ABSTRACT
Intraspinal synovial cysts are infrequent causes of back and radicular leg pain. Commonly associated with degenerative spinal disease, the majority of synovial cysts appear in the lumbar spine. Rarely, intracystic hemorrhage can occur through an unclear mechanism. Similarly rare, cysts may also become migratory. The pathogenesis of hemorrhagic synovial cysts remains uncertain and their potential for migration also remains unclear. A 36 year-old male presented to the clinic with 5 months of back pain and leg pain that began after a work-related injury. An initial MRI obtained by another surgeon 3 month prior demonstrated an epidural cystic mass with T1 hypointensity and T2 hyperintensity at L2-L3. With worsening pain, the patient came to our clinic for a second opinion. A second MRI demonstrated resolution of the L2-L3 epidural cystic mass and formation of a new epidural cystic mass at L3-L4 causing compression of the thecal sac. The patient subsequently underwent decompressive hemilaminectomy with cyst removal. We present a case of two lumbar synovial cysts, separated over time and a vertebral level and giving the appearance of a single, migratory cyst. This is the first case of an "occult migratory" synovial cyst with repeat MR imaging capturing spontaneous resolution of the initial cyst and formation of a hemorrhagic cyst one level below. We also present a summary of the 44 cases of hemorrhagic synovial cysts reported in the literature and propose a mechanism that may account for the hemorrhagic and migratory progression in some patients.
Key Words: lumbar synovial cyst, hemorrhagic, juxtafacet cyst
BACKGROUND AND IMPORTANCE
Spinal synovial cysts, also known as juxtafacet cysts, are an infrequent cause of radicular leg and back pain. The majority occur in the lumbar spine, especially the L4-L5 level, and are associated with facet joint osteoarthritis and spondylolisthesis1, 2). Rarely, synovial cysts can become hemorrhagic through an uncertain mechanism, though factors such as trauma and anticoagulation may play a role3, 4). Lumbar synovial cysts also may become migratory through an uncertain mechanism5, 6). We report on a patient who initially presented with a hemorrhagic lumbar cystic mass originating at L2-3 level which was read as lumbar epidural mass vs. hemorrhage; however, on repeated imaging obtained three months later, it had migrated to L3-4 level with further signs of acute hemorrhage. Due to the apparently migrated lesion, epidural hematoma was the tentative diagnosis but the final surgical pathology demonstrated L3-4 lesion to be consistent with synovial cyst with hemorrhage. Migration of a hemorrhagic lumbar synovial cyst demonstrated by repeated MR imaging has not been reported previously. We discuss the presenting symptoms, surgical management, and putative migratory etiology of hemorrhagic synovial cysts. We also conducted comprehensive literature review which found 44 published cases of lumbar hemorrhagic synovial cysts, and summarized the management strategy of this rare pathology.
CLINICAL PRESENTATION
A 36 year-old male with no past medical history presented with low back pain and bilateral leg pain and numbness that began five months earlier after a work related injury. His initial MRI lumbar spine demonstrated a cystic hemorrhagic epidural mass with T1 hypointensity and T2 hyperintensity on the L2-L3 level with considerable thecal sac and nerve root compression (Fig. 1). The lesion originated near the left L2-3 facet joint and extended under the lamina of L2 and L3 centrally.
Fig. 1.
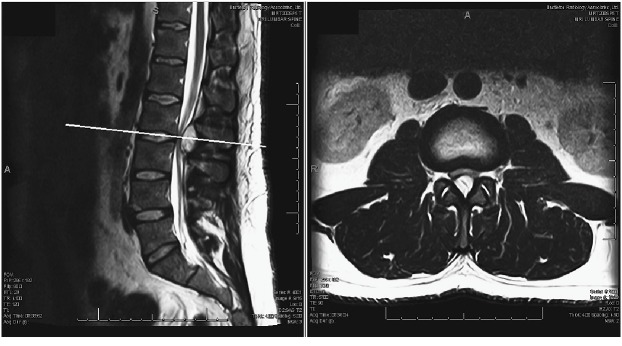
Initial lumbar spine sagittal magnetic resonance images performed 3 months prior to presentation. The T2-weighted image shows a hyperintense cystic mass at the L2-L3 dorsal epidural space.
After three months of progressive worsening of his pain and conservative management recommended by another surgeon, the patient came for a second opinion at our clinic due to newly developed worsening right leg weakness. The second MRI lumbar spine, performed three months after the initial, demonstrated complete resolution of the L2-3 hemorrhagic cystic mass but with a new hemorrhagic epidural mass formed at L3-4 causing compression of the thecal sac and L3 nerve root on the right side (Fig. 2). Additionally, the patient was noted to have bilateral spondylolysis at the L4 with pars defect along with bilateral facet hypertrophy at L3-L4. At this time, his right iliopsoas and quadriceps were 4/5 in motor strength.
Fig. 2.
