Abstract
Occludin is an essential integral transmembrane protein regulating tight junction (TJ) integrity in brain endothelial cells. Phosphorylation of occludin is associated with its localization to TJ sites and incorporation into intact TJ assembly. The present study is focused on the role of lipid rafts in polychlorinated biphenyl (PCB)-induced disruption of occludin and endothelial barrier function. Exposure of human brain endothelial cells to 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) induced dephosphorylation of threonine residues of occludin and displacement of occludin from detergent-resistant membrane (DRM)/lipid raft fractions within 1 h. Moreover, lipid rafts modulated the reduction of occludin level through activation of matrix metalloproteinase 2 (MMP-2) after 24 h h PCB153 treatment. Inhibition of protein phosphatase 2A (PP2A) activity by okadaic acid or fostriecin markedly protected against PCB153-induced displacement of occludin and increased permeability of endothelial cells. The implication of lipid rafts and PP2A signaling in these processes was further defined by co-immunoprecipitation of occludin with PP2A and caveolin-1, a marker protein of lipid rafts. Indeed, a significant MMP-2 activity was observed in lipid rafts and was increased by exposure to PCB153. The pretreatment of MMP-2 inhibitors protected against PCB153-induced loss of occludin and disruption of lipid raft structure prevented the increase of endothelial permeability. Overall, these results indicate that lipid raft-associated processes, such as PP2A and MMP-2 activation, participate in PCB153-induced disruption of occludin function in brain endothelial barrier. This study contributes to a better understanding of the mechanisms leading to brain endothelial barrier dysfunction in response to exposure to environmental pollutants, such as ortho-substituted PCBs.
Keywords: 2,2′,4,4′,5,5′-Hexachlorobiphenyl; Occludin; Lipid rafts; Protein phosphatase 2A; Matrix metalloproteinase-2; Brain endothelium
Introduction
Acute and chronic exposure to polychlorinated biphenyls (PCBs) may induce neurotoxicity resulting in morphological changes of neurons, developmental defects of the nervous system, behavioral changes, and learning deficits (Winneke, 2011; Doi et al., 2013; Selvakumar et al., 2013; Lesiak et al., 2014). The blood–brain barrier (BBB) is a highly specialized structural and functional barrier separating the microenvironment of the central nervous system (CNS) from the peripheral circulation by restricting paracellular movement of ions and solutes across the brain endothelium (Dodelet-Devillers et al., 2009). Disruption of the BBB is a common event in the development of several CNS disorders including stroke, septic encephalopathy, brain tumors, and ischemia/reperfusion injury (Forster, 2008; Banks, 2015; Shetty et al., 2014). Tight junctions (TJs) between adjacent brain endothelial cells are the critical structural components of the BBB, and perturbation of their integrity may affect neuronal homeostasis and contribute to neurotoxic effects.
Occludin was the first identified TJ protein and an invariant constituent of all mature epithelial and endothelial TJs (Van Itallie et al., 2010; Cummins, 2012). Although occludin knockout mice can form functional TJ strands, numerous abnormalities including growth retardation, testicular atrophy, calcification in the brain, and gastric inflammation were reported in these mice (Saitou et al., 2000; Schulzke et al., 2005). Overexpression of occludin in Madin–Darby canine kidney (MDCK) cells promoted TJ formation and barrier function (Balda et al., 2000). Furthermore, cytoplasmic TJ proteins such as ZO-1, ZO-2, and ZO-3 directly bind to the cytosolic carboxyl terminal domain of occludin, indicating that occludin anchors these cytoplasmic proteins to the membranes to maintain the intact TJ integrity (Fanning et al., 1998; Morita et al., 1998). Therefore, occludin is generally recognized as a critical regulator in the maintenance of TJ integrity and functions in both endothelial and epithelial cells (Rao, 2009; Dorfel and Huber, 2012).
Accumulating evidence has been reported that occludin is highly phosphorylated on serine and threonine residues at multiple sites in intact TJs, whereas dephosphorylated occludin is localized in the cytoplasm (Fischer et al., 2005; Rao, 2008). The phosphorylation status of these residues appears to be controlled by the balance between phosphorylation by protein kinases such as atypical protein kinase C (PKC), and dephosphorylation by protein phosphatases such as protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) (Seth et al., 2007; Jain et al., 2011). It has been observed that PP1 and PP2A directly interact with the carboxyl terminal tail of occludin and dephosphorylate occludin on the threonine and serine residues in MDCK and Caco-2 epithelial cell cultures (Nunbhakdi-Craig et al., 2002; Seth et al., 2007). Importantly, the molecular alterations of threonine/serine phosphorylation in occludin are associated with the disruption of TJ integrity.
Lipid raft domains are heterogeneous and highly dynamic biological microdomains within plasma membranes. They are composed of cholesterol- and sphingolipid-rich regions and are insoluble in detergents at 4 °C, forming detergent-resistant membrane (DRM) microdomains (Patel and Insel, 2009). Lipid rafts are implicated in a wide range of biological processes such as vesicular transport, regulation of signal transduction, membrane compartmentalization, cytoskeleton rearrangement, and TJ formation (Seegal, 2000; Jacobson et al., 2007). Indeed, these membrane microdomains were reported to be associated with a variety of signaling molecules including PKCs and protein phosphatases, which can control phosphorylation of TJ proteins (Farshori and Kachar, 1999; Han et al., 2010; Jain et al., 2011). Emerging data also indicate that lipid raft/caveolae microdomains are enriched with TJ proteins, and oligomerization of occludin and claudins in these domains is required for the formation of intercellular TJ complexes and barrier function (McCaffrey et al., 2007; Lesiak et al., 2014).
Highly chlorinated ortho-substituted PCBs (ortho-PCBs) have been shown to preferentially accumulate in the brain (Sparling and Safe, 1980; Saghir et al., 2000). One of the most representative ortho-PCBs is 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153), which is commonly found in environmental samples and accounts for 15–30% of the total PCB content in most human samples (Hansen, 1998; Saghir et al., 2000). Previous reports indicated that exposure to ortho-PCBs induced a decrease in TJ proteins and increased permeability across endothelial monolayers and the BBB (Eum et al., 2008; Seelbach et al., 2010; Cai et al., 2013). However, the mechanisms by which exposure to ortho-PCBs exerts TJ disruption are not fully understood.
Due to the role of lipid rafts in the maintenance of TJ integrity and functions, the present study is focused on the hypothesis that signaling pathways associated with lipid rafts modulate ortho-PCB-induced changes in occludin localization and expression in brain endothelial cells. Our data indicate that intact lipid rafts are prerequisites of PCB153-induced displacement and loss of occludin at TJs via PP2A and metalloproteinase-2 (MMP-2)-dependent pathways.
