Abstract
In November 1955 Geoffrey Harris published a paper based on the Christian A. Herter Lecture he had given earlier that year at Johns Hopkins University in Baltimore. The paper reviewed the contemporary research that was starting to explain how the hypothalamus controlled the pituitary gland. In the process of doing this Harris introduced a set of properties that would help define the neuroendocrine hypothalamus. They included: a) three criteria that putative releasing factors for adenohypophysial hormones would have to fulfill; b) an analogy between the representation of body parts in sensory and motor cortices and the spatial localization of neuroendocrine function in the hypothalamus; and c) the idea that neuroendocrine neurons were motor neurons, with the pituitary stalk functioning as a Sherringtonian final common pathway through which the impact of sensory and emotional events on neuroendocrine neurons had to pass to control pituitary hormone release. Were these properties a sign that the major neuroscientific discoveries being made in the early 1950s were beginning to influence neuroendocrinology? The present article discusses two main points: the context and significance of Harris's Herter Lecture for how our understanding of neuroendocrine anatomy (particularly as it relates to the control of the adenohypophysis) has developed since 1955; and within this framework, how novel and powerful techniques are taking our understanding of the structure of the neuroendocrine hypothalamus to new levels.
Keywords: Pituitary, Motor control, Neuroendocrinology, Neuropeptides, Neuroanatomy
1) INTRODUCTION
1-1) Geoffrey Harris the Anatomist
Anatomy played a major role throughout Geoffrey Harris's career. When he was a graduate student with Henry Harris, the chair of Anatomy at Cambridge, he was awarded the Marmaduke Shield Studentship in Anatomy. He then held positions in Cambridge University's Department of Anatomy and then the Physiological Laboratory before moving in 1952 to the newly created Laboratory of Neuroendocrinology at the Maudsley Hospital in South London. Geoffrey Harris's final move in 1962 was to the University of Oxford as Dr Lee's Professor of Anatomy, where he established and directed the MRC Neuroendocrinology Unit. Harris was also a strong advocate and enabler for improving methods used for teaching anatomy at Oxford [Vogt, 1972].
A hallmark of Harris's research was the continuous emphasis on establishing structure-function relationships between the hypothalamus and pituitary gland. This focus played a major part in his ability to consolidate his neurohumoral control hypothesis for the adenohypophysis. Harris recognized early on that establishing how the hypothalamus and pituitary gland were functionally connected required strong interactions between physiological and anatomical experiments. Together with other pioneering neuroendocrinologists in the UK and US, Harris's approach contrasted sharply with that used by ‘neurosecretionists’ (Ernst and Bertha Scharrer, Howard Bern, Manfred Gabe, etc.). These workers attempted to use morphology alone to establish neurosecretion as a process quite distinct from other forms of chemical signaling used by neurons in the brain [Watts, 2011].
While in the Cambridge University Physiological Laboratory in the late 1940s, much of Harris's work with John Green focused on clarifying the structural arrangement of the hypophysial portal vasculature [Green & Harris, 1947, 1949]. The reason was that it was impossible to establish that humorally mediated control was a viable explanation without first determining the structural means though which this control was exerted. What followed during the next five years was a series of very elegant and technically demanding experiments that began to establish the primacy of the hypophysial portal vasculature for conveying chemical signals from the hypothalamus to the adenohypophysis [Harris, 1950, Harris & Jacobsohn, 1950, 1952]. Harris's interpretations were certainly not universally accepted in 1955, but they were greatly strengthened by Nikitovich-Winer and Everett later in the decade [1957].
1-2) Neural Control of the Pituitary Gland
All of this work was discussed at various scientific meetings, documented in three substantial reviews [Harris, 1948, 1951a,b], before being presented in a more extensive form in Neural Control of the Pituitary Gland [Harris, 1955a]. For decades Neural Control of the Pituitary Gland has been recognized as a landmark publication in the development of neuroendocrinology. The book was published as the third contribution to what would become the long-running ‘Monographs of the Physiological Society’ series.
Harris stated that the book was an
‘attempt to analyse the mechanism by which the central nervous system, and the hypothalamus in particular, controls and integrates the activity of the [pituitary gland]‘.
Harris, G.W. [1955a, p 5]
As such it offered a comprehensive review of the contemporary state of the anatomical and functional bases of neuroendocrinology. But as far as the adenohypophysis was concerned, Harris's neurohumoral control hypothesis was still far from being universally accepted when the book was published [cf. Zuckerman, 1956; Sayers et al., 1958]. Indeed, this period was one of very vocal debate on the topic, with strong advocates in each of the opposing camps. Most famously Sir Solly Zuckerman dismissed the book in his review in Nature as ‘an edifice of speculation’ [Zuckerman, 1956]. (Zuckerman's review is well worth reading in its entirety to get a sense of the debate that was going on at this time.) So the book is not simply a description of a well-established concept. Instead it was a carefully presented account of the contemporary research results that Harris and others were producing to understand how the brain controlled the pituitary gland.
1-3) Neuroendocrinology as Neuroscience
From the standpoint of exactly how the hypothalamus controls the adenohypophysis Harris could not be particularly explicit in the book about detailed mechanisms:
The information as to the details of the mechanism involved is scanty but it seems likely that nerve fibers in the hypothalamus liberate some humoural substance into the primary plexus of the vessels, and that this substance is carried by the vessels to affect anterior pituitary activity.
