancement; Figs. 1-4).
Fig. 1.
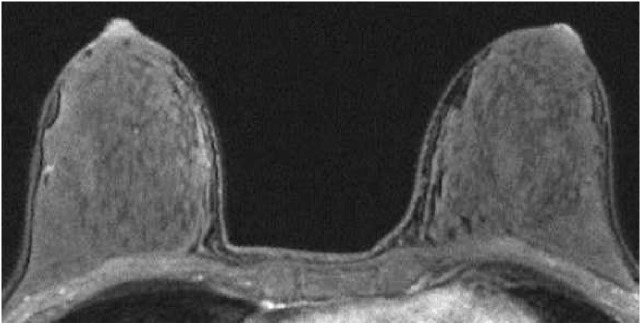
A 43-year-old premenopausal woman with invasive ductal carcinoma of the left breast who underwent preoperative breast MRI. The tumor is not shown on the image. The post-contrast, fat-suppressed T1-weighted image of the first phase of dynamic study shows minimal background enhancement.
Fig. 4.
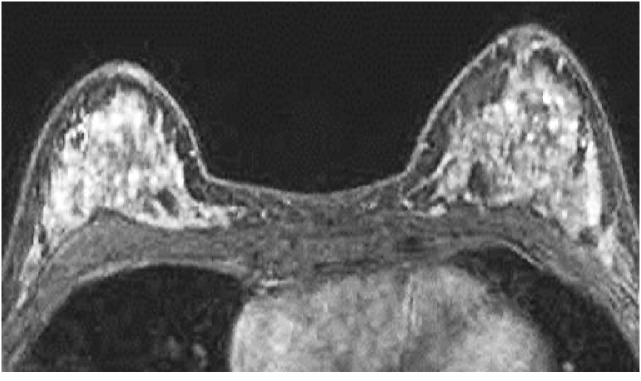
A 40-year-old premenopausal woman with invasive ductal carcinoma of the left breast who underwent preoperative breast MRI. The tumor is not shown on the image. The post-contrast, fat-suppressed T1-weighted image of the first phase of dynamic study shows marked background enhancement.
Fig. 2.
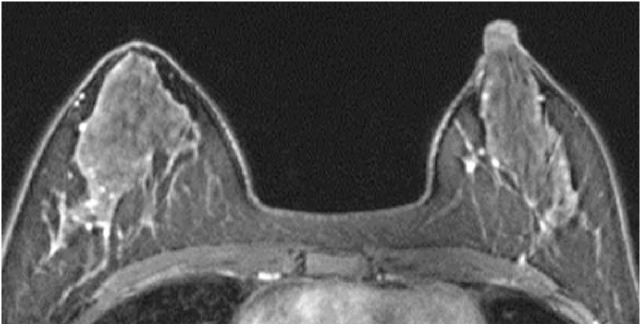
A 35-year-old premenopausal woman with invasive ductal carcinoma of the left breast who underwent preoperative breast MRI. The tumor is not shown on the image. The post-contrast, fat-suppressed T1-weighted image of the first phase of dynamic study shows mild background enhancement.
Fig. 3.
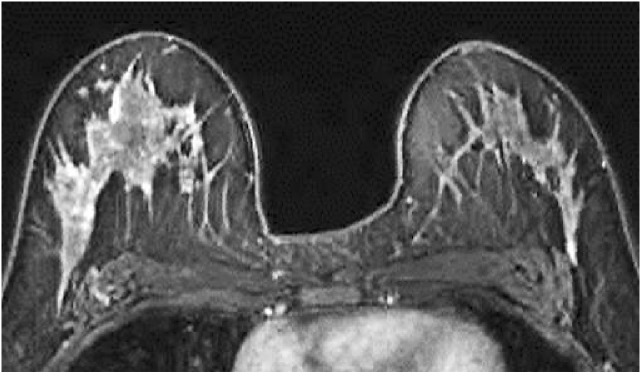
A 41-year-old premenopausal woman with invasive ductal carcinoma of the left breast who underwent preoperative breast MRI. The tumor is not shown on the image. The post-contrast, fat-suppressed T1-weighted image of the first phase of dynamic study shows moderate background enhancement.
Histopathological evaluation
The histopathologic specimens were selected for lesion evaluation. Samples for histopathological examination were prepared from five serial slices of the specimens measuring 5–10 mm each.