Material and methods
Materials
2,2′,4,4′,5,5′-Hexachlorobiphenyl (PCB153) was purchased from AccuStandard (New Haven, CT) and dissolved in dimethyl sulfoxide (DMSO). Collagen type I was obtained from BD Biosciences (Bedford, MA). Pharmacological inhibitors of PP2A were from Tocris Bioscience (Ellisville, MO). MMP-2 inhibitor II, ARP100 (MMP-2 inhibitor III), GM6001 and GM1489 were obtained from Calbiochem (La Jolla, CA). Anti-actin and anti-GAPDH antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-PP2AC antibody and PureProteome Protein A/G mix Magnetic Beads were purchased from Millipore (Temecular, CA). Anti-occludin antibodies were purchased from Invitrogen (Carmarillo, CA). All other chemicals and reagents, including MβCD and fibronectin, were purchased from Sigma (St. Louis, MO).
Cell cultures and PCB153 treatment
Human brain endothelial cells (hCMEC/D3 cells) were kindly provided by Dr. P.O. Couraud (INSERM, France). hCMEC/D3 cell is an immortalized cell line derived from human brain microvascular endothelial cells of a female patient through co-expression of hTERT and the SV40 large T-antigen (Weksler et al., 2013). This cell line retains most of the morphological and functional characteristics of human brain endothelial cells and has been used as a reliable in vitro model of human BBB (Toth et al., 2014; Chen et al., 2015). In this study, hCMEC/D3 cells were cultured in EBM-2 medium (Lonza, Walkersville, MD) supplemented with EGM-2 Bullet-kit, which contains basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), EGFR, hydrocortisone, ascorbate, gentamycin, and 2.5% fetal bovine serum (FBS). Prior to each experiment, the cells were pre-incubated in serum-free and phenol red-free EBM medium without supplements for at least 12 h.
Stock solution of PCB153 (50 mM) was prepared in DMSO. The same amount of DMSO (<0.02%) was added to control cultures. Serum concentrations of PCBs can reach approximately 3 μM during acute exposure to these toxicants (Wassermann et al., 1979). Therefore, cells were exposed to PCB153 at a concentration range of 1–10 μM in the present study. Similar levels of PCBs were used in our published manuscripts (Eum et al., 2006, 2008). Using the MTT conversion assay, we determined that PCB153 was not cytotoxic at these concentrations over the time course of the reported experiments (data not shown). In selected experiments, hCMEC/D3 cells were pretreated with pharmacological inhibitors against specific signaling molecules for 0.5–1 h prior to treatment with PCB153.
Preparation of detergent-resistant membrane (DRM) fractions
Cell monolayers were washed twice with ice-cold PBS and incubated for 5 min on ice in 0.5% NP-40 lysis buffer (40 mm Tris–HCl, pH 7.4, 0.5% NP-40, 1 mM Na3VO4, 1 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, 1 mM sodium pyrophosphate, and 1× protease inhibitor cocktail [Roche, Indianapolis, IN]). Cell lysates were centrifuged at 3000 ×g for 5 min at 4 °C. The pellets were suspended in NDS lysis buffer (40 mM Tris–HCl, pH 7.4, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 1 mM Na3VO4, 1 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, 1 mM sodium pyrophosphate, and 1× protease inhibitor cocktail) for 1 h at 4 °C and then centrifuged at 12,000×g for 20 min at 4 °C. Protein concentrations were determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL).
Isolation of lipid raft fractions
Detergent-free purification of lipid raft membrane fractions was performed as described previously (Song et al., 1996) with minor modifications. Briefly, cells were solubilized on ice in 1 ml of MES-buffered saline (25 mM MES [morpholineethanesulfonic acid, pH 6.5] in 150 mM NaCl) containing 500 mM Na2CO3. The cell lysate was then homogenized with a loose-fitting Dounce homogenizer and additionally subjected to sonication. The homogenate was centrifuged at 1800 ×g for 10 min at 4 °C and mixed with 1 ml of 90% sucrose in MES-buffered saline. The resulting 45% sucrose cell lysate was overlaid with 5 ml of 35% sucrose and 5 ml of 5% sucrose (w/v in separation buffer), followed by centrifugation for 20 h at 35,000 rpm at 4 °C using a Beckman SW41 rotor. Ten 1.2 ml fractions were collected at the meniscus (top to bottom), and aliquots of each fraction were subjected to SDS-PAGE and immunoblotting to determine the fractions corresponding to lipid rafts and caveolae using flotillin-2 and caveolin-1 as marker proteins.
Immunoblotting and immunoprecipitation
For measurements of occludin level, treated hCMEC/D3 cells were washed and lysed in NDS lysis buffer. The lysates were then centrifuged at 14,000 ×g for 20 min and the supernatants were collected. Protein concentration was determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL). Protein samples were separated on SDS-PAGE and transferred onto a Hybond-ECL membrane (Amersham Biosciences, Piscataway, NJ). Immunoreactive protein bands were visualized with the enhanced chemiluminescence system (Amersham). Caveolin-1 or actin levels were determined as internal standards. The band intensity was determined by the densitometric analysis using Image J program. For co-immunoprecipitation experiments, 500 μg of total protein extract was immunoprecipitated with 1 μg of anti-occludin antibody (Invitrogen, Carmarillo, CA) or 1 μg of anti-phospho-threonine antibody (Santa Cruz Biotech, Dallas, TX) overnight at 4 °C. Then, 20 μl of Protein A/G mix magnetic beads (EMD Millipore, Darmstadt, Germany) was added to each sample, and immunoprecipitation was performed for 2 h at 4 °C. The beads were recovered by centrifugation at 1000 ×g for 5 min at 4 °C, and washed three times with 1 ml of phosphate-buffered saline containing 0.1% Tween 20 (PBS-T). Bound proteins were eluted by boiling in 2× sodium dodecyl sulfate sample buffer for 5 min.
Zymogram/MMP-2 activity assay
MMP-2 activity was determined by zymographic analysis. Briefly, aliquots of 400 μl of the sucrose gradient fractions were concentrated using Nanosep centrifugal device (10 kDa cutoff, Pall Life Sciences, Ann Arbor, MI) and mixed with 2× non-reducing Zymogram sample buffer (Bio-Rad Laboratories). Zymographic analysis was performed by electrophoresis on 10% SDS-polyacrylamide gel containing gelatin for MMP-2. After electrophoresis, SDS was removed by incubating the gel twice with 1× zymogram renaturation buffer and MMP-2 activity was revealed by three days incubation at 37° with zymogram development buffer (both from Bio-Rad), followed by staining with 0.1% Coomassie Brilliant Blue solution.
Changes in total (active and pro-) levels of MMP-2 activities were determined using the AnaSpec SensoLyte 520 MMP-2 Assay Kit (AnaSpec, San Jose, CA), according to the manufacturer's instructions. Following treatment, the total cell lysates were prepared and stored at −70 °C. Pro-MMP-2 was activated with 1 mM p-aminophenylmercuric acetate (APMA). The kit uses a 5-FAM/QXL520 fluorescence resonance energy transfer (FRET) peptide as an MMP-2 substrate. In the intact FRET peptide, the fluorescence of 5-FAM was quenched by QXL520. Upon cleavage into separate fragments by MMP-2, fluorescence intensity was measured at 490/520-nm using a microplate spectrofluorometer (SPECTRAMax Gemini EM).