Experiments were only just beginning to examine the nature of the chemical signals, which parts of the hypothalamus were responsible for controlling which pituitary hormones, how they were controlled by inputs to the hypothalamus and the rest of the brain, etc. Instead the book presents a systematic account of the anatomical arrangement of the connections between the hypothalamus and the pituitary gland together with the evidence that the functional connection to the adenohypophysis had to be neurohumoral.
Neural Control of the Pituitary Gland was published at a time when many of the seminal discoveries that would eventually shape neuroscience as we now know it were being made: Eccles confirmed the primacy of chemical neurotransmission at spinal motoneuron synapses [Brock et al., 1952]; the formal description of the ionic basis for action potential propagation [Hodgkin & Huxley 1952] and synaptic transmission [Fatt & Katz, 1952]; the first visualization of the synapse using electron microscopy [Palade & Palay, 1954]; the introduction of the Nauta stain for tracing neural pathways [Nauta & Gygax, 1954]. The book was therefore published when the physiological basis of chemical neurotransmission was on the minds of many people and was already starting to influence neuroendocrinologists.
Harris's view on the hypothalamic control of the pituitary gland from what we would now recognize as a neuroscience perspective, is presented rather more clearly in a review entitled The Function of the Pituitary Stalk [Harris, 1955b] published in November of 1955. This paper was developed from the Herter Lecture Harris delivered at Johns Hopkins University in March 1955. It was most likely written later than Neural Control of the Pituitary Gland, the preface of which says that the book was based on an series of teaching lectures that Harris gave in Cambridge before he moved to the Maudsley in 1952.
Harris's thoughts about hypothalamo-hypophysial interactions are better developed in the Johns Hopkins paper, and he makes clearer statements about the topic than he did in his book. The way he discusses these control processes shows that he was fully aware of the rapidly developing progress in chemical neurotransmission, pathway organization, functional localization etc—concepts that we now associate with neuroscience. It seems to have been the first time that he presented a view of hypothalamic control of the pituitary in this manner.
Harris discussed three concepts or properties that he considered essential for understanding how the hypothalamus controls the pituitary gland [Harris, 1955b]:
-
a)
Three criteria that all releasing factors (hormones) would have to fulfill if they were to be regarded as mediators of chemical signal transmission to the adenohypophysis,
-
b)
The spatial localization of function in the hypothalamus,
-
c)
The pituitary stalk as a final common motor pathway to the pituitary gland.
He continued to investigate and refine these properties for the rest of his career [cf. Harris, 1972], and they have had a major influence on neuroendocrinology ever since. We will now discuss in more detail how each of these three concepts has influenced the progress in understanding neuroendocrine anatomy since Harris first presented them in 1955.
2) THE STRUCTURE OF THE NEUROENDOCRINE HYPOTHALAMUS
2-1) Chemical Neurotransmission and The Releasing Factors
The nature of neurotransmission at central, autonomic, and neuromuscular synapses, together with the chemical nature of the signals that mediated these processes had to have been on Harris's mind in 1955. The view that the most well-accepted neurotransmitters at the time—acetylcholine and the adrenergic catecholamines—contributed to hypothalamic control of gonadotropin secretion had already been presented by Charles Sawyer and his colleagues in the US a few years earlier [Sawyer et al., 1949; Markee et al., 1952] (see also [Watts, 2011]). Wilhelm Feldberg had made seminal contributions towards establishing acetylcholine as a neurotransmitter [eg. Feldberg, 1951], and he was a colleague and collaborator of Harris's [Feldberg & Harris, 1953]. Presumably there must have been opportunities for the two of them to discuss chemical neurotransmission and the emerging concepts of neuroendocrinology.
In 1955 Harris stated three requirements that must be met for a compound to be accepted as a releasing factor of adenohypophysial hormones.
‘Several suggestions have been put forward as to the nature of a transmitter substance, but such suggestions and the neurohumoral view as a whole will only be established if it is possible to (...)
- a)
show this substance is present in the blood in the hypophysial portal vessels in greater amount than in systemic blood,
- b)
show that the concentrations of this substance in the blood of the hypophysial portal vessels varies according to electrical or reflex activation of the hypothalamic nerve tracts,
- c)
demonstrate that activity of the adenohypophysis is correlated with this varying concentration.’
Adapted from Harris 1955b, p368
Although they provided a framework for much of Harris's later work, it wasn't until twenty years later (and five years after his death) that all three requirements were fulfilled for a releasing hormone: GnRH [Fink, 1976; Sarkar et al., 1976]. More generally, they are logical criteria for any chemical signal, and in this context they make an interesting comparison to those first proposed for neurotransmitters by William Paton in 1958 [Paton, 1958]. Paton was the Chair of Pharmacology at Oxford University at the same time Harris was the Chair of Human Anatomy.
Harris mentioned ‘adrenergic substance’, histamine, and other compounds, including ‘neurosecretory material associated with the neurohypophysis’, as potential neurochemical signals that could control the pituitary [1955b]. However, he was careful to say that evidence was not yet available to make any firm statements about the chemical nature of the signals in the hypophysial vasculature [Harris, 1955a,b]. It took another fifteen years before the structure of the first adenohypophysial releasing factors was determined, and then a further five years before these findings were able to have a significant impact on experiments that could finally elucidate the fine structure of the neuroendocrine hypothalamus.