Medical record review
The patient records were reviewed using the hospital database system, and the following information was recorded: patient age; menopausal status; surgical management; histological diagnosis; maximum tumor diameter; and T classification. In cases of breast conservation therapy, information concerning the surgical margin status was also collected. In cases where surgery was changed to an extended excision or mastectomy, the additional suspicious MRI findings were categorized as follows: (1) true positive if the malignancy was confirmed on histology (in situ or invasive carcinoma); or (2) false positive if additional malignant lesions were not pathologically proven.
Statistical analysis
The correlation between the BPE and age or index tumor size was determined using the Shapiro-Wilk and Kruskal Wallis tests. The relationship between the BPE and the menopausal status, change in surgical approach, positive predictive value (PPV) of additional MRI findings when surgical management was changed, and the surgical margin status in cases of breast conservation therapy was evaluated using the Chi-square test. All analyses were performed using SPSS statistics version 20, and a p-value < 0.05 was considered statistically significant.
RESULTS
Of 91 cases, 49 (53.8%) showed minimal BPE, 27 (29.7%) showed mild BPE, 7 (7.7%) showed moderate BPE, and 8 (8.8%) showed marked BPE.
The mean patient age was 55.5 years overall, 58.0 years in the minimal group, 56.1 years in the mild group, 46.6 years in the moderate group, and 46.5 years in the marked group. A total 41.8% of patients (38/91) were premenopausal overall; 28.6% (14/49) were premenopausal in the minimal group, 37.0% (10/27) in the mild group, 85.7% (6/7) in the moderate group, and 100% (8/8) in the marked group. The high-grade BPE groups comprised significantly younger patients (p = 0.002) and had more premenopausal patients (p < 0.001) than the low-grade BPE groups.
Table 1 shows the T classification and maximum tumor dimensions. There were no significant variations in the tumor size between the groups (p = 0.410). On pathological examination, 16 ductal carcinoma in situ (DCIS) tumors (17.6%), 69 invasive ductal carcinomas (IDCs) (75.8%), 3 lobular carcinomas (3.3%), and 3 mucinous carcinomas (3.3%) were diagnosed.
Table 1.
T Classification & the maximum dimension
| BPE | |||||
|---|---|---|---|---|---|
| Minimal | Mild | Moderate | Marked | total | |
| n=49 | n=27 | n=7 | n=8 | n=91 | |
| Tis | 9 | 5 | 1 | 1 | 16 |
| T1mic | 1 | 2 | 0 | 0 | 3 |
| T1a | 4 | 1 | 0 | 1 | 6 |
| T1b | 10 | 7 | 0 | 1 | 18 |
| T1c | 17 | 10 | 3 | 4 | 34 |
| T2 | 8 | 2 | 3 | 1 | 14 |
| T3 | 0 | 0 | 0 | 0 | 0 |
| Mean dimension | 14.7mm | 12.8mm | 18.3mm | 12.9mm | 14.2mm |
Table 2 shows the correlation between the BPE grade and the changes in surgical management based on the MRI findings. Surgical treatment was changed in 24 of 91 patients (26.4%) after discovery of suspicious lesions on breast MRI. In 12 patients (13.2%), the excision was wider than initially planned, and in the other 12 patients (13.2%), the surgery was changed from breast conservation therapy to mastectomy. Although the mastectomy rate was greater in the high-grade BPE groups, there was no significant correlation between the BPE and the rates of conversion from breast conservation therapy to more aggressive procedures due to the MRI results (p = 0.202).
Table 2.
Correlation of BPE and changes in operative procedures as a consequence of additional MRI findings
| BPE | |||||
|---|---|---|---|---|---|
| Change in operative procedure | Minimal | Mild | Moderate | Marked | total |
| n=49 | n=27 | n=7 | n=8 | n=91 | |
| No change | 37 | 21 | 5 | 4 | 67 |
| (75.5%) | (77.8%) | (71.4%) | (50.0%) | (73.6%) | |
| Extended excision | 7 | 3 | 1 | 1 | 12 |
| (14.3%) | (11.1%) | (14.3%) | (12.5%) | (13.2%) | |
| Mastectomy | 5 | 3 | 1 | 3 | 12 |
| (10.2%) | (11.1%) | (14.3%) | (37.5%) | (13.2%) | |
Table 3 shows the surgical margin status in patients who underwent breast conservation therapy. Six of 79 patients (7.6%) had a positive surgical margin histologically, and none had their surgery altered due to the MRI results; two patients were in the minimal group (two cases of DCIS), three were in the mild group (one DCIS, one IDC, and one lobular carcinoma), and one patient was in marked group (DCIS). One patient in the mild group, who was diagnosed with IDC, underwent reoperation. One patient in the minimal group with DCIS received hormonal therapy postoperatively, and the remaining four patients received additional (so-called boost) radiation. No significant correlation was observed between the BPE grade and the surgical margin status (p = 0.324).