Permeability assay
hCMEC/D3 cells (1 × 106 cells/insert) were seeded on fibronectin-coated Transwell polyester filter insert (12 mm diameter, 0.4 μm pore size, Corning Costar) and allowed to grow to confluence. PP2A inhibitors were added to both the lower and the upper compartments of the Transwell system 30 min prior to exposure to PCB153 for 1 h. After the cultures were rinsed with phenol red-free EBM medium, 0.5 ml of FITC-dextran 70 (70 kDa) or FITC-dextran 20 (20 kDa) (0.5 mg/ml in phenol red-free EBM medium) was loaded into the upper chamber. The system was incubated for 60 min at 37 °C in humidified atmosphere (5% CO2), and the assay was stopped by removing the upper chambers. Aliquots (100 μl) from the lower chambers were transferred to new wells of 96-well fluorescence plate, and the fluorescence intensity of FITC-dextran was determined with a microplate spectrofluorometer (Molecular Devices SPECTRA-Max Gemini EM) using 490 nm and 520 nm as excitation and emission wavelengths, respectively. Relative permeability was expressed by the ratio of FITC-dextran transported into the lower chamber compared to the untreated-control group. All assays were performed at least in triplicate.
Animals and PCB153 administration
All animal protocols in this study were approved by the Institutional Committee on Animal Care and abide by the NIH guidelines. Male C57BL/6 mice (12–14 weeks of age; Charles River Laboratories, Wilmington, MA) were housed under 12 h light:12 h dark conditions with free access to food and water ad libitum. Mice were fasted overnight prior to PCB153 treatment (150 μmol/kg). The 150 μmol/kg dose of PCB153 results in 5 μM PCB153 level in the plasma, reflecting the concentration that was used in vitro previously (Choi et al., 2010). PCB153 congener was dissolved in tocopherol-stripped safflower oil (Dyets Inc., Bethlehem, PA) and administered in a 0.1-ml volume via oral gavage using an 18-gauge gavage needle, 3 inch long, curved, 2.25 mm ball diameter (Popper and Sons, New Hyde Park, NY). Control animals received safflower oil as vehicle. 25 mg/kg dose of SB-3CT (general MMP inhibitor) was treated twice through intraperitoneal injection at 0.5 h prior to PCB153 administration and at 24 h post PCB153 administration. Mice were sacrificed for brain microvessel isolation at 48 h after PCB153 or vehicle treatment.
Isolation of brain microvessels and Immunofluorescence
Isolation of brain microvessels and immunofluorescence detection of occludin in isolated microvessels were performed as described earlier (Choi et al., 2012). Briefly, after removal of the choroid plexus, meninges, cerebellum, and brainstem, brains were homogenized in buffer containing 103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15 mM HEPES, 25 mM NaHCO3, 10 mM glucose, 1 mM Na pyruvate, and 10 g/l dextran. Following homogenization, the samples were mixed with 26% dextran, centrifuged at 5800 ×g for 20 min, and filtered through a 70-μm mesh filter. Following isolation, microvessels were spread onto glass slides, heat-immobilized at 95 °C for 10 min, and fixed with 4% formaldehyde in PBS for 10 min. Microvessels were permeabilized with 0.1% Triton X-100 for 30 min and incubated with anti-occludin antibody overnight at 4 °C. Occludin was visualized with a Texas Red-conjugated secondary antibody.
Statistical analysis
Results are expressed as mean ± S.D. Comparisons among treatments were made by analysis of variance (ANOVA) test followed by post hoc Tukey's pairwise comparison procedure. A statistical significance level was set at two-sided alpha = 0.05.
Results
Exposure to PCB153 displaces occludin from TJ assembly
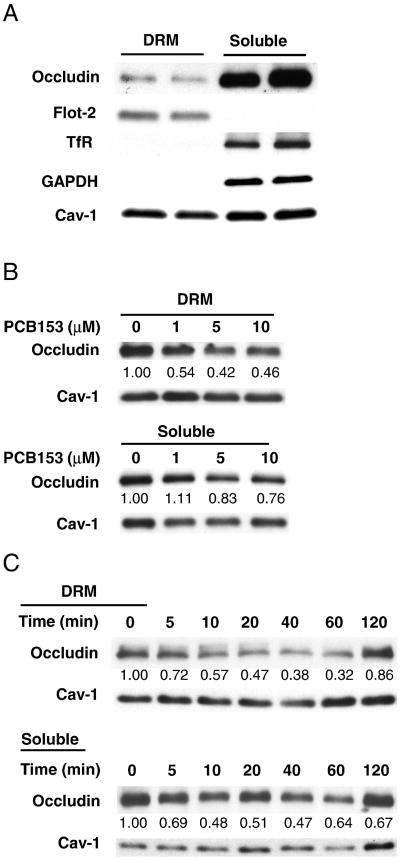
Occludin is known to be located in the DRM domains in intercellular tight junction complexes of endothelial or epithelial cells (Rao, 2008; Lesiak et al., 2014). Therefore, detergent insolubility of occludin is commonly used as an indicator of protein incorporation into intact TJs. The purity of DRM fractions were identified by the presence of flotillin-2 and caveolin-1 as lipid raft marker proteins, and confirmed by the absence of both transferrin receptor and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a non-lipid raft transmembrane protein and a cytosolic protein, respectively (Fig. 1A). To assess short-term changes of occludin, cells were exposed to PCB153 at the indicated concentrations for 60 min, followed by isolation of the NP-40 insoluble (i.e., the DRM) and soluble fractions and determination of occludin levels by immunoblotting. As shown in Fig. 1B, exposure to PCB153 markedly and dose-dependently reduced occludin levels in the DRM fractions. Caveolin-1 was used in these experiments as an internal standard for protein expression analysis. In addition, exposure to 5 μM PCB153 resulted in a time-dependent decrease of occludin in the DRM fractions and NP-40-soluble fractions within 1 h after PCB153 treatment and occludin level was recovered at 120 min (Fig. 1C).
Fig. 1.
PCB153 induces loss of occludin from tight junction assembly. (A) Detergent-resistant membrane fractions (DRM fractions) and detergent-soluble fractions (soluble fractions) of hCMEC/D3 cells were prepared based on solubility in 0.5% NP-40 as described in the Material and methods section. Both fractions were processed for immunoblotting and probed for occludin, flotillin-2 (flot-2, a lipid raft marker), transferrin receptor (TfR, a non-lipid raft transmembrane protein), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, a cytosolic protein) and caveolin-1 (Cav-1) expression. (B) hCMEC/D3 cells were incubated with PCB153 at the indicated concentrations for 60 min. The level of occludin in DRM or detergent-soluble fractions was assessed by immunoblotting. Caveolin-1 levels were used as an internal standard. (C) Cells were exposed to 5 μM PCB153 for the indicated time periods, followed by occludin determination in DRM and detergent-soluble fractions. The blots are representative from at least three experiments. The band intensity was determined by the densitometric analysis using Image J program.