As morphological techniques became more sensitive and sophisticated, it became possible to examine the organization of hypothalamic neuroendocrine neurons in detail (Section 2-2). Once this happened, a finding that was unexpected at the time was that all of these neurons seemed to express other chemical signals in addition to the primary signal active in the pituitary gland. These included other peptides [Sawchenko et al., 1984; Everitt et al., 1986; Hokfelt et al., 1986; Coen et al.,1990]. More recent evidence supports the idea that some neuroendocrine neurons also express fast-acting single amino acid-derived neurotransmitters [Hrabovszky et al., 2005; Krashes et al., 2014].
The presence of these multiple chemical signals in neuroendocrine neurons quickly led to the idea that differential regulation by hormone feedback and various stimuli provided a way for their release mechanisms to switch between these different chemical signals, thereby increasing their response adaptation [Swanson, 1983, 1991]. Differential switching of peptide biosynthetic mechanisms was then demonstrated in CRH neuroendocrine neurons in the hypothalamic paraventricular nucleus (PVH) in response to combinations of glucocorticoid and various stressors [Sawchenko & Swanson, 1985; Watts & Sanchez-Watts, 1995, 2002; Watts, 2005].
2-2) Spatial Localization of Function in the Neuroendocrine Hypothalamus
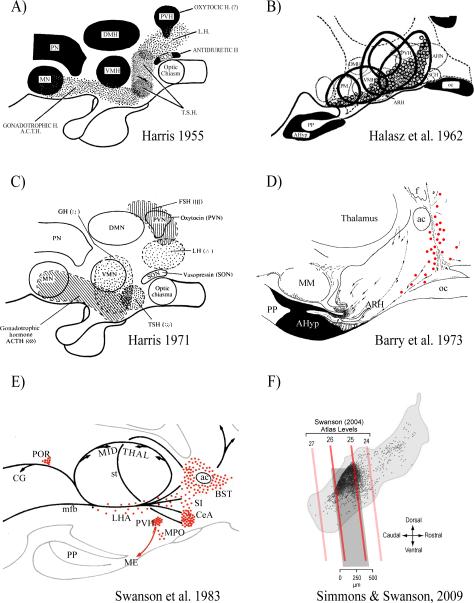
Experimental support for the spatial representation of function in the brain emerged during the late 19thC with the first reports about the localization of cortical function [eg. Ferrier, 1876, 1890]. In his 1955 Johns Hopkins review Harris makes an explicit comparison between the representation of the various parts of the human body in sensory and motor cortices, and the location of control mechanisms within the hypothalamus for the different pituitary hormones. He then went on to summarize contemporary knowledge about the hypothalamic locations of these control functions—poor as it was—by presenting the first map to show which parts of the hypothalamus were associated with controlling the various pituitary hormones (Fig. 1A) [Harris, 1955b]. The map was based on results from what were the only experimental techniques then available to investigate the locations of brain functions: lesions and electrical stimulation. It was followed seven years later by a map derived from pituitary transplantations published by Bela Hálasz and his colleagues (Fig. 1B [Hálasz et al., 1962]). In 1971 Harris presented a second version of his map (Fig. 1C [Harris, 1972]). Although he modifies some of the locations associated with particular pituitary hormones, the second map is little changed from the first, despite a sixteen year interval and the transplantation studies from the Hungarian group [Hálasz et al., 1962; Szentagothai et al., 1968]. What was the reason for this?
Figure 1.
Six maps from 1955 to 2009 show the hypothalamic locations of regions containing neuroendocrine neurons and pituitary hormone control mechanisms. They illustrate the dramatic improvement in the resolution of these representations since 1955.
A) Diagram of a midline sagittal section through the hypothalamus and pituitary gland. The stippled areas indicate the sites where electrical stimulation or lesions have resulted in changes of pituitary secretion. Abbreviations: ACTH, adrenocorticotrophic hormone; DMH, dorsomedial nucleus of the hypothalamus; LH, luteinizing hormone; MN, mammillary nuclei; PN, ‘posterior nucleus’; PVH, paraventricular nucleus of the hypothalamus; TSH, thyroid stimulating hormone; VMH, ventromedial nucleus of the hypothalamus. Adapted from Harris [1955b].
B) The hypophysiotropic area of the hypothalamus. The solid black line represents the borders of five relatively midline pituitary grafts in which considerable cellular integrity was maintained despite their ectopic site. The dotted circles are the locations of periodic acid-Schiff-positive basophils. Abbreviations as A) except: AHN, anterior hypothalamic area; AHyp, adenohypophysis; ARH, arcuate nucleus; oc, optic chiasm; PM, premammillary nucleus; PP, posterior pituitary; SCH, suprachiasmatic nucleus. Adapted from from Halasz et al. [1962].
