Abstract
Introduction
Presacral venous bleeding is an uncommon but potentially life threatening complication of rectal surgery. During the posterior rectal dissection, it is recommended to proceed into the plane between the fascia propria of the rectum and the presacral fascia. Incorrect mobilisation of the rectum outside the Waldeyer’s fascia can tear out the lower presacral venous plexus or the sacral basivertebral veins, causing what may prove to be uncontrollable bleeding.
Methods
A systematic search of the MEDLINE® and Embase™ databases was performed to obtain primary data published in the period between 1 January 1960 and 31 July 2013. Each article describing variables such as incidence of presacral venous bleeding, surgical approach, number of cases treated and success rate was included in the analysis.
Results
A number of creative solutions have been described that attempt to provide good tamponade of the presacral haemorrhage, eliminating the need for second operation. However, few cases are reported in the literature.
Conclusions
As conventional haemostatic measures often fail to control this type of haemorrhage, several alternative methods to control bleeding definitively have been described. We propose a practical comprehensive classification of the available techniques for the management of presacral bleeding.
Keywords: Presacral bleeding, Rectal surgery, Pelvic packing
Presacral venous bleeding (PSB) is a potentially life threatening complication of rectal surgery with an incidence of 3% to 9.4%.1–4 Rapid control is important to prevent a fatal outcome.
The presacral venous plexus (PSVP) is usually exposed during rectal mobilisation. During the posterior rectal dissection, it is recommended to proceed into the plane between the fascia propria of the rectum and the presacral fascia.5 An incorrect mobilisation of the rectum outside of Waldeyer’s fascia, the ‘holy plane’ of dissection,6 can tear the lower PSVP7 or the sacral basivertebral veins, causing what may prove to be uncontrollable bleeding. Moreover, the vessel may retract into the sacrum, making conventional haemostatic manoeuvres of little use. Blunt dissection in the retrorectal space by blindly stripping the posterior rectal wall with the surgeon’s fingers is the most common cause of haemorrhage.
Methods
A systematic search of the MEDLINE® and Embase™ databases was performed to obtain primary data published between January 1960 and July 2013. Search terms such as presacral bleeding, presacral haemorrhage, rectal surgery, pelvic surgery, pelvic packing and PSVP were used. The reference lists of retrieved publications were reviewed for other relevant papers. Each article describing variables such as incidence of PSB, surgical approach, number of cases treated and success rate was included in the analysis. Owing to paucity of suitable detailed publications, a conventional systematic review and meta-analysis was not possible.
Results
Anatomy
Bleeding from the PSVP occurs because of damage to the variable and complex interlacing venous networks both beneath and on the surface of the sacral periosteum.8,9
The PSVP is the lowest portion of the anterior external vertebral venous system.10 It is formed by the two lateral sacral veins, the middle sacral vein and the interconnecting communicating veins (Fig 1), and it is anastomosed with the internal vertebral system through the basivertebral vessels emerging from the sacral foramina.11 The PSVP lies posterior to the fascia propria of the rectum and just below the presacral fascia,12 running over the anterior aspect of the sacrum.
Figure 1.