PP2A mediates PCB153-induced dephosphorylation of occludin and loss of occludin from DRM fractions
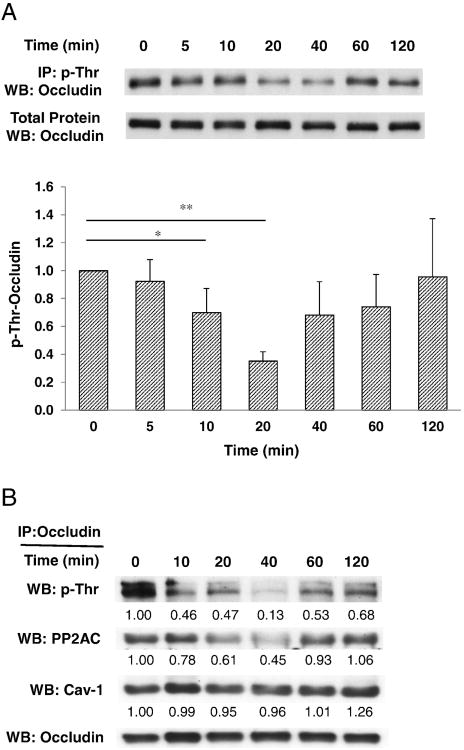
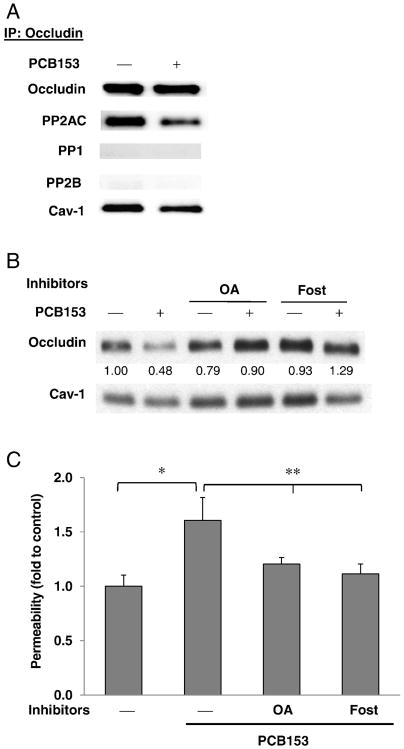
Highly phosphorylated threonine and serine residues and/or low levels of phosphotyrosine are characteristic features of occludin localized at intact TJs, promoting the barrier functions of endothelial and epithelial cell monolayers (Forster, 2008; Rao, 2009). During the disruption of tight junctions, occludin is dephosphorylated on serine and threonine residues. Using immunoprecipitation analysis with anti-phosphothreonine antibody or anti-occludin antibody, dephosphorylation of threonine residues of occludin was confirmed at 10–60 min after PCB153 treatment (Fig. 2A and B). Phosphorylation of occludin is modulated by several kinases and protein phosphatases such as c-Src, PP1, PP2A, and PP2B. Among these enzymes, PP2AC (a catalytic subunit of PP2A) is abundantly co-immunoprecipitated with occludin (Figs. 2B and 3A). In contrast, level of PP1 and PP2B were low or undetectable in occludin immunoprecipitates of total cell lysates in both normal and PCB153-treated human brain endothelial cells (Fig. 3A). To address a question whether protein phosphatase 2A (PP2A) is involved in PCB153-induced loss of occludin from the DRMs, confluent hCMEC/D3 cultures were pretreated for 1 h with pharmacological inhibitors (okadaic acid or fostriecin) against PP2A followed by exposure to 5 μM PCB153 for 1 h. PP2A inhibitors prevented PCB153-induced loss of occludin in the DRM microdomains (Fig. 3B). Moreover, PP2A inhibitors attenuated the increased permeability of hCMEC/D3 monolayers after 1 h treatment of PCB153, as determined by FITC dextran 70 (70 kDa) (Fig. 3C).
Fig. 2.
PCB153 induces dephosphorylation on threonine residues of occludin. (A) Confluent hCMEC/D3 cells were exposed to 5 μM PCB153 for the indicated time periods. The level of phosphothreonine of occludin in total cell lysates was measured by immunoprecipitation with anti-phosphothreonine antibody in total cell lysates, followed by immunoblotting for occludin. The occludin levels in total cell lysates were assessed as a loading control. Values (means ± SD) are expressed as fold change compared to control (0 min); n = 4. *p < 0.05 vs control and **p < 0.01 vs control. (B) Confluent hCMEC/D3 cells were exposed to 5 μM PCB153 for the indicated time periods. Occludin in total cell lysates was isolated using immunoprecipitation with anti-occludin antibody. The level of phosphothreonine of occludin was assessed with anti-phosphothreonine antibody and the co-immunoprecipitation of a catalytic subunit of PP2A (PP2AC) and caveolin-1 (Cav-1) with occludin was determined by immunoblotting with anti-PP2AC antibody and anti-caveolin-1 antibody. Occludin levels were assessed as an internal standard.
Fig. 3.
PP2A mediates PCB153-induced displacement of occludin and disruption of barrier function. (A) Confluent hCMEC/D3 cultures were treated for 1 h with 5 μM PCB153, followed by immunoprecipitation with anti-occludin antibody and immunoblotting for PP2AC, protein phosphatase 1 (PP1), protein phosphatase 2B (PP2B), and caveolin-1 (Cav-1). (B) Confluent hCMEC/D3 cultures were pretreated for 1 h with PP2A inhibitors (okadaic acid [10 nM, OA] or fostriecin [100 nM, Fost]) followed by exposure to 5 μM PCB153 for 1 h. The level of occludin in the DRM fraction was evaluated as in Fig. 1. (C) Confluent hCMEC/D3 cultures on the upper side of fibronectin-coated Transwell inserts were pretreated with OA or Fost and exposed to PCB153 for 1 h as in B. Then, integrity of hCMEC/D3 monolayers was assessed using FITC-dextran (70 kDa) as the permeability marker. Values are mean ± SD, n = 4, *p < 0.05 when compared to control cells (vehicle treated cells), **p < 0.01 when compared to PCB153 treated cells.