Table 3.
Correlation of BPE and surgical margin status
| BPE | ||||||
|---|---|---|---|---|---|---|
| Change in operative procedure | Surgical margin status | Minimal | Mild | Moderate | Marked | total |
| n=44 | n=24 | n=6 | n=5 | n=79 | ||
| No change | positive / total | 2/37 | 3a/21 | 0/5 | 1/4 | 6/67 |
| Extended excision | positive / total | 0/7 | 0/3 | 0/1 | 0/1 | 0/12 |
a One patient in the mild group, who was diagnosed with IDC, underwent reoperation.
Table 4 shows the histological accuracy of additional suspicious MRI lesions in cases where the surgical management was changed. In total, the PPV for the additional tumor foci was 91.7% in the minimal group and 66.7% in the mild group. In the moderate and marked groups, all of the MRI-detected foci were false positives, and the PPV was 0% in both groups. A higher BPE grade was significantly correlated with a decreased PPV for additional MRI findings in mastectomy cases (p = 0.026) and overall (p = 0.002). There was no significant correlation between the BPE grade and cases of extended excision (p = 0.162). In the cases of false positive MRI findings, histopathology revealed sclerosing adenosis and atypical hyperplasia without carcinoma.
Table 4.
Correlation of BPE and histological accuracy of additional MRI findings
| BPE | ||||||
|---|---|---|---|---|---|---|
| Change in operative procedure | Additional MRI findings | Minimal | Mild | Moderate | Marked | total |
| n=12 | n=6 | n=2 | n=4 | n=24 | ||
| Extended excision | true positive | 6 | 2 | 0 | 0 | 8 |
| false positive | 1 | 1 | 1 | 1 | 4 | |
| PPV | 85.7% | 66.7% | 0% | 0% | 66.7% | |
| Mastectomy | true positive | 5 | 2 | 0 | 0 | 7 |
| false positive | 0 | 1 | 1 | 3 | 5 | |
| PPVa | 100% | 66.7% | 0% | 0% | 58.3% | |
| Total | true positive | 11 | 4 | 0 | 0 | 15 |
| false positive | 1 | 2 | 2 | 4 | 9 | |
| PPVb | 91.7% | 66.7% | 0% | 0% | 62.5% | |
ap = 0.026
bp = 0.002.
DISCUSSION
We demonstrated that the degree of BPE affected the clinical assessment of preoperative MRI. For patients in the moderate and marked groups, the PPV for otherwise occult findings diagnosed by MRI was 0%, and the overestimation of the tumor extent based on the MRI results resulted in overly radical surgery. In contrast, a significant number of women in the minimal and mild groups had true positive MRI findings, and generally underwent the appropriate surgical procedure.
When choosing the surgical procedure, identification of multifocal and multicentric disease within the breast is important. Several studies have reported that MRI is more sensitive for detecting multiple malignant foci than are MMG and US. Liberman et al. reported that 20% of multifocal cancers and 7.1% of multicentric cancers were evident on MRI alone.7) However, while MRI is potentially beneficial, it is also associated with false-positive findings and can lead to overly extensive surgery.8, 9) One plausible reason for false-positive findings is the degree of BPE.