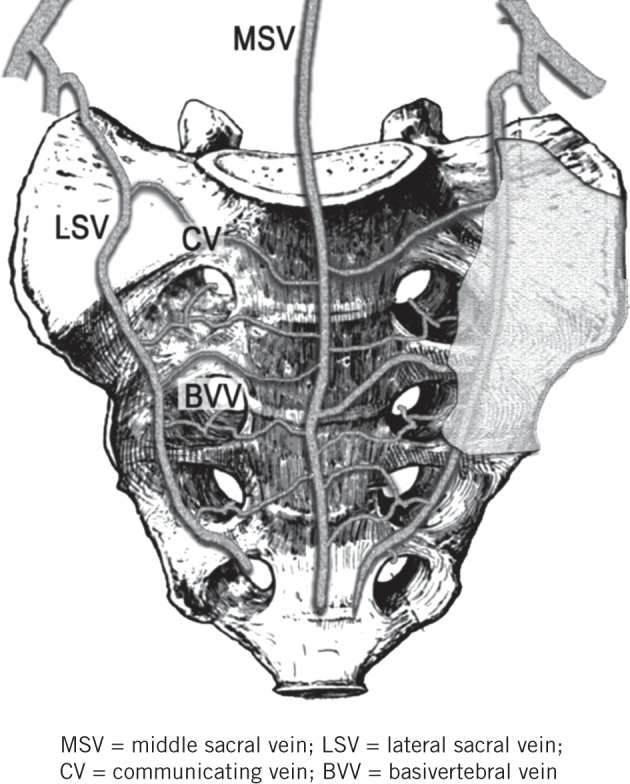
Anatomy of the presacral venous plexus: The presacral fascia, shown on the left sacral wing, has been removed. The basivertebral veins, emerging from the sacral foramina, anastomose the presacral venous plexus with the internal vertebral system.
The adventitia of presacral veins is blended intimately with sacral periosteum adjacent to the sacral foramina and can be lacerated easily.13 The most likely area of injury is the lower sacrum, where Waldeyer’s fascia is thick and may attach directly to the presacral fascia.14
As this entire pelvic venous system lacks valves,15 it has been estimated that with the patient in the lithotomy position, the hydrostatic pressure in the presacral space is up to three times that of the inferior vena cava.16 Experimental studies have demonstrated that blood loss from presacral veins measuring 2–4mm in diameter can reach up to 1,000ml/min and increasing the diameter of the vein by just 1mm can increase the blood loss almost threefold.1 Owing to the lack of valves and increased hydrostatic pressure, bleeding from small vessels in this area can be torrential and extremely difficult to control.
Surgical management
Whenever PSB occurs, the first temporary manoeuvre is direct pressure at the point of bleeding together with aspiration of the accumulated blood. Conventional haemostatic methods often fail and may occasionally further shear the delicate veins, extend the area of active haemorrhage and significantly worsen the problem. Ligation of the internal iliac artery is ineffective, and may cause bladder and gluteal necrosis17 while ligation of the internal iliac vein obstructs the drainage of the tributary veins, increasing the pressure in the PSVP and exacerbating the bleeding.18 When conventional methods prove inadequate, alternative methods for stopping PSB include packing and the use of thumbtacks.
The aim of this review was to systematically describe all the published techniques to control PSB in order to guide surgeons through the possible strategies for dealing with this challenging, life threatening complication. We propose a classification of techniques for management of PSB: packing techniques, tacking techniques, topical haemostatic agents, and direct/indirect electrocoagulation and suture (Table 1).
Table 1.
Classification of the techniques available to control presacral bleeding
| Packing techniques |
| Traditional pelvic packing |
| Silastic tissue expander |
| Perineal Sengstaken–Blakemore tube |
| Inflatable sterile saline bag |
| Breast implant sizer |
| Muscle tamponade |
| Tacking techniques |
| Metallic thumbtacks |
| Topical haemostatic agents |
| Haemostatic matrix + adsorbable haemostat |
| Oxidised cellulose + cyanoacrylate glue |
| Bone cement |
| Bone wax |
| Direct/indirect electrocoagulation and suture |
| Muscle fragment welding |
| Spray electrocautery |
| Argon beam coagulation |
| Bipolar coagulation |
| Circular suture ligation |
Packing techniques
Pelvic packing controls PSB effectively and may be life saving;19 multiple laparotomy pads are applied over bleeding sites and the abdomen is closed under tension. Packing has the disadvantage of requiring reoperation for removing the packs 24–48 hours later and risk of rebleeding. Furthermore, pelvic packing involves the implantation of a foreign material, which may increase the risk of pelvic sepsis.