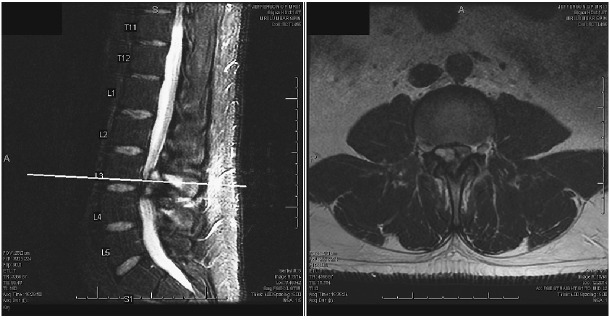
Second lumbar spine magnetic resonance imaging three months after the initial imaging. T2-weighted sequence shows a heterogeneous hyperintense cystic mass at the L3-L4 dorsal epidural space and the initial cystic mass at L2-3 level has resolved.
The patient was offered surgical treatment and subsequently underwent right L3-L4 hemilaminectomy and medial facetectomy under a microscope. After opening the ligamentum flavum at L3, bloody fluid egressed out of the epidural space. Thickened tissue associated with the L2-3 facet joint was resected. Intraoperative pathology was consistent with fibrinous tissue as well as blood suggestive of synovial cyst. The post-operative lumbar MRI demonstrated complete decompression of thecal sac after resection of the synovial cyst originating at L2-3 right facet (Fig. 4). With the possibility of malignancy, the L4 pars defect was not repaired during the procedure. After confirming no malignancy on final pathology, the patient was taken back to operative room one week later for L4 pars repair with bilateral L4 transpedicular screws and sublaminar hooks. Right iliac crest bone graft was harvested for bone grafting of pars defects (Fig. 5)
Fig. 4.
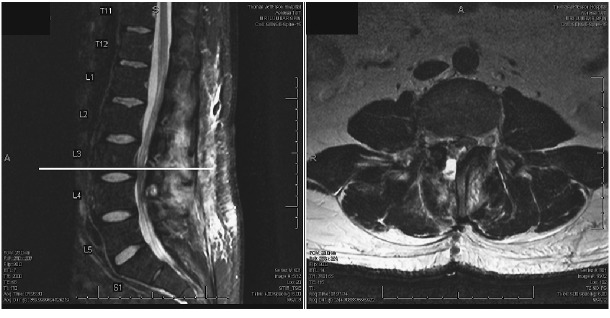
Post-operative T2 MRI sequence demonstrating decompressed lumbar spinal canal with total resection of the synovial cyst
Fig. 5.
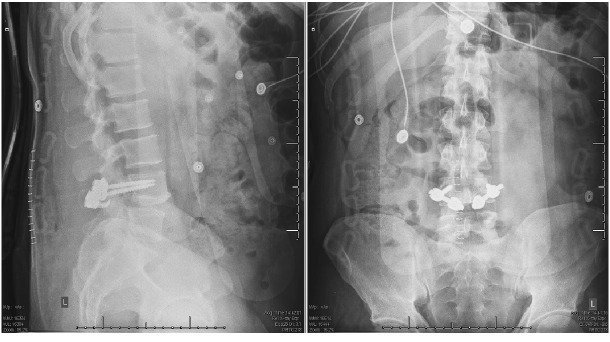
Post-operative lateral and AP view of lumbar radiograph after the pars repair at L4
Final pathological findings were fibroadipose tissue with reactive changes including focal hemosiderin deposition, along with fragments of tissue with spindle cells consistent with ligament and synovial tissue. Figure 3 shows the H&E stein of the resection hemorrhagic synovial cyst.
Fig. 3.
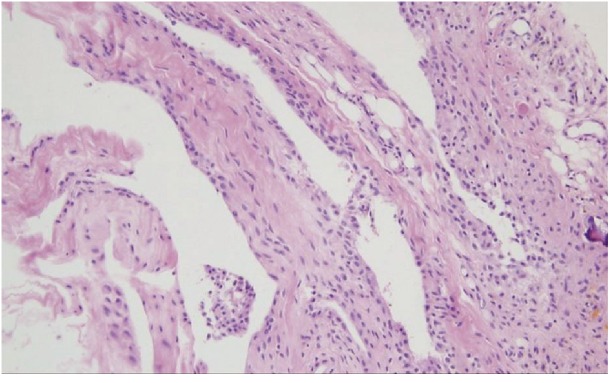
Histological appearance of the hemorrhagic synovial cyst showing synovial cell lining, fibroconnective tissue, neoangiogenesis, and hemosiderin microdeposits (H&E stain, ×200).
At 3 weeks follow-up, the patient’s leg pain and weakness had resolved with mild low-back pain at the site of the incision.