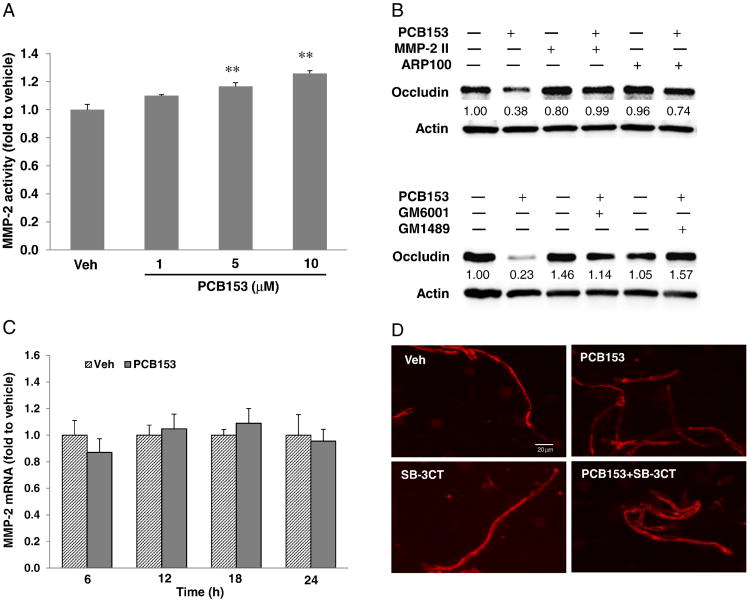
MMP-2 mediates PCB153-induced reduction of occludin
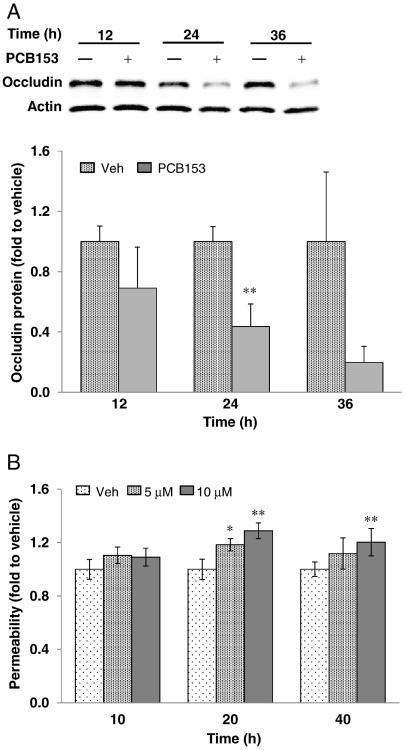
In addition to the displacement of occludin from the DRM and dephosphorylation of occludin threonine residues within 1 h, longer exposure to PCB153 markedly decreased total occludin levels in the cells at 24 h and 36 h after treatment (Fig. 4A), which were accompanied with the increase of permeability dose- and time-dependently (Fig. 4B). Matrix metalloproteinases (MMPs) including MMP-2 are known to play an important role in the loss of occludin in epithelial cells and endothelial cells (Lu et al., 2013; Yang et al., 2013; Eum et al., 2014). Therefore, a potential involvement of MMPs in PCB153-induced loss of occludin was addressed in the present study. Exposure to PCB153 induced dose-dependent increases of MMP-2 activity at 20 h post treatment (Fig. 5A) without significant changes in mRNA levels of MMP-2 under the employed experimental conditions (Fig. 5C). The contribution of MMP-2 to PCB153-induced loss of occludin was further evaluated using general MMP inhibitors (GM6001, GM1489), or specific pharmacological inhibitors against MMP-2 (MMP-2 inhibitor II, ARP100). Cells were pretreated for 1 h with these MMP inhibitors, followed by exposure to 5 μM PCB153 for 20 h. MMP inhibitors were effective in protecting against PCB153-induced loss of occludin (Fig. 5B). Consistent with the results of in vitro cell culture experiments, oral exposure of mouse to PCB153 for 48 h decreased the immunofluorescence reactivity of occludin in brain microvessels and MMP-2 inhibition by SB-3CT attenuated the loss of immunoreactivity of occludin (Fig. 5D).
Fig. 4.
PCB153 decreases the expression of occludin. (A) Confluent hCMEC/D3 cells were treated with 5 μM PCB153 for the indicated time periods. The levels of occludin in total cell lysates were assessed by immunoblotting and the occludin levels in PCB153-treated cells were compared to that of vehicle-treated cells at the indicated time point. Actin levels were assessed as an internal standard. The blots are representative from three experiments. (B) Confluent hCMEC/D3 cultures on the upper side of Transwell inserts were exposed to 5 μM or 10 μM PCB153 for the indicated time periods. Then, integrity of hCMEC/D3 monolayers was assessed using FITC-dextran (20 kDa) as the permeability marker. Values are mean ± SD, n = 4, *p < 0.05 and **p < 0.01 when compared to control cells (vehicle treated cells).
Fig. 5.
PCB153 induces loss of occludin through MMP-2 activation. (A) Cells were incubated with PCB153 at the indicated concentration for 20 h, and then MMP-2 activity was determined using activity assay kits as described in the Material and methods section. Values are mean ± SD, n = 4, *p < 0.05 and **p < 0.01 when compared to control cells (vehicle treated cells). (B) Cells were pretreated with specific MMP-2 inhibitors (2 μM MMP-2 inhibitor II (MMP-2II) or 2 μM ARP100) or general MMP inhibitors (2 μM GM6001 or 2 μM GM1489) for 1 h prior to exposure to PCB153 at 5 μM for 24 h. Controls were incubated with vehicle (0.05% DMSO). Expression of occludin was assessed by immunoblotting. Representative blots of three independent experiments. (C) The level of MMP-2 mRNA was assessed by real-time RT-PCR in hCMEC/D3 cells treated with 5 μM PCB153 for the indicated time periods. Values are mean ± SD, n= 4. (D) Mice were exposed to PCB153 (150 μmol/kg) via oral gavage. 25 mg/kg dose of SB-3CT (general MMP inhibitor) was treated twice through intraperitoneal injection at 0.5 h prior to PCB153 administration and at 24 h post PCB153 administration. The mice were sacrificed for the isolation of brain microvessels at 48 h after PCB153 or vehicle treatment. Occludin immunoreactivity was stained in red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
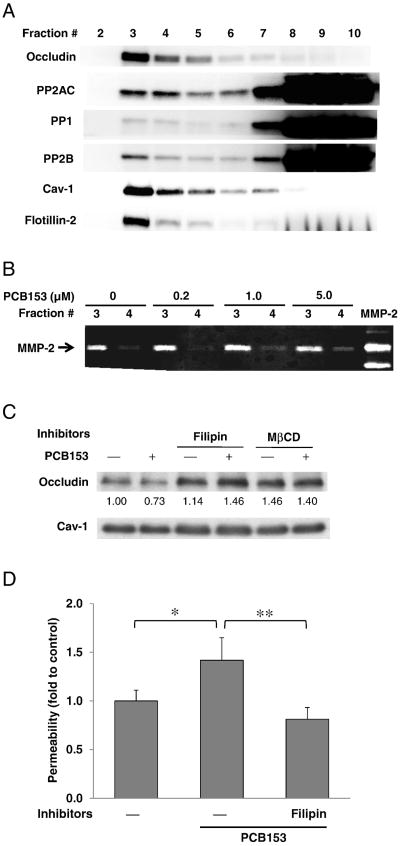
Intact lipid rafts are prerequisites for PCB153-induced occludin loss and endothelial barrier disruption
Lipid raft domains were reported to be the platforms for occludin oligomerization and incorporation into TJ assembly (McCaffrey et al., 2009). Therefore, we next investigated whether lipid rafts are required for PCB153-induced disruption of occludin function. Lipid rafts/caveolae were isolated using a detergent-free sucrose gradient ultracentrifugation method and were confirmed by the presence of caveolin-1 and flotillin-2 as the marker proteins. Lipid raft fractions were present at the interface of 5% and 35% sucrose concentrations that preferentially corresponded to fractions 3 and 4 in Fig. 6A. The majority of occludin was found in lipid raft fractions of brain endothelial cells. Although protein phosphatases (namely, PP1, PP2A, and PP2B) were predominantly present in non-lipid raft domains, considerable level of PP2A was also detected in lipid raft fractions (Fig. 6A). Moreover, prominent MMP-2 activity in lipid raft fractions was detected by zymography (Fig. 6B). To evaluate whether lipid raft domains contribute to loss of occludin in response to PCB153, hCMEC/D3 cells were pretreated for 1 h with Filipin III or methyl-β-cyclodextrin (MβCD) which disrupts lipid raft structures. This procedure effectively prevented PCB153-induced loss of occludin in the DRM (Fig. 6C). Importantly, disruption of lipid rafts by pretreatment with Filipin III attenuated an increase in permeability across human brain endothelial monolayers exposed to PCB153 (Fig. 6D). These observations suggest that functional lipid raft membrane domains are required for PCB153-induced displacement of occludin from TJ and disruption of endothelial barrier function.