C) As A), except both stippling and cross-hatching are used to indicate the sites where electrical stimulation or lesions have resulted in changes of pituitary secretion. Adapted from Harris [1972]
D) General diagram of the system of LRF (GnRH)-producing cells in a paramedian sagittal section of guinea-pig hypothalamus. Red dots show specifically immunoreactive perikarya. The dotted lines show the pathway of LRF axons (the arrows showing direction of transport). Abbreviations as A) and B), except: ac, anterior commissure; MM, median mammillary nucleus. Adapted from Barry et al. [1973].
E) A schematic illustration of the major CRF (CRH)-stained cell groups (red dots) and fiber systems (black lines) represented on a sagittal view of the rat diencephalon. Abbreviations as A) B), and D) except: BST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; CG, central gray; LHA, lateral hypothalamic area; ME, median eminence; mfb, medial forebrain bundle. MPO, medial preoptic area; POR, periocularmotor nucleus; SI, substantia innominata; st, stria terminalis. Adapted from Swanson et al. [1983].
F) The location of individual CRH neuroendocrine neurons (black dots) shown on a sagittal view of the rat paraventricular nucleus of the hypothalamus (PVH; light gray outline). The oblique red/dark gray lines show the corresponding positions of four atlas levels (24–26) from Swanson [2003]. Note that the vast majority of CRH neurons are found in the dorsal aspect of the PVH between levels 25 and 26 (darker gray box). In the coronal plane, most CRH neuroendocrine neurons are found in the dorsal zone of the medial parvicellular (mpd) part of the PVH. Adapted from Watts & Khan et al. [2013] using results originally published in Simmons & Swanson [2009].
Identifying the hypothalamic origins of the chemical signals responsible for controlling the pituitary gland with any detail required neuroanatomical methods that could reveal neurons with a clarity similar to that permitted by the histofluorescence techniques developed in 1962 by Bengt Falck and Nils-Åke Hillarp for catecholamines. Central catecholaminergic neurons were seen for the first time when these methods were applied to the brain [Dahlström & Fuxe, 1964]. Knowledge of catecholamine structure and chemistry was required to achieve these revolutionary findings. Without knowing the precise structure of the various hypothalamic releasing factors, it was impossible to develop appropriate visualization techniques for them. Therefore the reason why the maps changed so little was simple: the techniques needed for determining the locations of the hypothalamic neuroendocrine neurons with improved spatial resolution were still unavailable in 1971. But all was to change just as Harris's final review was published posthumously [Harris, 1972].
2-2-1) Immunohistochemistry and Radioimmunoassay
By 1971 Guillemin and Schally had determined the chemical structure of the two releasing factors—as Harris argued they should be called [Harris 1972]—for three adenohypophysial hormones: luteinizing hormone, follicle-stimulating hormone (LRF/GnRH), and thyroid-stimulating hormone (TRF/TRH). Those for prolactin (dopamine, a release-inhibiting factor), ACTH (CRF/CRH), and growth hormone (GRF/GHRH and somatostatin) were identified at various times during the following decade. The structures of oxytocin and vasopressin had been worked out in the 1950s [Du Vigneaud, 1954-1955]. This information quickly led to the generation of specific antibodies, which in turn meant that two methods with effective sensitivity and spatial resolution—immunohistochemistry (IHC) and radioimmunoassay (RIA)—could now be used to determine the location of hypothalamic neurons containing releasing factors and the neural lobe hormones.
Beginning with TRH and GnRH in 1974, Brownstein, Palkovits, and their colleagues used a micropunch technique combined with RIA to document the content of neuroendocrine peptides in hypothalamic and extrahypothalamic brain regions [Brownstein et al., 1974; Palkovits et al., 1974, 1976, 1983]. These studies were part of a huge series of experiments that measured peptides and neurotransmitter content—particularly catecholamines and their synthetic enzymes—in the brain. Before larger numbers of suitable antibodies for IHC became available in the 1980s, the Palkovits brain micropunch technique provided the highest resolution data for the spatial location of these neurochemicals.
The first immunohistochemical reports of the hypothalamic location of GnRH neurons appeared in 1973 [Barry et al., 1973; Leonardelli et al., 1973]. These results immediately and dramatically improved the resolution of hypothalamic maps (Fig. 1D). Over the next 12 years detailed maps were published showing the brain locations of oxytocin, vasopressin, and all the releasing factors, with CRH (CRF; Fig 1E) and GHRH (GRF) being the final ones to be mapped [Swanson et al., 1983; Sawchenko et al., 1985].
Despite the superior spatial resolution of IHC compared to other techniques, it quickly became apparent that the cell bodies of many neuroendocrine neurons contained levels of peptide that were below the sensitivity of IHC. The first solution to this problem was to pre-treat animals with intercerebroventricular injections of colchicine to block microtubule formation, which then confined peptides to cell bodies. This widely used method was very helpful for those peptides whose cell body staining was problematic [eg. Lechan et al., 1982; Swanson et al., 1983; Sawchenko et al., 1985]. But because colchicine is a toxin that interferes with normal neuronal function and structure [Rho & Swanson, 1989; Watts, 1996], it is difficult to use in experiments investigating the physiology of neuroendocrine peptides. New approaches were required.