DISCUSSION
The two possible etiologies of this patient’s presentation is either spontaneous hemorrhagic epidural bleed that migrated caudally over three months or a hemorrhagic synovial cyst initially originating at left L1-2 facet joint and extending to epidural space at L2-3 level that resolved spontaneously and another different hemorrhagic synovial cyst formed at the level below upon new injury to the lumbar spine three months later. Since synovial joints are confined to each facet joints, it would be unlikely that the single synovial cyst actually migrated caudally. Furthermore, the new MRI also showed intense STIR signal at L2-3 and L3-4 interspinous ligaments which was not evident on the first MRI suggesting another injury in between the MRIs. This makes the development of two separate synovial cysts more likely than migration of epidural bleed, especially considering intraoperative visualization of right sided synovial cyst and resection pathology. Hemorrhagic conversion of the cyst content could be attributable to the fact that the patient was taking large amounts of aspirin for his back pain during this period. Regarding the apparent migration of the cyst, it’s likely that an occult phenomenon occurred where two separate cysts formed in proximity with one resolving while the other worsened making it appear to be the same lesion. Synovial cyst is a known pathology but symptomatic, hemorrhagic synovial cysts are rare. Two large, symptomatic, hemorrhagic synovial cysts forming within three months of each other with one completely resolving has not been reported.
Synovial or ganglion cysts usually present in the extremities and have been known to occur with joint disease and hypermobility38, 39). Once considered rare, spinal synovial cysts, which, as noted, are mostly of lumbar origin, have been increasing in prevalence along with the increase in use of CT and MRI for spinal disorders. Imaging in symptomatic and highly-active, asymptomatic populations has shown the incidence to be in the range of 7.0–22.4%, with some patients having multiple cysts40, 41). Increasing prevalence on imaging suggests that the majority of these cysts resolve or remain asymptomatic, and there are reports of spontaneous resolutions of these cysts 42). However, synovial cysts may also undergo rapid enlargement through both hemorrhagic as well as nonhemorrhagic mechanisms causing acute cauda equina syndrome18, 43).
From our literature review, we found 44 previously published cases of hemorrhagic synovial cysts, defined by intraoperative finding of blood in the cyst or the observation of blood or hemosiderin on pathological analysis. Table 1 summarizes each case’s presentation, surgical managements, and outcomes as well as patients’ satisfaction according to the Macnab criteria. The average age at presentation was 63.4±3.2 (SEM) years, with 39/43 (91%) cases occurring after age 50. There were 25 males (60%) and 17 females (40%) in the cases. The average age of the females at presentation was 69.2±2.2 years compared to 59.6±3.1 years for males. Of note, the four cases presenting before age 50 (ages 24, 29, 36, 42) were all male.
Table 1.
Review of the Literature: Summary of 44 cases of Hemorrhagic Lumbar Synovial Cysts
| Authors/Year | Age/Gender | Level | Symptom Duration | Back Pain |
Leg Pain |
Weakness | Sensory Change | Decreased Reflexes | Other | MRI* (T1/T2) |
Surgical Treatment – Laminectomy | Follow- up |
Outcome | Applied Macnab Score† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brish and Payan, 19725) |
51/M | L4-L5 | 3 mo | + | + | + | + | NR | Complete resolution of presenting symptoms | Excellent | ||||
| Pendleton et al., 198331) | 50/F | L3-L4 | 3 mo -> 2wks | + | + | + | + | + | + | 1 mo | Improved symptoms | Good | ||
| Kjerulf et al., 198619) | 56/F | L5-S1 | 5 mo | + | + | + | + | 1 yr | Complete resolution of presenting symptoms | Excellent | ||||
| Franck et al., 198712) | 62/F | L4-L5 | 2 mo | + | + | + | NR | Complete resolution of presenting symptoms | Excellent | |||||