The same principle of traditional packing (but without the need for reoperation) has been used in two creative methods inspired by the technique of Cosman et al in which a silastic tissue expander was used20 and the technique of McCourtney et al in which a perineal Sengstaken–Blakemore tube was employed.21 Ng et al used an inflatable sterile saline bag filled with 850ml of saline for compression of PSB that occurred during an abdominoperineal excision.2 The port of the saline bag was brought out through the perineal incision and the surgery was concluded. From five days after surgery, the bag was gradually emptied and removed at the patient’s bedside.
Braley et al have reported the use of a breast implant sizer to control PSB during an abdominoperineal resection.23 The sizer was placed into the presacral space and inflated with saline until haemostasis was obtained. The infusion port of the sizer was brought out through the perineal incision and traction was applied to the sizer by attaching the port to a litre bag of saline. The sizer was deflated and easily removed at the bedside two days after surgery.
The above techniques have been reported for patients who had abdominoperineal resection and a permanent stoma. When there is a fresh colorectal anastomosis, packing increases the risk of anastomotic disruption.19 An alternative innovative method of haemostasis has been described by Remzi et al, who used the rectus abdominis muscle to tamponade PSB.24 A 4cm × 2cm × 1cm piece of rectus abdominis muscle is harvested as a free flap and sewn over the bleeding area.
Tacking techniques
Metallic thumbtacks were first used to control PSB by Wang et al in 1985.1 Several reports have proven the efficacy of this method25,26 and an instrument for thumbtack application has been described.27 The technique has gained favour among gynaecologists28 and improvements have been developed such as the addition of a synthetic coagulant under the tack.29 There are, however, reports of thumbtack displacement resulting in chronic pain, anastomotic disruption and anastomotic fistulas.30,31 Another limitation is that thumbtacks cannot be applied to bleeding points originating from a sacral neural foramen or near vital structures (eg the ureters). They are also ineffective for diffuse haemorrhage.32
Multiple technical difficulties can be encountered using thumbtacks such as poor accessibility of the lower sacrum, obscured visualisation caused by bleeding, inability in a limited space to drive the tip of the tack and bending of the tacks. Van der Vurst et al used a ProTack™ device (Covidien, Dublin, Ireland) to fix to the sacrum haemostatic sponges with endoscopic helical tackers.33 This long and thin device is used extensively in laparoscopic hernia surgery and tacks can be applied with minimal obstruction of visibility.
Topical haemostatic agents
The use of glue combined with other topical haemostatic materials has proved its effectiveness in a number of reports. Germanos et al described the successful haemostasis obtained in three cases with the combined use of a haemostatic matrix (Floseal®; Baxter, Hayward, CA, US) and an absorbable haemostat (Surgicel® Fibrillar™; Ethicon, Somerville, NJ, US).34 The matrix haemostatic sealant is applied over the bleeding site followed by the absorbable haemostat on top as a pad. The pelvis is then packed for temporary haemostasis and preventing the sealant from being washed out. After three minutes, the packs are removed and the operation can continue. There is no risk of infection or secondary complication from foreign bodies because the materials used are absorbable. Moreover, they can be used in several bleeding sites conforming to irregular wound surfaces.