2-2-2) Using Gene Products and Gene Manipulations to Study Neuroendocrine Neurons
A huge step forward in studying the location, morphology, and physiological regulation of neuroendocrine neurons began at the end of the 1970s with the cloning of the genes encoding hypothalamic peptide hormones [Roberts et al., 1979]. Genes for all the neuropeptides involved with pituitary gland function, along with a host of other neuropeptides were sequenced during the next decade (see [MacLean & Jackson, 1988] for review). This information led directly to two techniques that have dramatically improved our ability to study the physiology of neuroendocrine neurons: in situ hybridization in the 1980s; and later, the transgenic expression of fluorescent and other reporter proteins under the control of neuropeptide gene promoters [Spergel et al., 1999; Young III et al., 1999; Herbison et al., 2001].
In Situ Hybridization
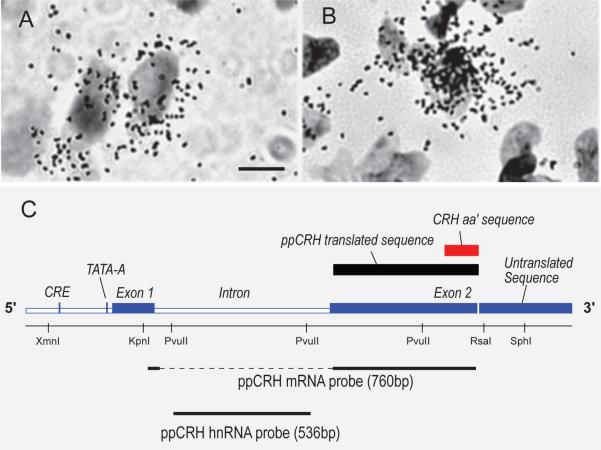
In situ hybridization (ISH) uses radio-isotopically or chemically labeled DNA or RNA sequences that are complementary to the RNAs transcribed from peptide genes. This technique can detect mRNAs and the heteronuclear (hn) RNAs that are the primary RNA products of gene transcription (Fig. 2). It has provided enormous insights into the location and control of neuroendocrine neurons during the past 30 years.
Figure 2.
Brightfield photomicrographs of hybridization for CRH mRNA (A) and CRH hnRNA (B) on sections counterstained with thionin to show cell nuclei. Note the cytoplasmic labeling for the mRNA and the nuclear labeling for the hnRNA (scale bar = 5 μm). C) A schematic representation of the rat pre-procorticotropin releasing hormone (ppCRH) gene. It shows the location of exon 1, the intronic sequence, exon 2, the cyclic AMP response element (CRE), and the TATA-core promoter sequence (TATA-A). Also shown are coding regions for the ppCRH translated sequence (black box) and the CRH amino acid (aa’) sequence (red box). The black dashed and solid lines at the bottom of the diagram show the sequences targeted by a 760 base-pair cRNA probe for ppCRH mRNA and a 536 base-pair cRNA probe for ppCRH hnRNA that detects the primary transcribed (intronic) sequence. Adapted from Tanimura et al. [1998].
In the first instance the technique was used to locate those neurons that expressed the genes for various neuropeptides, particularly for those where IHC could only provide equivocal results [Gee et al., 1983]. A flurry of mapping papers using ISH then followed. But the technique was also quickly applied to investigating physiologically relevant changes in gene expression, particularly the effects of hormone feedback, physiological challenges, and changes over time [eg. Young et al., 1986; Koller et al., 1987; Lightman & Young, 1988; Zoeller & Young, 1988; Swanson & Simmons, 1989; Watts & Swanson, 1989].
More recently non-isotopic methods, including colorimetric and particularly fluorescent ISH (FISH), have become more popular than radio-isotopic ISH. These methods offer greater flexibility for multi-labeling and imaging [eg. Yue et a., 2008; Babb et al., 2011]. But for intact tissue sections, non-isotopic ISH still has difficulty detecting some low-abundance RNAs such hnRNAs or the mRNAs for some receptors.
Transgenic Expression of Reporter Proteins
During the 1990s it became possible to drive the neuronal expression of various reporter genes under the control of specific neuropeptide gene promoters. While ß-galactosidase (encoded by lacZ) has been employed to investigate neuroendocrine neurons (eg. Schwartz et al., 1998; Skynner et al., 1999), this reporter has largely been superseded for morphological and electrophysiological studies by the transgenic expression of genes encoding fluorescent proteins (FPs) [Spergel et al., 1999 Young 3rd et al,. 1999; Cowley et al., 2001].
Using specific neuropeptide gene promoters to drive FP gene expression enables the fluorescent labeling of neurons—often in their entirety—that express a particular peptide gene at some point in their lifetime. The resultant labeling is permanent and often robust, which mitigates problems with antibody sensitivity. However, it should be remembered that because FP-labeling does not directly correlate with peptide or mRNA content at a particular time—indeed that is why the technique is often so useful as a specific marker—it is not a proxy for the types of quantitative information that can be obtained with IHC or ISH.