| Onofrio and Mih, 198827) | 64/F | L4-L5 | 3 mo | + | + | + | + | 3 mo | Pain free | Excellent | ||||
| 71/F | L4-L5 | 3 mo | + | + | + | 3 mo | Mild residual foot pain | Good | ||||||
| Reust et al., 198835) | 77/F | L4-L5 | 1 mo | + | + | + | 1 y | Complete resolution of presenting symptoms | Excellent | |||||
| 24/M | L4-L5 | 5 mo | + | + | + | 1 yr | Progressive improvement | Good | ||||||
| Eyster and Scott, 198910) |
60/M | L4-L5 | 3 mo | + | + | + | NR | Complete resolution of presenting symptoms | Excellent | |||||
| Lemish et al., 198920) | 78/F | L5-S1 | 3 mo | + | + | NR | Complete resolution of presenting symptoms | Excellent | ||||||
| 53/M | L4-L5 | 21 d | + | spinal claudication | + | NR | Complete resolution of presenting symptoms | Excellent | ||||||
| Rousseaux et al., 198936) | 29/M | L5-S1 | 7 mo | + | + | NR | NR | NR | ||||||
| Jackson et al., 198916) |
NR/NR | L4-L5 | NR | + | + | Hi/Hi | + | NR | Improvement in pain and relief of radiculopathy | Good | ||||
| Summers and Quint, 199238) |
60/M | L2-L3 | 6 mo | + | + | + | hHi/hLo | + | NR | NR | NR | |||
| Tatter and Cosgrove, 199441) |
59/M | L3-L4 | 5 d | + | + | + | + | Hi/hLo | + | 2 mo | Complete resolution of presenting symptoms | Excellent | ||
| Yarde et al., 199547) | 42/M | L5-S1 | 12 mo | + | + | NR | + | 4 mo | Improved symptoms | Good | ||||
| Howling and Kessel, 199715) | 51/M | L4-L5 | 21 d | + | + | + | + | + | hHi/hHi | + | NR | Complete resolution of presenting symptoms | Excellent | |
| Radatz et al., 199732) | 65/M | L4-L5 | >1 mo | + | + | + | + | Lo/Hi | + | 1 mo | Complete resolution of presenting symptoms | Excellent | ||
| Henriet and Nobourgh, 199813) |
56/F | L4-L5 | 14 d | + | + | + | NR | NR | NR | |||||
| Bandiera et al., 19993) | 81/F | L3-L4 | 4 mo | + | + | + | spinal claudication | Hi/hHi | + | 1 yr | Complete resolution of presenting symptoms | Excellent | ||
| Kaneko and Inoue, 200017) |
55/M | L3-L4 | 6 mo -> 3 d | + | + | + | + | Hi/NR | + | 2.5 yr | Complete resolution of presenting symptoms | Excellent | ||
| Wang et al., 200445) | 76/F | L4-L5 | 4 mo -> few days | + | Hi/Lo | + | NR | Complete resolution of pain | Excellent | |||||
| 82/F | L4-L5 | 3 mo -> 24 hr | + | + | + | Hi/Lo | + | NR | Complete resolution of pain | Excellent | ||||
| Eck and Triantafyllou, 20059) | 77/M | L3-L4 | 2 wk | + | + | + | NR/Hi | + | 1 yr | Complete resolution of presenting symptoms | Excellent | |||
| Wait et al., 200544) | 62/M | L3-L4 | 6 mo -> 2 mo | + | + | + | Hi/NR | + | NR | Full recovery | Excellent | |||
| 69/M | L3-L4 | 8 mo -> 2 wk | + | + | + | + | + | urinary retention | Hi/NR | + | 4 mo | Full recovery | Excellent | |
| Khan et al., 200518) | NR/NR | NR | NR | NR | ||||||||||
| NR/NR | NR | NR | NR | |||||||||||
| Ramieri et al., 200634) | 71/F | L5-S1 | 15 d | + | + | + | + | hHi/NR | + | 1 yr | Complete resolution of presenting symptoms | Excellent | ||
| 75/F | L3-L4 | 10 d | + | + | + | + | + | hHi/NR | + | 1 yr | Recovery of sensation, reflexes. Partial recovery of strength | Good | ||
| 68/M | L4-L5 | 14 d | + | + | hHi/NR | + | 2 yr | No radicular pain, continued back pain | Good | |||||
| Nourbakhsh and Garges, 200726) | 67/M | L4-L5 | 1.5 mo | + | + | + | + | clonus | Hi/hLo | + | 1 yr | Complete resolution of presenting symptoms | Excellent | |
| Arantes et al., 20082) | 61/M | L4-L5 | 2 mo -> 8 d | + | + | + | Hi/Lo | + | 6 mo | Complete resolution of neurological symptoms | Excellent | |||
| Miyatake et al., 200925) | 86/M | L1-L2 | 3 mo -> 1mo | + | Hi/Hi | + | NR | Complete resolution of neurological symptoms | Excellent | |||||
| 56/M | L4-L5 | 1 mo -> 4 d | + | + | cauda equina | Hi/Hi | + | NR | Complete resolution of neurological symptoms | Excellent | ||||
| 84/M | L4-L5 | 2 mo -> 1 mo | + | + | Hi/Hi | + | NR | Complete resolution of neurological symptoms | Excellent | |||||
| 57/M | L5-S1 | 3 mo -> 2 mo | + | Hi/Hi | + | NR | Complete resolution of neurological symptoms | Excellent | ||||||
| Cicuendez et al., 20107) | 72/F | L3-L4 | 3 mo -> 1 wk | + | + | + | + | Hi/h | + | ~4 wk | Full recovery | Excellent | ||