Oxidised cellulose is also used in combination with cyanoacrylate glue in a similar method of haemostasis described by Chen et al.35 The patch of oxidised cellulose is pressed over the lesion by a Kelly clamp, and cyanoacrylate glue is applied evenly over the surface and tissue surrounding the oxidised cellulose pieces. Zhang et al obtained complete control of the bleeding in five patients with the use of an absorbable haemostatic gauze, made of chemically treated cellulose, spread with medical adhesive (alpha-cyanoacrylate) compressed on to the bleeding vessel.36 Cyanoacrylate glue has also been used successfully in three cases by Losanoff et al.37 Furthermore, applying bone cement (polymethyl methacrylate) used for orthopaedic procedures38 and the use of bone wax have been reported.39
Direct/indirect electrocoagulation and suture
Many studies report the failure of direct coagulation in arresting PSB. A study in 2007 reassessed the role of spray electrocautery applied slightly above the target bleeders at the presacral fascia, delivering a high frequency electrical current in combination with drainage suction.40 The method resulted in successful haemostasis in four cases. Kandeel et al reported a case of PSB controlled with an argon beam coagulator.41 Li et al obtained complete haemostasis in seven patients with the use of direct bipolar coagulation.42
A well validated technique is indirect coagulation through muscle fragment.43 Harrison et al reported eight cases of PSB controlled with muscle fragment welding.44 A 1.5cm × 2cm segment of rectus abdominis muscle is harvested from the incision and held in place with a forceps over the bleeding area; electrocautery at high setting is then applied to the forceps and transmitted to the muscle fragment, welding the bleeding site. The muscle has the advantage of being soft so it can conform to the bleeding site and pressure can be applied effectively. This is the only non-traditional technique that has demonstrated its effectiveness in a larger number of cases45 and it has the advantage of being effective on multiple bleeding sites. As an alternative, an epiploic appendix can be used instead of the muscle fragment.46
Direct suture has been shown to be an unsuccessful method and concerns regarding the chance of worsening the bleeding have been raised. Jiang et al have recently described the circular suture ligation method to control PSB by suture ligating the venous plexus with a 4/0 silk suture in one or more circles in the area with intact presacral fascia that surrounds the bleeding site.47
Discussion
The anatomy of the PSVP makes it vulnerable to damage, generating serious bleeding that can often be difficult to control using conventional methods. Contributing factors are bulky disease in the rectum, a narrow pelvis, posterior infiltration and reoperation. The higher incidence of this complication in patients who had radiotherapy before surgery might be owing to more difficult dissection related to increased pararectal fibrosis.
This is the first comprehensive review of all the methods described to control PSB. Traditional methods for stopping PSB include pelvic packing and the use of sterile thumbtacks. Several creative solutions have been described in an attempt to provide a good tamponade and eliminate the need for reoperation but few cases are reported. Drainage and compression are the first manoeuvres to be carried out; poor access to and poor visibility in the pelvis add to the challenge, and rectal resection should be considered to obtain better exposure. The muscle fragment welding method has a high success rate and is our preferred technique. Applicator devices for tacks and topical haemostatic agents should be available in all operative rooms (Fig 2).
Figure 2.
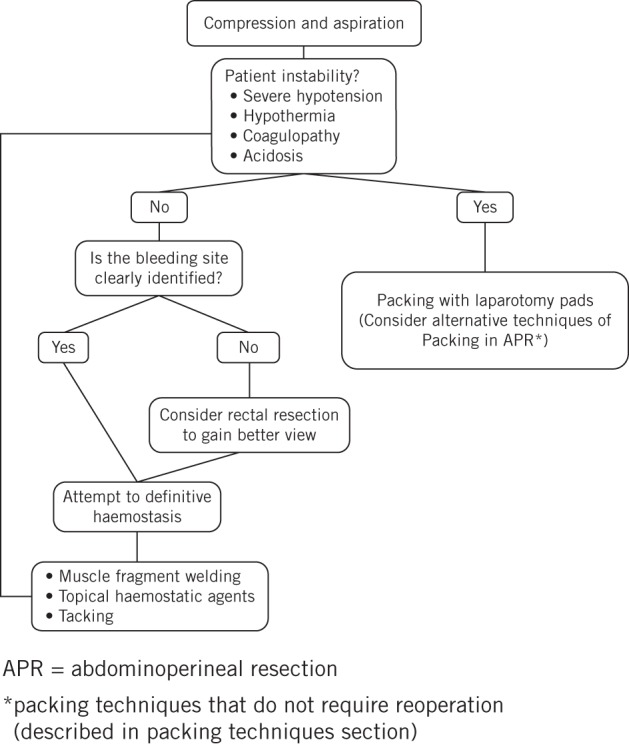
Algorithm for surgical management of presacral bleeding. Different techniques can be applied in a single patient sequentially.