Fig. 6.
Intact lipid rafts are prerequisites for PCB153-induced disruption of endothelial barrier function. (A) Confluent hCMEC/D3 cells were fractionated using a detergent-free sucrose ultracentrifugation method, followed by immunoblotting to determine the presence of occludin, PP2AC, PP1, and PP2B. Flotillin-2 and caveolin-1 (Cav-1) were determined as marker proteins of lipid rafts/caveolae (primarily fractions 3 and 4). (B) hCMEC/D3 cells were exposed to PCB153 at indicated concentration for 12 h, and then were fractionated using a detergent-free sucrose ultracentrifugation method. The fractions 3 and 4, which correspond to lipid rafts, were concentrated as described in the Material and methods section. MMP-2 activity was determined by gelatin zymogram. (C) Confluent hCMEC/D3 cells were pretreated for 1 h with Filipin III (5 μg/ml, Filipin) or methyl-β-cy-clodextrin (MβCD) at 0.5 mg/ml to disrupt lipid rafts, followed by exposure to 5 μM PCB153 for 1 h. Occludin expression in the DRM fractions were evaluated by immunoblotting. (D) Confluent hCMEC/D3 cultures on the upper side of Transwell inserts were pretreated with Filipin III (2.5 μg/ml, Filipin) for 1 h and exposed to PCB153 for 18 h. Then, integrity of hCMEC/D3 monolayers was assessed using FITC-dextran (20 kDa) as the permeability marker. Values are mean ± SD, n = 4, *p < 0.05 when compared to control cells (vehicle treated cells), **p < 0.01 when compared to PCB153 treated cells.
Discussion
Blood–brain barrier (BBB) is a physical and metabolic barrier which separates the microenvironments of central nervous system (CNS) from the peripheral circulation to protect homeostasis of CNS (Rubin and Staddon, 1999; Banks, 2015). Brain endothelial cells are fundamental components of the BBB and TJ formation between brain endothelial cells are largely responsible for the barrier function of the BBB (Forster, 2008). We previously reported that exposure to specific PCB congeners can alter expression of TJ proteins and lead to permeability of the endothelial and epithelial cells in in vivo mouse model and in vitro cell cultures (Eum et al., 2008; Choi et al., 2010, 2012; Seelbach et al., 2010; Zhang et al., 2012). Moreover, it is important to note that exposure to ortho-substituted PCBs induces transmigration of inflammatory cells or tumor cells across the endothelial cells (Sipka et al., 2008; Eum et al., 2009), which can exaggerate the disruption of TJ integrity in the brain endothelial cells. However, the molecular mechanisms by which PCB congeners disrupt TJ barrier functions are not fully understood.
Results of the present study indicate that lipid raft domains play a key role in PCB153-induced disruption of occludin function in TJ integrity through PP2A-dependent mechanisms in the early stage and through MMP-2-mediated mechanisms in the late stage. Short-term exposure to PCB153 reduced occludin levels in the DRM fractions of brain endothelial cells and dephosphorylated threonine residues of occludin within less than 1 h. In addition, total protein expression of occludin in brain endothelial cells was markedly decreased after relative long-term exposure to PCB153 (i.e. 24 h and 36 h). The alteration of occludin levels was accompanied with the increase in permeability across brain endothelial monolayers after both short-time and long-time exposure. Detergent insolubility of TJ proteins is commonly used as an indicator of protein incorporation into intact TJ assemblies (Lesiak et al., 2014). Thus, our results suggest that PCB153 can acutely induce the displacement of occludin from TJ complexes through lipid raft-mediated PP2A-dependent mechanism, which might be implicated in disassembly of TJs and the disruption of the barrier functions of brain endothelial cells. These results are in line with reports indicating that the displacement of occludin from the DRM fractions is accompanied by TJ disassembly and increases paracellular permeability in response to various stimuli, including exposure to cytokines, inflammation, and oxidative stress (Rao, 2008; McCaffrey et al., 2009; Sheth et al., 2009).
Several lines of evidence highlight the importance of occludin phosphorylation status in the incorporation of occludin into the TJ integrity. For example, dephosphorylation of threonine/serine residues by protein phosphatases is associated with occludin redistribution from intact TJs as it was shown in the models of calcium depletion, bacterial infection, or glutamate treatment (Simonovic et al., 2000; Andras et al., 2007). In contrast, phosphorylation of tyrosine residues by protein kinases can induce delocalization of occludin from TJs and destabilization of TJ integrity (Elias et al., 2009; Rao, 2009). Along with co-localization of occludin and protein phosphatases in lipid raft domains, the result of occludin immunoprecipitation in this study demonstrated that occludin in brain endothelial cells formed a complex with PP2A and caveolin-1. The level of PP2A in occludin immunoprecipitates was corresponding to the phosphorylation status of occludin threonine residues. This observation suggests that PP2A directly binds occludin in the lipid raft domains and activation of PP2A induces dephosphorylation of threonine residues and displacement of occludin from TJ assemblies. Indeed, pretreatment of the cells with okadaic acid and fostriecin, both well-established pharmacological inhibitors of PP2A, efficiently protected against occludin displacement from the DRM fractions of human brain endothelial cells. These results are in agreement with previous reports (Fonnum and Mariussen, 2009; Han et al., 2010) demonstrating that activation of microvascular endothelial cells by septic insult stimulated PP2A-dependent dephosphorylation of TJ-associated occludin and decreased occludin levels at the cell-cell borders. PP1 was shown to modulate phosphorylation levels of TJ proteins (Seth et al., 2007; Sallee and Burridge, 2009). Occludin immunoprecipitates in this study contained PP1 at low but detectable level and PP1 was present in lipid raft domains of human brain endothelial cells. Therefore, we cannot exclude the possibility that PP1 also contributes the displacement of occludin from TJ complex through modification of occludin phosphorylation.