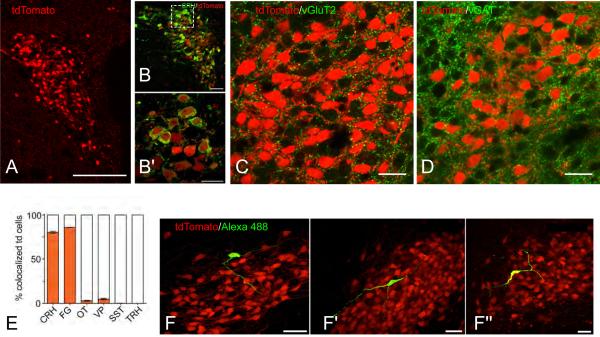
As an example of the clarirty and spatial resolution provided by transgenic FP labeling, Figure 3 shows CRH neurons labeled with TdTomato in the paraventricular nucleus of the hypothalamus (PVH) of Crh-IRES-Cre;Ai14 mice [Wamsteeker et al., 2013]. Robust TdTomato labeling is evident only in CRH neurons (Fig. 3A,B,E), and close appositions can be visualised between VGluT2 (glutamatergic) or vGAT (GABAergic) elements, and CRH neurons (Fig. 3C,D). FP-labeled CRH neurons are easily sampled for electrophysiological recording (Fig. 3F). In addition, they can be optogenetically manipulated by viral Cre-driven expression of channelrhodopsin [Wamsteeker et al., 2013]. For other neuroendocrine peptides, transgenic expression of FPs has been used very effectively to examine the connections, electrophysiology, morphology of GnRH neurons [Suter et al., 2000; Herbison et al., 2001; Campbell & Herbison, 2007; Iremonger et al.,2010], and to identify the neurotransmitter phenotypes of POMC neurons in the arcuate nucleus [Hentges et al., 2009].
Figure 3.
Anatomical distribution and CRH protein expression in tdTomato labelled cells in the paraventricular nucleus of the hypothalamus (PVH) of Crh-IRES-Cre;Ai14 mice.
A) Confocal image (20× magnification) of the PVH in a Crh-IRES-Cre;Ai14 (tdTomato, red) mouse (level 61 of Dong [2007]). B) Confocal image (40×) of a colchicine-treated Crh-IRES-Cre;Ai14 mouse PVN. Immunostaining for corticotropin-releasing hormone (CRH) is shown in green. B’) Higher magnification (100×) of the box inset in (B). Confocal images of the PVH in Crh-IRES-Cre;Ai14 (tdTomato, red) mouse labeled for vGluT2 (C, green), or vGAT (D, green). Note the numerous close appositions (yellow) between tdTomato (CRH) neurons and glutamaterigic (vGlutT2) and GABAergic (vGAT) elements. E) Bar graph showing the percent of tdTomato positive cells that coexpress various neuropeptides, each from n=5 colchicine-treated mice. Abbreviations: CRH, corticotropin-releasing hormone; FG, fluorogold; OT, oxytocin; SST, somatostatin; TRH, thyrotropin-releasing hormone. F) Confocal Images (60× magnification), from 3 mouse PVH sections (F – F”), showing morphology of single tdTomato neurons (red) filled with Alexa488- (green) and biocytin (yellow) during whole-cell patch clamp recordings.
Scale bars are 100 μm (A), 50 μm (B, F - F”), and 20 μm (B’, C, and D). Panels A), B), E), and F) are adapted from Wamsteeker et al. [2013]. Panels C) are D) are unpublished photomicrographs from the author's laboratory (C.S. Johnson & A.G. Watts).
2-2-3) Mapping and Neuroinfomatic Techniques
The first atlases of the hypothalamus were published in the 1930s [Krieg, 1932; Le Gros Clark, 1936; Rioch et al., 1940]. These remained definitive well into the 1950s, and they clearly influenced the maps produced by Harris and Halasz (Fig 1A-C). But even a cursory glance at any examples of spatial representation of neuroendocrine function from this time shows that these locations are projected onto what are rather rudimentary maps. More accurate atlases started to emerge in the 1950s and 1960s [de Groot, 1959; Christ, 1969], and these helped support increasingly accurate maps of neuroendocrine topography in the hypothalamus (Figs. 1 D,E).
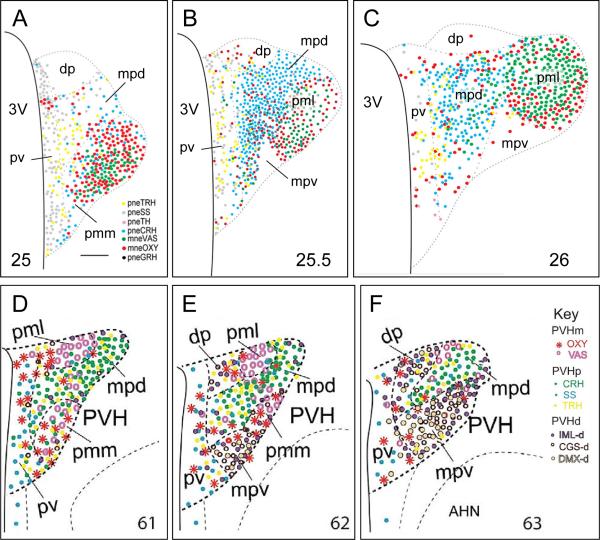
As greater amounts of high resolution spatial data were generated from IHC, ISH, and FP-expression these results required atlases with equivalent accuracy for investigators to take full advantage of their new findings. This started in the early 1980s when new rat and mouse brain atlases began to be published [Paxinos & Watson, 1982; Franklin & Paxinos, 1996; Swanson, 1992, 2003; Dong, 2007]. These atlases have made it possible to produce high precision topographies of neuroendocrine neurons. Figures 1F and 4 shows recent topographies of the neuroendocrine PVH in the rat and mouse using maps from these atlases [Simmons & Swanson, 2009; Biag et al., 2012; Watts & Khan, 2013].