Typically, BPE is bilateral, symmetric, and diffuse in distribution. The degree of overall enhancement is usually minimal or mild, with slow early and persistently-delayed kinetic curves. There is little diagnostic difficulty in classifying these patterns as normal background enhancement. Similar areas of enhancement bilaterally, regardless of distribution, are more characteristic of benign enhancement, such as fibrocystic changes or hormonally mediated background enhancement, than of malignancy.5,6) However, an atypical pattern of BPE, such as moderate and marked enhancement, asymmetric or nondiffuse distribution, and rapid-early with plateau or washout-delayed kinetic features, is difficult to diagnose.10)
Uematsu et al. reported that the accuracy for evaluating the tumor extent in patients with moderate/marked BPE (52%) was significantly lower than in patients with minimal/mild BPE (84%; p = 0.002).11) In contrast, most studies of surgical impact due to preoperative breast MRI do not consider the degree of BPE. Houssami et al. performed the most widely known systemic review and meta-analysis on the impact of MRI on surgical treatment and found that 11.3% of patients were switched to a more extensive surgery due to true positive detection. However, 5.5% of patients underwent more extensive surgery due to false positive detection.12) In the present study, the rate of conversion to more extensive surgery due to true positive detection on MRI was 0% (0/15) in the moderate/marked BPE groups, whereas conversion due to false-positive detection was 40.0% (6/15). Conversely, in the minimal/mild BPE groups, the rate of conversion to more extensive surgery was 19.7% (15/76) due to true-positive detection by MRI and 3.9% (3/76) due to false-positive detection by MRI. Our result is consistent with previous reports in finding that BPE on breast MRI affects the accuracy of breast cancer extent assessment.11) Our findings suggest that strong BPE (i.e., the moderate/marked groups) may cause false positives results for tumor detection and thus, unnecessary and more radical breast surgery. Additionally, we suspect that strong BPE may mask actual breast cancer. Conversely, in patients with weak BPE, such as those in the minimal and mild groups, therapy changes based on MRI findings generated favorable outcomes. It is likely that strong BPE would negatively affect and impact surgical planning.
However, preoperative breast MRI has limitation. Emerging data indicate that MRI is not associated with a significant reduction in positive margins and re-excision rates after local excision.2, 3) In our study, six out of 79 patients had a positive margin, and their surgical treatment was not changed due to MRI findings. One out of the six patients underwent reoperation. These results were unrelated to degree of BPE. Therefore, false negative detection may lead to underestimation of tumor extent and cause a positive surgical margin and reoperation.
There are a few limitations in the present study. First, this was a single-site, retrospective study, which potentially limits the generalizability of the results for prospective assessment of BPE in a diverse group of radiologists and patients. Another limitation was the small size of the moderate and marked BPE groups. Therefore, these findings should only be considered preliminary.
In summary, the degree of BPE affects the clinical assessment of preoperative MRI. Strong BPE causes false-positive breast MRI findings and may lead to overly extensive surgery, whereas MRI may be beneficial in select patients with weak BPE. When using preoperative MRI to help select the surgical treatment, the degree of BPE should be considered.
ACKNOWLEDGEMENTS
The authors would like to thank the radiological technologists of the MRI and MMG/US unit at our institute. In addition, the author also would like to express appreciation for breast surgeons for their great support.
Conflict of Interest
None
REFERENCES
- 1).Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology, 2007; 244: 672–691. [DOI] [PubMed]
- 2).Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, Hanby A, Brown J. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet, 2010; 375: 563–571. [DOI] [PubMed]
- 3).Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin, 2009; 59: 290–302. [DOI] [PubMed]
- 4).Molleran V, Mahoney MC. The BI-RADS breast magnetic resonance imaging lexicon. Magn Reson Imaging Clin N Am, 2010; 18: 171–185. [DOI] [PubMed]
- 5).Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, Schild HH. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology, 1997; 203: 137–144. [DOI] [PubMed]
- 6).Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology, 2007; 244: 356–378. [DOI] [PubMed]
- 7).Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol, 2003; 180: 901–910. [DOI] [PubMed]
- 8).Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast, 2007; 16: 34–44. [DOI] [PubMed]
- 9).Lim HI, Choi JH, Yang JH, Han BK, Lee JE, Lee SK, Kim WW, Kim S, Kim JS, Kim JH, Choe JH, Cho EY, Kang SS, Shin JH, Ko EY, Kim SW, Nam SJ. Does pre-operative breast magnetic resonance imaging in addition to mammography and breast ultrasonography change the operative management of breast carcinoma? Breast Cancer Res Treat, 2010; 119: 163–167. [DOI] [PubMed]
- 10).Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol, 2011; 196: 218–224. [DOI] [PubMed]
- 11).Uematsu T, Kasami M, Watanabe J. Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol, 2011; 21: 2261–2267. [DOI] [PubMed]
- 12).Houssami N, Ciatto S, Macaskill P, Lord SJ, Warren RM, Dixon JM, Irwig L. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol, 2008; 26: 3248–3258. [DOI] [PubMed]