We have previously reported that ortho-substituted PCBs including PCB153 and PCB118, induced the disruption of TJ integrity and the loss of TJ proteins including ZO-1, ZO-2, occludin and claudin-5 in brain microvessels (Choi et al., 2012; Zhang et al., 2012, 2013). In addition, exposure of hCMEC/D3 cells to PCB congeners including PCB153 for 24 h induced the loss of selective TJ proteins, such as ZO-1, ZO-2, and AF6, in isolated plasma membrane fractions of the cells and total cell lysates without significant changes of mRNA levels of these TJ proteins (Eum et al., 2008). These results suggested the possibility that PCBs disrupt TJ integrity through degradation of TJ proteins in brain endothelial cells. However, the underlying mechanisms of PCB-induced TJ disruption are not clear.
Besides that short-time exposure to PCB153 induced PP2A-mediated loss of occludin in the DRM fractions accompanied with the increase of permeability across human brain endothelial cells, the results of this study showed that PCB153 treatment for more than 24 h remarkably reduced the levels of total occludin and significantly increased the permeability through lipid raft/MMP-2-dependent mechanisms. We observed that exposure to PCB153 induced dose-dependent increase in MMP-2 activities without the changes of MMP-2 mRNA level. In addition, the inhibition of MMP-2 activity using specific MMP-2 inhibitors or general MMP inhibitors protected against PCB153-induced reduction of total occludin levels in brain endothelial cells. Consistent with the results of in vitro cell culture experiments, oral administration of PCB153 diminished the immunoreactivity of occludin in mouse brain microvessels. MMP-2 inhibition by treatment of SB-3CT alleviated PCB153-induced loss of occludin in mouse brain microvessels. The results of the present studies suggest that exposure to PCB153 induces occludin degradation through MMP-2 activation in brain endothelia cells. MMP-2 (gelatinase A) is a member of MMPs which are zinc-dependent endopeptidases involved in the remodeling of extracellular matrix under physiological and pathological conditions (Lu et al., 2013). Recent observations indicated that MMPs can degrade TJ proteins such as occludin, ZO-1 and claudins, resulting in impairment of the barrier function in endothelial and epithelial cells (Naito and Yoshikawa, 2005; Lischper et al., 2010). For example, MMP-2 mediated degradation of TJ proteins including occludin and claudin-5 in the BBB during ischemia and reperfusion injury (Liu et al., 2012; Lu et al., 2013).
The presence of caveolin-1, a key component of caveolae, in occludin immunoprecipitates indicates the direct interaction between occludin and caveolae domains. Tight junction disruption in endothelial or epithelial cells can be regulated by vesicle trafficking processes including caveolae-mediated endocytosis (Shen and Turner, 2005; Schwarz et al., 2007). Caveolae has been demonstrated to modulate TNF-α-induced occludin loss from TJs through myosin light chain kinase-dependent caveolae endocytosis (Wang et al., 2005; Marchiando et al., 2010). In addition, MβCD and Filipin III, used in this study can disrupt the structure and function of both caveolae and lipid rafts. Therefore, we cannot exclude the possibility that lipid rafts/caveolae-mediated endocytosis contributed, at least in part, to PCB153-induced loss of occludin in human brain endothelial cells. Moreover, while the results of the present study and our previous reports suggest that paracellular transport by TJ disruption is critical in PCB-induced increase in permeability (Eum et al., 2008; Choi et al., 2012), it should note that alterative mechanisms such as caveolae-mediated transcytosis or micropinocytosis can be implicated.
While cell membrane lipid raft domains are integral parts of the TJ spatial assemblies (Dodelet-Devillers et al., 2009; Li et al., 2009; Eum et al., 2014), lipid raft domains also form signaling platforms to trigger redox-signaling cascades. Specifically, membrane lipid rafts in endothelial cells serve as a platform to recruit and activate NADPH oxidase systems, causing cellular oxidative stress (Patel and Insel, 2009). Oxidative stress induced by hydrogen peroxide or ischemia/reperfusion resulted in altered occludin phosphorylation and activation of MMP-2, resulting in occludin cleavage in brain microvascular endothelial cells (Lischper et al., 2010; Liu et al., 2012). We previously reported that exposure to PCB153 increased oxidative stress through lipid raft-mediated activation of NADPH oxidases and related signaling molecules including Src kinases, EGFR and JAK kinases (Eum et al., 2009; Choi et al., 2010), which are known to modulate activity of PP2A or MMP-2 (Park et al., 2006; Fedida-Metula et al., 2012). In addition, intact lipid raft structures were required for MMPs-mediated occludin degradation by a bacterial quorum sensing molecule, C12-homoserine lactone, in intestinal epithelial cells (Eum et al., 2014). These observations suggest the possibility that lipid raft-mediated redox-signaling modulates PCB153-induced occludin alteration through activation of PP2A and MMP-2.
Recently, ortho-substituted PCBs including PCB153 have been acknowledged as potential ligands for ryanodine receptors (RyRs), which can mediate ortho-substituted PCB-induced perturbation in cellular Ca2+ signaling. Due to the fundamental roles of Ca2+ signaling in cell homeostasis, cellular metabolism, proliferation, gene expression and modulation of cell responses (Pessah et al., 2010), activation of RyR-mediated Ca2+ signaling mechanisms can be, at least in part, responsible for PCB toxicity on endothelial cells.
In summary, our results indicate that lipid raft domains modulate PCB153-induced disruption of brain endothelial barrier function through PP2A-mediated displacement of occludin from intact TJs in early stage and MMP-2-mediated reduction of occludin level in late stage after exposure to PCB153.
Acknowledgments
This study was supported in part by the American Heart Association (09SDG2300037) and the NIH grants (P42 ES 07380, MH63022, MH072567, and NS39254).
Abbreviations
- PCB153
2,2′,4,4′,5,5′-hexachlorobiphenyl
- PCBs
polychlorinated biphenyls
- TJ
tight junction
- DRM
detergent-resistant membrane
- PP2A
protein phosphatase 2A
- MMP
matrix metalloproteinase
- BBB
blood–brain barrier
- MβCD
methyl-beta-cyclodextrin
- FBS
fetal bovine serum
- CNS
central nervous system
Footnotes
Transparency document: The Transparency document associated with this article can be found, in the online version.