Minimally invasive approaches have been increasingly applied to colorectal sugery and D’Ambra et al described a method to control PSB laparoscopically.48 The initial use of bipolar cautery or suturing is followed by cautery through an absorbable fabric mesh and if bleeding does not stop, indirect cautery through an epiploic appendix or a piece of omentum is performed. If the second step fails, a small scrap of bovine pericardium graft is finally tacked to the bleeding site.
Although a multitude of strategies have been employed successfully to control PSB, it is imperative to consider the stability of the patient when using potentially time consuming techniques to control such haemorrhage. When a patient begins developing the lethal triad of acidosis, coagulopathy and hypothermia, the surgeon must always consider packing of the pelvis to rapidly control haemorrhage and prevent further deterioration.49
Conclusions
This classification based on more than 50 years of scientific literature is a step towards understanding the incidence and factors predisposing to presacral haemorrhage. This is the first comprehensive classification of all the methods available and can guide the surgeons through the possible strategies to deal with a challenging, life threatening complication.
Acknowledgement
The authors are grateful to Salvatore Marra (architect), who kindly drew Figure 1.
References
- 1.Wang QY, Shi WJ, Zhao YR et al. New concepts in severe presacral hemorrhage during proctectomy. Arch Surg 1985; 120: 1,013–1,020. [DOI] [PubMed] [Google Scholar]
- 2.Barras JP, Fellman T. Massive hemorrhage from presacral veins during resection of the rectum. Helv Chir Acta 1992; 59: 335–339. [PubMed] [Google Scholar]
- 3.Pollard CW, Nivatvongs S, Rojanasakul A, Ilstrup DM. Carcinoma of the rectum. Profiles of intraoperative and early postoperative complications. Dis Colon Rectum 1994; 37: 866–874. [DOI] [PubMed] [Google Scholar]
- 4.Jorge JM, Habr-Gama A, Souza AS et al. Rectal surgery complicated by massive presacral hemorrhage. Arq Bras Cir Dig 1990; 5: 92–95. [Google Scholar]
- 5.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery – the clue to pelvic recurrence? Br J Surg 1982; 69: 613–616. [DOI] [PubMed] [Google Scholar]
- 6.Heald RJ. The ‘Holy Plane’ of rectal surgery. J R Soc Med 1988; 81: 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacon HE, Gutierrez RR. Cancer of the rectum and colon. Review of 2,402 personal cases. Dis Colon Rectum 1967; 10: 61–64. [DOI] [PubMed] [Google Scholar]
- 8.Flynn MK, Romero AA, Amundsen CL, Weidner AC. Vascular anatomy of the presacral space: a fresh tissue cadaver dissection. Am J Obstet Gynecol 2005; 192: 1,501–1,505. [DOI] [PubMed] [Google Scholar]
- 9.Corman ML. Colon and Rectal Surgery Baltimore: Lippincott Williams & Wilkins; 1993. pp1–23. [Google Scholar]
- 10.Crock HV, Yoshizawa H. The Blood Supply of the Vertebral Column and Spinal Cord in Man New York: Springer; 1977. pp66–69. [Google Scholar]
- 11.Edwards EA, Malone PD, Macarthur JD. Operative Anatomy of Abdomen and Pelvis Philadelphia: Lea & Febiger; 1975. pp73–75. [Google Scholar]
- 12.Muntean V. The surgical anatomy of the fasciae and the fascial spaces related to the rectum. Surg Radiol Anat 1999; 21: 319–324. [DOI] [PubMed] [Google Scholar]
- 13.Church JM, Raudkivi PJ, Hill GL. The surgical anatomy of the rectum - a review with particular relevance to the hazards of rectal mobilisation. Int J Colorectal Dis 1987; 2: 158–166. [DOI] [PubMed] [Google Scholar]