| Utsunomiya et al., 201143) | 65/F | L3-L4 | 2 mo -> days | + | + | + | + | hHi/Lo | + | 1 yr | Complete recovery | Excellent | ||
| Xu et al., 201146) | 68/M | L3-L4 | 4 mo -> 1 wk | + | + | + | + | Hi/Lo | + | 8 mo | Resolution of majority of radicular symptoms | Good | ||
| Tofuka et al., 201142) | 84/M | L4-L5 | >2 mo | + | + | + | urinary disturbance | Hi/h | + | 1 wk | Symptoms improved, Pt could walk without support at 1 wk post-op | Good | ||
| Park et al., 201230) | 72/F | L2-L3 | acute | + | + | Hi/Lo | + | 1 mo | Full recovery | Excellent | ||||
| Machino et al., 201222) | 65/M | L2-L3 | 3 mo -> 1 wk | + | + | + | mild incontinence of bowel and bladder | Hi/Lo | + | 1 mo | Full recovery | Excellent | ||
| Hoover and Pirris, 201414) |
68/F | S1-S2 | 3 wk | + | + | + | Lo/Hi | + | NR | Significant improvement in leg pain | Good |
NR = Not recorded by authors
*h = heterogeneous, Hi = hyperintense, Lo = hypointense
†According to Macnab score23)
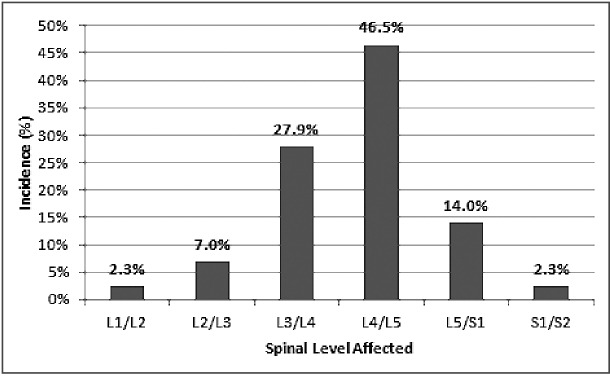
The most commonly involved vertebral levels were L4-L5 (46.5%) followed by L3-L4 (27.9%), L5-S1 (14.0%), and L2-L3 (7.0%). There has been one reported case at S1-S2 and one reported case at L1-L2 (Fig. 6). Previous literature reviews have shown the majority (64%) of non-hemorrhagic lumbar synovial cysts to be located at L4-L52).
Fig. 6.
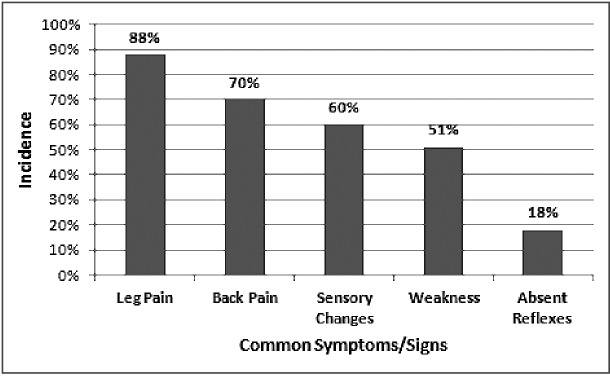
Incidence of the most common symptoms and physical findings associated with hemorrhagic synovial cysts. Data collected from 43 cases published in the literature.
The most common presenting symptoms and physical findings were leg pain (88%), back pain (70%), sensory changes including numbness, tingling, paresthesia (60%) and weakness (51%) (Fig. 7). Absent reflexes were reported in 18% of cases, while incontinence or other urinary changes were rare with only two reported cases.
Fig. 7.
Spinal levels affected by hemorrhagic synovial cysts. Data collected from 43 cases published in the literature.
Among cases with recorded MRI findings, most cysts (93%, 26/28) were found to be hyperintense or heterogeneously hyperintense on T1-weighted imaging. In 6 cases, contrast enhancement on the peripheral rim of the cyst, the capsule, was noted on T1-weighted imaging. With T2-weighting, there is a mixed picture; among 23 cases, 30.4% were hypointense, 34.7% were heterogeneous and 34.7% were hyperintense. On MR imaging, non-hemorrhagic synovial cysts are typically T1-hypointense and T2-hyperintense, but the signal varies depending on factors including protein content, blood products, and timing of hemorrhage within the cyst2). T2 imaging of cysts is known to have varying signal intensity, and previous reports have similarly surmised previous old hemorrhages, elevated protein content, and calcification can all be causes of this varying signal44).
Surgical decompression via laminectomy and cyst excision is the current standard and was the treatment of choice in majority of the cases reviewed. Depending on cyst size, medial facetectomy was also performed. Posterior fusion and stabilization was performed in only 3 cases. Of the 40 cases with documented postoperative outcomes, a majority of patients (75%) had complete resolution of symptoms. Improved symptoms were noted in the remaining 25% of cases. There were no recorded cases of fair or poor outcomes. For patients with recurrent cyst formation, persistent back pain or leg pain symptoms due to concurrent pathology such as spondylolysis or pars defect as in our patient, additional lumbar fusion or pars repair for stabilization are often carried out to stabilize the spine. Pars repair offers benefit of not losing the segmental motion at the involved level thereby exposing patients to less chance of developing adjacent level disease in the future. We believe this was a good option for this particular young patient who has extremely physically challenging career. Percutaneous rupture of intact lumbar synovial cysts is also an option, but has not been used or recommended in hemorrhagic cases with the risk of causing epidural bleed45).