References
- Andras IE, Deli MA, Veszelka S, Hayashi K, Hennig B, Toborek M. The NMDA and AMPA/KA receptors are involved in glutamate-induced alterations of occludin expression and phosphorylation in brain endothelial cells. J Cereb Blood Flow Metab. 2007;27:1431–1443. doi: 10.1038/sj.jcbfm.9600445. [DOI] [PubMed] [Google Scholar]
- Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. [PubMed] [Google Scholar]
- Banks WA. The blood–brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Wang C, Huang L, Chen M, Zuo Z. A novel effect of polychlorinated biphenyls: impairment of the tight junctions in the mouse epididymis. Toxicol Sci. 2013;134:382–390. doi: 10.1093/toxsci/kft106. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu W, Wang P, Xue Y, Su Q, Zeng C, Shang X. Endophilin-1 regulates blood–brain barrier permeability via EGFR-JNK signaling pathway. Brain Res. 2015;1606:44–53. doi: 10.1016/j.brainres.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect. 2010;118:976–981. doi: 10.1289/ehp.0901751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Choi YJ, Chen L, Zhang B, Eum SY, Abreu MT, Toborek M. Lipopoly-saccharide potentiates polychlorinated biphenyl-induced disruption of the blood–brain barrier via TLR4/IRF-3 signaling. Toxicology. 2012;302:212–220. doi: 10.1016/j.tox.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodelet-Devillers A, Cayrol R, van Horssen J, Haqqani AS, de Vries HE, Engelhardt B, Greenwood J, Prat A. Functions of lipid raft membrane microdomains at the blood–brain barrier. J Mol Med. 2009;87:765–774. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- Doi H, Nishitani S, Fujisawa TX, Nagai T, Kakeyama M, Maeda T, Shinohara K. Prenatal exposure to a polychlorinated biphenyl (PCB) congener influences fixation duration on biological motion at 4-months-old: a preliminary study. PLoS One. 2013;8:e59196. doi: 10.1371/journal.pone.0059196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Rha GB, Hennig B, Toborek M. c-Src is the primary signaling mediator of polychlorinated biphenyl-induced interleukin-8 expression in a human microvascular endothelial cell line. Toxicol Sci. 2006;92:311–320. doi: 10.1093/toxsci/kfj194. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras IE, Couraud PO, Hennig B, Toborek M. PCBs and tight junction expression. Environ Toxicol Pharmacol. 2008;25:234–240. doi: 10.1016/j.etap.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Andras I, Hennig B, Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol Appl Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Jaraki D, Bertrand L, Andras IE, Toborek M. Disruption of epithelial barrier by quorum-sensing N-3-(oxododecanoyl)-homoserine lactone is mediated by matrix metalloproteinases. Am J Physiol Gastrointest Liver Physiol. 2014;306:G992–G1001. doi: 10.1152/ajpgi.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012;33:740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol. 2005;84:687–697. doi: 10.1016/j.ejcb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J Neurochem. 2009;111:1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, Wilson JX. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radic Biol Med. 2010;48:128–135. doi: 10.1016/j.freeradbiomed.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect. 1998;106(Suppl. 1):171–189. doi: 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Czeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J. 2011;437:289–299. doi: 10.1042/BJ20110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci. 2014;34:717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang Q, Wang C, Liu X, Qu L, Gu L, Li N, Li J. Altered distribution of tight junction proteins after intestinal ischaemia/reperfusion injury in rats. J Cell Mol Med. 2009;13:4061–4076. doi: 10.1111/j.1582-4934.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–127. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood–brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Suofu Y, Guan F, Broderick JP, Wagner KR, Clark JF. Matrix metalloproteinase-2 deletions protect against hemorrhagic transformation after 1 h of cerebral ischemia and 23 h of reperfusion. Neuroscience. 2013;253:361–367. doi: 10.1016/j.neuroscience.2013.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, II, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood–brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood–brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110:58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–866. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Asp Med. 2005;26:379–390. doi: 10.1016/j.mam.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS, Lee SH, Park IC, Rhee CH, Hong SI. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66:8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- Patel HH, Insel PA. Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid Redox Signal. 2009;11:1357–1372. doi: 10.1089/ars.2008.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Saghir SA, Hansen LG, Holmes KR, Kodavanti PR. Differential and non-uniform tissue and brain distribution of two distinct 14C-hexachlorobiphenyls in weanling rats. Toxicol Sci. 2000;54:60–70. doi: 10.1093/toxsci/54.1.60. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee JL, Burridge K. Density-enhanced phosphatase 1 regulates phosphorylation of tight junction proteins and enhances barrier function of epithelial cells. J Biol Chem. 2009;284:14997–15006. doi: 10.1074/jbc.M901901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF. The neurotoxicological consequences of developmental exposure to PCBs. Toxicol Sci. 2000;57:1–3. doi: 10.1093/toxsci/57.1.1. [DOI] [PubMed] [Google Scholar]
- Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt blood–brain barrier integrity and promote brain metastasis formation. Environ Health Perspect. 2010;118:479–484. doi: 10.1289/ehp.0901334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar K, Bavithra S, Ganesh L, Krishnamoorthy G, Venkataraman P, Arunakaran J. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: anxiolytic-like effects of quercetin. Toxicol Lett. 2013;222:45–54. doi: 10.1016/j.toxlet.2013.06.237. [DOI] [PubMed] [Google Scholar]
- Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth P, Samak G, Shull JA, Seth A, Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J. 2009;421:59–70. doi: 10.1042/BJ20081951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Mishra V, Kodali M, Hattiangady B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci. 2014;8:232. doi: 10.3389/fncel.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, Toborek M. Oral administration of PCBs induces proinflammatory and prometastatic responses. Environ Toxicol Pharmacol. 2008;25:251–259. doi: 10.1016/j.etap.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Sparling J, Safe S. The effects of ortho chloro substituents on the retention of PCB isomers in rat, rabbit, Japanese quail, guinea pig and trout. Toxicol Lett. 1980;7:23–28. doi: 10.1016/0378-4274(80)90080-6. [DOI] [PubMed] [Google Scholar]
- Toth AE, Toth A, Walter FR, Kiss L, Veszelka S, Ozsvari B, Puskas LG, Heimesaat MM, Dohgu S, Kataoka Y, Rakhely G, Deli MA. Compounds blocking methylglyoxal-induced protein modification and brain endothelial injury. Arch Med Res. 2014;45:753–764. doi: 10.1016/j.arcmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci. 2010;123:2844–2852. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci. 2011;308:9–15. doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, Yang Y, Estrada EY, Rosenberg GA. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chen L, Choi JJ, Hennig B, Toborek M. Cerebrovascular toxicity of PCB153 is enhanced by binding to silica nanoparticles. J Neuroimmune Pharmacol. 2012;7:991–1001. doi: 10.1007/s11481-012-9403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Choi JJ, Eum SY, Daunert S, Toborek M. TLR4 signaling is involved in brain vascular toxicity of PCB153 bound to nanoparticles. PLoS One. 2013;8:e63159. doi: 10.1371/journal.pone.0063159. [DOI] [PMC free article] [PubMed] [Google Scholar]