- 14.Crapp AR, Cuthbertson AM. William Waldeyer and the rectosacral fascia. Surg Gynecol Obstet 1974; 138: 252–256. [PubMed] [Google Scholar]
- 15.Bagué P, Karimdjee B, Iannelli A et al. Anatomy of the presacral venous plexus: implications for rectal surgery. Surg Radiol Anat 2004; 26: 355–358. [DOI] [PubMed] [Google Scholar]
- 16.Hill AD, Menzies-Gow N, Darzi A. Methods of controlling presacral bleeding. J Am Coll Surg 1994; 178: 183–184. [PubMed] [Google Scholar]
- 17.Binder SS, Mitchell GA. The control of intractable pelvic hemorrhage by ligation of the hypogastric artery. South Med J 1960; 53: 837–843. [DOI] [PubMed] [Google Scholar]
- 18.Hata M, Kawahara N, Tomita K. Influence of ligation of the internal iliac veins on the venous plexuses around the sacrum. J Orthop Sci 1998; 3: 264–271. [DOI] [PubMed] [Google Scholar]
- 19.Zama N, Fazio VW, Jagelman DG et al. Efficacy of pelvic packing in maintaining hemostasis after rectal excision for cancer. Dis Colon Rectum 1988; 31: 923–928. [DOI] [PubMed] [Google Scholar]
- 20.Cosman BC, Lackides GA, Fisher DP, Eskenazi LB. Use of tissue expander for tamponade of presacral hemorrhage. Report of a case. Dis Colon Rectum 1994; 37: 723–726. [DOI] [PubMed] [Google Scholar]
- 21.McCourtney JS, Hussain N, Mackenzie I. Balloon tamponade for control of massive presacral haemorrhage. Br J Surg 1996; 83: 222. [PubMed] [Google Scholar]
- 22.Ng X, Chiou W, Chang S. Controlling a presacral hemorrhage by using a saline bag: report of a case. Dis Colon Rectum 2008; 51: 972–974. [DOI] [PubMed] [Google Scholar]
- 23.Braley SC, Schneider PD, Bold RJ et al. Controlled tamponade of severe presacral venous hemorrhage: use of a breast implant sizer. Dis Colon Rectum 2002; 45: 140–142. [DOI] [PubMed] [Google Scholar]
- 24.Remzi FH, Oncel M, Fazio VW. Muscle tamponade to control presacral venous bleeding: report of two cases. Dis Colon Rectum 2002; 45: 1,109–1,111. [DOI] [PubMed] [Google Scholar]
- 25.Nivatvongs S, Fang DT. The use of thumbtacks to stop massive presacral hemorrhage. Dis Colon Rectum 1986; 29: 589–590. [DOI] [PubMed] [Google Scholar]
- 26.Sutton GP, Addison WA, Livengood CH, Hammond CB. Life-threatening hemorrhage complicating sacral colpopexy. Am J Obstet Gynecol 1981; 140: 836–837. [DOI] [PubMed] [Google Scholar]
- 27.Timmons MC, Kohler MF, Addison WA. Thumbtack use for control of presacral bleeding, with description of an instrument for thumbtack application. Obstet Gynecol 1991; 78: 313-315. [PubMed] [Google Scholar]
- 28.Patsner B, Orr JW. Intractable venous sacral hemorrhage: use of stainless steel thumbtacks to obtain hemostasis. Am J Obstet Gynecol 1990; 162: 452. [DOI] [PubMed] [Google Scholar]
- 29.Lucarotti ME, Armstrong CP, Bartolo DC. Control of presacral bleeding in rectal surgery. Ann R Coll Surg Engl 1991; 73: 289–290. [PMC free article] [PubMed] [Google Scholar]
- 30.Lacerda-Filho A, Duares LC, Santos HF. Displacement and per-anal extrusion of a hemostatic sacral thumbtack. J Pelvic Med Surg 2004; 10: 319-322. [Google Scholar]
- 31.Suh M, Shaikh JR, Dixon AM, Smialek JE et al. Failure of thumbtacks used in control of presacral hemorrhage. Am J Forensic Med Pathol 1992; 13: 324–325. [DOI] [PubMed] [Google Scholar]
- 32.Stolfi VM, Milsom JW, Lavery IC et al. Newly designed occluder pin for presacral hemorrhage. Dis Colon Rectum 1992; 35: 166–169. [DOI] [PubMed] [Google Scholar]