Figure 4.
A – C) Three maps of the rat PVH taken from a reference atlas of neuroendocrine neuron type labeled with antibodies for neuropeptides. The locations of each type of peptidergic neuroendocrine neuron are plotted onto three levels of PVH (designated by the number at the bottom of each panel) from Swanson [2003]. Panels A - C) are adapted from Simmons & Swanson [2009].
D - F) Schematic drawings illustrating the delineations of three levels of the PVH in the mouse brain, which are determined based on distributions of eight neuronal phenotypes in two major neuroendocrine divisions (magnocellular [m] PVHm, including OXY and VAS; and parvicellular [p] PVHp, including CRH, SS, and TRH) and three descending preautonomic populations (PVHd) that project to the intermediolateral column of the spinal cord (IML-d), to the central gray of the spinal cord (CGS-d), and to the dorsal motor nucleus of the vagus nerve (DMX-d). The mouse atlas levels [Dong, 2007] are designated by the number at the bottom of each panel. D - F) are adapted from Biag et al. [2012].
Other abbreviations: 3V, third ventricle; AHN, anterior hypothalamic nucleus; CRH, corticotropin-releasing hormone; dp, ,dorsal parvicellular part of the PVH (descending division); GRH, growth hormone-releasing hormone; mne, magnocellular neuroendocrine; mpd, medial parvicellular part of the PVH, dorsal zone; mpv, medial parvicellular part of the PVH, ventral zone; OXY, oxytocin; pml, posterior magnocellular part of the PVH, lateral zone; pmm, posterior magnocellular part of the PVH, medial zone; pne, parvicellular neuroendocrine; pv, periventricular part of the PVH; SS, somatostatin; TRH, thyrotropin-releasing hormone;VAS, vasopressin.
The importance of using accurate maps now goes further than simply facilitating topographic analyses. Neuroinformatics provides new and powerful tools for analyzing the highly complex brain connectional and topographic data now being generated [eg. Zingg et al., 2014; Bota et al., 2015]. This makes it increasingly important to represent data on accurate, and of particular importance, standard and widely available—often online—brain atlases. Accurate topographies greatly facilitate data input into databases, atlases, and neuroinformatics tools. But critically, they also enable neuroanatomical data to be directly compared between different experiments, experimenters, labs etc. This is a central feature of experimental science, and one that is much more difficult to achieve with the types of unique single investigator-generated maps sometimes used to present results.
2-3) The Pituitary Stalk As A Final Common Motor Pathway To The Pituitary Gland
2-3-1) Neuroendocrine Neurons as Motor Neurons
An important step towards investigating how any neural system is organized is to establish a conceptual framework as the basis for structural and functional experiments. For the neuroendocrine system a sound framework of this nature took a long time to develop. Reports in the 1920s and 1930s showing the existence of ‘nerve-gland cells’ in the hypothalamus of many vertebrate species [Scharrer & Scharrer, 1940] came from efforts to explain how environmental stimuli could alter ‘internal secretions’ [Watts, 2011]. The result of these investigations was neurosecretion.
During the 1930s and 1940s neurosecretion was a concept with numerous flaws and inconsistencies [Watts, 2011]. It was unable to make any meaningful impact towards understanding how the hypothalamus controlled the pituitary gland until about 1950 when the results of Palay [1945] and Bargmann [1949] were assimilated into the field [Watts, 2011]. Nevertheless the emphasis on explaining how sensory information controlled the pituitary gland remained an essential theme for the entire field and showed that projections from many parts of the brain to the hypothalamus must at some point influence neuroendocrine neuronal function. Results from experiments in the 1950s showed that interosensory and extrasensory information converged on integrative mechanisms in the hypothalamus, which then directly controlled hormone release from the pituitary gland [eg. Sayers et al., 1958].
Moving the field forward with novel structural and functional experiments needed a conceptual framework to explain how this control occurred. To this end, in 1955 Harris [1955b] compared neuroendocrine control to the classic voluntary motor control system established during the previous hundred years.
In 1954 [Harris & Fortier, 1954] Harris proposed that the pituitary stalk formed the connecting link
‘between the external environment and the central nervous system on the one hand, and the pituitary gland and its target organs on the other.’
Harris [1955b, p371]
He then likened sensorimotor integration in the cortex (ie. the representation of different parts of the body in the sensory and motor cortex) with the locations of the different mechanisms responsible for controlling the secretion of the various pituitary hormones. Harris continued this line of thinking by noting the similarity of the pituitary stalk to the ventral horn of the spinal cord. A logical extension of this comparison was to say that the supraopticohypophysial tract (and most likely the hypophysial portal vessels) was a final common pathway—in the Sherringtonian sense—used by the brain to control hormone release from the pituitary gland [Harris, 1955b, p360]. Harris therefore considered that the nature of the hypothalamic neurons controlling the posterior pituitary was motor. He made the explicit comparison between the posterior pituitary and a voluntary muscle, stating that
‘both structures are dependent on their innervation for any functional activity, even to the extent that they undergo atrophy if denervated.’