Non-hemorrhagic synovial cysts are thought to be related to degenerative spinal changes and segmental instability, with retrospective studies supporting these hypotheses1, 9). MRI prevalence studies have also shown cysts to have significant associations with facet arthropathy, facet joint osteoarthritis, and spondylolisthesis40). The pathogenesis of hemorrhagic cysts is less well understood. While trauma and anticoagulation therapy have been implicated as factors, they have not been associated in a majority of cases3). The literature review of 44 cases found that cyst hemorrhage more often occurs in men and at an earlier age, lending support to a micro-trauma hypothesis if occupation and lifestyle is considered. In our case, a 36 year-old male police officer with bilateral spondylolysis at the L4 level, it is likely that degenerative changes in his lumbar spine along with daily microtrauma caused by his occupation led to his initial synovial cyst and eventual hemorrhagic transformation.
Regarding our patient’s apparent migratory cyst, previous reports of migratory synovial cysts have shown migration to the erector spinae as well as between spinous processes5,6). In our case, cyst migration is likely an occult phenomenon because the synovial cyst is apparently occurring at two distinct levels. Our hypothesis is that the patient had initial trauma that induced first hemorrhagic synovial cyst at L1-2 level on left extending to L2-3 epidural level which resolved and had another trauma on work duty that induced completely different hemorrhagic synovial cyst at the level below at L3-4 on right. This is both consistent with the MRI finding of new posterior ligamentous injury as well as the patient developing new symptoms of worsened right leg pain and right quadriceps weakness during the intervening three months.
Unlike in our case, true synovial cyst migration around a facet, under the epidural space, or into the erector spinae muscle, is likely to involve mechanical as well as chemical mechanisms. An inflammatory process in or near the cyst may cause an increase in the proteolytic enzymatic activity of the cystic fluid. Synovial fluid contains metalloproteases, hyaluronidase, and other enzymes capable of digesting through tissue, giving it the capability to create new pathways through which it propagates via gravity and pressure-related mechanisms46). This migration of inflammatory, enzymatic cystic fluid may be involved with the spontaneous hemorrhage observed as vascular structures exposed are vulnerable to digestion. Previous reports have hypothesized that anti-inflammatories such as NSAIDs may play a role in regression of synovial cysts47). This certainly could be the case for our patient’s spontaneous resolution of the cyst at L2-3 as he was primarily taking NSAIDs to cope with his low back pain. The role of migration in hemorrhagic transformation remains unclear. With increasing use of MRI, it is likely more cases with repeated imaging will capture previously unseen migration.
CONCLUSION
Lumbar synovial cysts are an increasingly common finding on MR imaging in both symptomatic and asymptomatic populations. Trauma may play a role in their formation and a higher index of suspicion may be warranted in males with active occupations. While the majority of synovial cysts can be observed, acute worsening or change in symptoms may herald either a hemorrhagic transformation or migration to a different level. Patients should be counseled regarding these rare possibilities. As our case demonstrates, repeated MRI imaging is worthwhile. Surgical decompression with cyst excision remains the treatment of choice. In patients with associated pars defect or spondylolisthesis, lumbar decompression with resection of cysts may not be enough to reduce back pain symptoms warranting concurrent fusion or pars repair for better symptomatic relief.
CONFLICTS OF INTEREST
All authors have no financial support or relationships that may pose a conflict of interest to disclose for this work.