- 33.der Vurst van TJ, Bodegom ME, Rakic S. Tamponade of presacral hemorrhage with hemostatic sponges fixed to the sacrum with endoscopic helical tackers: report of two cases. Dis Colon Rectum 2004; 47: 1,550–1,553. [DOI] [PubMed] [Google Scholar]
- 34.Germanos S, Bolanis I, Saedon M, Baratsis S. Control of presacral venous bleeding during rectal surgery. Am J Surg 2010; 200: e33-e35. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Chen F, Xie P et al. Combined oxidized cellulose and cyanoacrylate glue in the management of severe presacral bleeding. Surg Today 2009; 39: 1,016–1,017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang CH, Song XM, He YL et al. Use of absorbable hemostatic gauze with medical adhesive is effective for achieving hemostasis in presacral hemorrhage. Am J Surg 2012; 203: e5-e8. [DOI] [PubMed] [Google Scholar]
- 37.Losanoff JE, Richman BW, Jones JW. Cyanoacrylate adhesive in management of severe presacral bleeding. Dis Colon Rectum 2002; 45: 1,118–1,119. [DOI] [PubMed] [Google Scholar]
- 38.Becker A, Koltun L, Shulman C, Sayfan J. Bone cement for control of massive presacral bleeding. Colorectal Dis 2008; 10: 409–410. [DOI] [PubMed] [Google Scholar]
- 39.Civelek A, Yeğen C, Aktan AO. The use of bonewax to control massive presacral bleeding. Surg Today 2002; 32: 944–945. [DOI] [PubMed] [Google Scholar]
- 40.Filippakis GM, Leandros M, Albanopoulos K et al. The use of spray electrocautery to control presacral bleeding: a report of four cases. Am Surg 2007; 73: 410–413. [PubMed] [Google Scholar]
- 41.Kandeel A, Meguid A, Hawasli A. Controlling difficult pelvic bleeding with argon beam coagulator during laparoscopic ultra low anterior resection. Surg Laparosc Endosc Percutan Tech 2011; 21: e21–e23. [DOI] [PubMed] [Google Scholar]
- 42.Li YY, Chen Y, Xu HC et al. A new strategy for managing presacral venous hemorrhage: bipolar coagulation hemostasis. Chin Med J 2010; 123: 3,486–3,488. [PubMed] [Google Scholar]
- 43.Xu J, Lin J. Control of presacral hemorrhage with electrocautery through a muscle fragment pressed on the bleeding vein. J Am Coll Surg 1994; 179: 351–352. [PubMed] [Google Scholar]
- 44.Harrison JL, Hooks VH, Pearl RK et al. Muscle fragment welding for control of massive presacral bleeding during rectal mobilization: a review of eight cases. Dis Colon Rectum 2003; 46: 1,115–1,117. [DOI] [PubMed] [Google Scholar]
- 45.Ayuste E, Roxas MF. Validating the use of rectus muscle fragment welding to control presacral bleeding during rectal mobilization. Asian J Surg 2004; 27: 18–21. [DOI] [PubMed] [Google Scholar]
- 46.Lou Z, Zhang W, Meng RG, Fu CG. Massive presacral bleeding during rectal surgery: from anatomy to clinical practice. World J Gastroenterol 2013; 19: 4,039–4,044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang J, Li X, Wang Y et al. Circular suture ligation of presacral venous plexus to control presacral venous bleeding during rectal mobilization. J Gastrointest Surg 2013; 17: 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Ambra L, Berti S, Bonfante P et al. Hemostatic step-by-step procedure to control presacral bleeding during laparoscopic total mesorectal excision. World J Surg 2009; 33: 812–815. [DOI] [PubMed] [Google Scholar]
- 49.McPartland KJ, Hyman NH. Damage control: what is its role in colorectal surgery? Dis Colon Rectum 2003; 46: 981–986. [DOI] [PubMed] [Google Scholar]