REFERENCES
- 1).Boviatsis EJ, Stavrinou LC, Kouyialis AT, et al. Spinal synovial cysts: pathogenesis, diagnosis and surgical treatment in a series of seven cases and literature review. Eur Spine J. 2008; 17(6): 831–7. [DOI] [PMC free article] [PubMed]
- 2).Lyons MK, Atkinson JL, Wharen RE, Deen HG, Zimmerman RS, Lemens SM. Surgical evaluation and management of lumbar synovial cysts: the Mayo Clinic experience. J Neurosurg. 2000; 93 (1 Suppl): 53–7. [DOI] [PubMed]
- 3).Xu R, Solakoglu C, Maleki Z, Mcgirt MJ, Gokaslan ZL, Bydon A. Hemorrhagic synovial cyst: the possible role of initial trauma and subsequent microtrauma in its pathogenesis: case report. Neurosurgery. 2011; 68(3): E858–65. [DOI] [PubMed]
- 4).Eck JC, Triantafyllou SJ. Hemorrhagic lumbar synovial facet cyst secondary to anticoagulation therapy. Spine J. 2005; 5(4): 451–3. [DOI] [PubMed]
- 5).Palmieri F, Cassar-pullicino VN, Lalam RK, Tins BJ, Tyrrell PN, Mccall IW. Migrating lumbar facet joint cysts. Skeletal Radiol. 2006; 35(4): 220–6. [DOI] [PubMed]
- 6).Hoover J, Pirris S. Synovial cyst mimicking an intraspinal sacral mass. Case Rep Neurol Med. 2014; 2014: 953579. [DOI] [PMC free article] [PubMed]
- 7).Brish A, Payan HM. Lumbar intraspinal extradural ganglion cyst. J Neurol Neurosurg Psychiatr. 1972; 35(6): 771–5. [DOI] [PMC free article] [PubMed]
- 8).Pendleton B, Carl B, Pollay M. Spinal extradural benign synovial or ganglion cyst: case report and review of the literature. Neurosurgery. 1983; 13(3): 322–6. [DOI] [PubMed]
- 9).Kjerulf TD, Terry DW, Boubelik RJ. Lumbar synovial or ganglion cysts. Neurosurgery. 1986; 19(3): 415–20. [DOI] [PubMed]
- 10).Franck JI, King RB, Petro GR, Kanzer MD. A posttraumatic lumbar spinal synovial cyst. Case report. J Neurosurg. 1987; 66(2): 293–6. [DOI] [PubMed]
- 11).Onofrio BM, Mih AD. Synovial cysts of the spine. Neurosurgery. 1988; 22(4): 642–7. [DOI] [PubMed]
- 12).Reust P, Wendling D, Lagier R, et al. Degenerative spondylolisthesis, synovial cyst of the zygapophyseal joints, and sciatic syndrome: report of two cases and review of the literature. Arthritis Rheum. 1988; 31(2): 288–94. [DOI] [PubMed]
- 13).Eyster EF, Scott WR. Lumbar synovial cysts: report of eleven cases. Neurosurgery. 1989; 24(1): 112–5. [DOI] [PubMed]
- 14).Lemish W, Apsimon T, Chakera T. Lumbar intraspinal synovial cysts. Recognition and CT diagnosis. Spine. 1989; 14(12): 1378–83. [DOI] [PubMed]
- 15).Jackson DE, Atlas SW, Mani JR, Norman D. Intraspinal synovial cysts: MR imaging. Radiology. 1989; 170(2): 527–30. [DOI] [PubMed]
- 16).Rousseaux P, Durot JF, Pluot M, et al. [Synovial cysts and synovialomas of the lumbar spine. Histo-pathologic and neuro-surgical aspects apropos of 8 cases]. Neurochirurgie. 1989; 35(1): 31–9. [PubMed]
- 17).Summers RM, Quint DJ. Case report 712: Hemorrhagic synovial cyst arising from right L2-3 facet joint. Skeletal Radiol. 1992; 21(1): 72–5. [DOI] [PubMed]
- 18).Tatter SB, Cosgrove GR. Hemorrhage into a lumbar synovial cyst causing an acute cauda equina syndrome. Case report. J Neurosurg. 1994; 81(3): 449–52. [DOI] [PubMed]
- 19).Yarde WL, Arnold PM, Kepes JJ, O’boynick PL, Wilkinson SB, Batnitzky S. Synovial cysts of the lumbar spine: diagnosis, surgical management, and pathogenesis. Report of eight cases. Surg Neurol. 1995; 43(5): 459–64. [DOI] [PubMed]
- 20).Howling SJ, Kessel D. Case report: acute radiculopathy due to a haemorrhagic lumbar synovial cyst. Clin Radiol. 1997; 52(1): 73–4. [DOI] [PubMed]
- 21).Radatz M, Jakubowski J, Cooper J, Powell T. Synovial cysts of the lumbar spine: a review. Br J Neurosurg. 1997; 11(6): 520–4. [DOI] [PubMed]
- 22).Henriet M, Nobourgh Y. Hemorragie dans un kyste synovial lombaire avec compression polyradiculaire aigue. Rachis 1998; 10: 89–90.
- 23).Bandiera S, Campanacci L, De iure F, Bertoni F, Picci P, Boriani S. Hemorrhagic synovial lumbar cyst: a case report and review of the literature. Chir Organi Mov. 1999; 84(2): 197–203. [PubMed]
- 24).Kaneko K, Inoue Y. Haemorrhagic lumbar synovial cyst. A cause of acute radiculopathy. J Bone Joint Surg Br. 2000; 82(4): 583–4. [DOI] [PubMed]
- 25).Wang YY, Mckelvie P, Trost N, Murphy MA. Trauma as a precipitant of haemorrhage in synovial cysts. J Clin Neurosci. 2004; 11(4): 436–9. [DOI] [PubMed]
- 26).Wait SD, Jones FD, Lonser RR, Lee KS. Symptomatic epidural hematoma caused by lumbar synovial cyst rupture: report of two cases and review of the literature. Neurosurgery. 2005; 56(5): E1157. [PubMed]
- 27).Khan AM, Synnot K, Cammisa FP, Girardi FP. Lumbar synovial cysts of the spine: an evaluation of surgical outcome. J Spinal Disord Tech. 2005; 18(2): 127–31. [DOI] [PubMed]
- 28).Ramieri A, Domenicucci M, Seferi A, Paolini S, Petrozza V, Delfini R. Lumbar hemorrhagic synovial cysts: diagnosis, pathogenesis, and treatment. Report of 3 cases. Surg Neurol. 2006; 65(4): 385–90, discussion 390. [DOI] [PubMed]
- 29).Nourbakhsh A, Garges KJ. Lumbar synovial joint hematoma in a patient on anticoagulation treatment. Spine. 2007; 32(9): E300–2. [DOI] [PubMed]
- 30).Arantes M, Silva RS, Romão H, et al. Spontaneous hemorrhage in a lumbar ganglion cyst. Spine. 2008; 33(15): E521–4. [DOI] [PubMed]
- 31).Miyatake N, Aizawa T, Hyodo H, Sasaki H, Kusakabe T, Sato T. Facet cyst haematoma in the lumbar spine: a report of four cases. J Orthop Surg (Hong Kong). 2009; 17(1): 80–4. [DOI] [PubMed]
- 32).Cicuendez M, Alen JF, Ramos A, Lobato RD, Lagares A. Spontaneous hemorrhage into a lumbar synovial cyst. Eur Spine J. 2010; 19 Suppl 2: S190–2. [DOI] [PMC free article] [PubMed]
- 33).Utsunomiya R, Sakai T, Wada K, et al. Hemorrhagic facet cyst in the lumbar spine causing contralateral leg symptoms: a case report. Asian Spine J. 2011; 5(3): 196–200. [DOI] [PMC free article] [PubMed]
- 34).Tofuku K, Koga H, Komiya S. Facet arthrography in an unusual presentation of a lumbar hemorrhagic synovial cyst. J Neurointerv Surg. 2012; 4(6): e40. [DOI] [PubMed]
- 35).Park HS, Sim HB, Kwon SC, Park JB. Hemorrhagic lumbar synovial cyst. J Korean Neurosurg Soc 2012; 52(6): 567–9.Machino et al. [36] (2012) [DOI] [PMC free article] [PubMed]
- 36).Machino M, Yukawa Y, Ito K, Kanbara S, Kato F. Spontaneous hemorrhage in an upper lumbar synovial cyst causing subacute cauda equina syndrome. Orthopedics. 2012; 35(9): e1457–60. [DOI] [PubMed]
- 37).Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971; 53(5): 891–903. [PubMed]
- 38).Sze CI, Kindt G, Huffer WB, Chang M, Wang M, Kleinschmidt-demasters BK. Synovial excrescences and cysts of the spine: clinicopathological features and contributions to spinal stenosis. Clin Neuropathol. 2004; 23(2): 80–90. [PubMed]
- 39).Steiner E, Steinbach LS, Schnarkowski P, Tirman PF, Genant HK. Ganglia and cysts around joints. Radiol Clin North Am. 1996; 34(2): 395–425, xi–xii. [PubMed]
- 40).Doyle AJ, Merrilees M. Synovial cysts of the lumbar facet joints in a symptomatic population: prevalence on magnetic resonance imaging. Spine. 2004; 29(8): 874–8. [DOI] [PubMed]
- 41).Rajeswaran G, Turner M, Gissane C, Healy JC. MRI findings in the lumbar spines of asymptomatic elite junior tennis players. Skeletal Radiol. 2014; [DOI] [PubMed]
- 42).Swartz PG, Murtagh FR. Spontaneous resolution of an intraspinal synovial cyst. AJNR Am J Neuroradiol. 2003; 24(6): 1261–3. [PMC free article] [PubMed]
- 43).Paolini S, Ciappetta P, Santoro A, Ramieri A. Rapid, symptomatic enlargement of a lumbar juxtafacet cyst: case report. Spine. 2002; 27(11): E281–3. [DOI] [PubMed]
- 44).Apostolaki E, Davies AM, Evans N, Cassar-pullicino VN. MR imaging of lumbar facet joint synovial cysts. Eur Radiol. 2000; 10(4): 615–23. [DOI] [PubMed]
- 45).Cambron SC, Mcintyre JJ, Guerin SJ, Li Z, Pastel DA. Lumbar facet joint synovial cysts: does T2 signal intensity predict outcomes after percutaneous rupture?. AJNR Am J Neuroradiol. 2013; 34(8): 1661–4. [DOI] [PMC free article] [PubMed]
- 46).Fernandes JC, Martel-pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002; 39(1–2): 237–46. [PubMed]
- 47).Mattei TA, Goulart CR, Mccall TD. Pathophysiology of regression of synovial cysts of the lumbar spine: the ‘anti-inflammatory hypothesis’. Med Hypotheses. 2012; 79(6): 813–8. [DOI] [PubMed]