Harris [1955b, p359]
Therefore the basic premise of Harris's discussion was that the neuroendocrine neurons responsible for controlling pituitary hormone release were, in principle, motor neurons like those in the ventral horn of the spinal cord: both directly control the activity of an organ located outside the brain.
One prediction of this model is that neuroendocrine control mechanisms are organized along similar lines to voluntary movement and autonomic motor control [Saper, 2002; Thompson & Swanson, 2003]. In this way, neuroendocrine motor neurons are directly controlled by pre-motor neurons and pattern generators, which in turn are then influenced by a comprehensive set of inputs that allows the many and varied interosensory, exterosensory, and emotional influences to control pituitary hormone release.
Harris does not seem to have pursued the implications of his comparison any further, and it does not appear to have been picked up to any extent by others in the field during his lifetime. As we have seen in Section 2-2, this was not surprising because sufficient details about the nature, morphology, location, and hodology of hypothalamic neuroendocrine neurons were still unknown when Harris died in 1971. While a general understanding of the complex inputs from the rest of the brain to the hypothalamus that controlled ACTH secretion for example, was already reasonably well developed in the late 1950s [Sayers et al., 1958], many of the other key structural elements of neuroendocrine motor control still needed to be elucidated. Since 1971 however, a multitude of studies have characterized these features in great detail thereby allowing the development of more extensive conceptual models of neuroendocrine control based on Harris's original premise [eg. Watts & Swanson, 2002; Thompson & Swanson, 2003; Watts 2005; Watts & Khan, 2013].
2-3-2) Pre-Motor Control Networks as Hormone Release Pattern Generators
It has been known for decades that LH secretion is comprised of two modes: a pulsatile (or episodic) pattern that is most apparent during basal conditions; and a surge pattern seen during stimulation that is superimposed upon pulsatile release. Although all pituitary hormones show these two patterns to a greater or lesser degree (with the nature and timing of the patterns varying from hormone to hormone), the basic organization is nevertheless maintained [Watts, 2005].
If a model of neuroendocrine control based on voluntary movement and autonomic motor control is tenable then we should be able to identify distinct sets of pre-motor neurons that are responsible for each of these two release patterns, along with direct connections between each of the different components. For most neuroendocrine neurons this continues to be extremely difficult, not least because directly measuring their output into hypophysial portal blood in response to experimental manipulations remains a considerable technical challenge. Furthermore, neuroanatomical tracing techniques for identifying projections to and from a single neuronal cell type have until very recently, been unavailable.
Support for the idea that neuroendocrine neurons are controlled by pre-motor neurons and motor pattern generators (or analogues thereof) is perhaps best developed for GnRH neurons. The fact that immortalized GnRH neurons organize themselves in a manner that supports pulsatile release in the absence of all other inputs [Wetsel et al., 1992] is consistent with the idea that pulsatility is the fundamental release pattern of these particular neuroendocrine motor neurons. In whole animals pulsatile release from GnRH neurons (and perhaps all types of neuroendocrine neurons) is then modified by various pre-motor inputs into the more complex surge patterns. In turn, the actions of these pre-motor neurons are shaped further by their own particular sets of inputs [Watts, 2005].
Investigating the structural and functional organization of the GnRH control networks was made much clearer when kisspeptin was indentified as a potent activator of GnRH neurons and an essential component for the onset of puberty [reviewed in Dungan et al., 2006]. Kisspeptin neurons were then found in the rodent anteroventral periventricular (AVPV) and arcuate (ARH) nuclei [Lehman et al., 2010]. This arrangement is broadly maintained across diverse mammalian species [Goodman & Lehman, 2012]. Kisspeptin strongly stimulates the firing rate of GnRH neurons [reviewed in Piet et al., 2015], and the two populations of kisspeptin neurons directly innervate or have some form of interaction with GnRH neurons [Clarkson & Herbison, 2006; Lehman et al., 2010]. These two kisspeptin populations are implicated in directly controlling either the pulsatile (ARH) or the surge (AVPV) release modes [reviewed in Piet et al., 2015], and both are required for normal surge activity and estrous cyclicity [Hu et al., 2015].
Much of the detailed hodology of kisspeptin neurons remains to the determined, but the fact that the LH surge is superimposed on the more fundamental pulsatile release [Fox & Smith, 1985; Hoeger et al., 1999] would require connections from the AVPV kisspeptin to the ARH kisspeptin populations. Although projections of this type between these two nuclei exist [Gu & Simerly, 1997] and they involve kisspeptin AVPV neurons [Yeo & Herbison, 2011], whether there are direct projections from AVPV kisspeptin to ARH kisspeptin neurons remains to be determined [Lehman et al., 2010].
3) FUTURE CHALLENGES
Chemical Neurotransmission and Neuroendocrine Motor Neurons
The vast majority of our current understanding about brain function rests on the foundation of chemical neurotransmission at the synapse, which is a rapid and spatially restricted means of communication. As the fundamentals of neuroendocrinology emerged in the 1930s and 1940s the thought was that neurosecretory systems communicated in ways that were different from other parts of the brain. At that time signalling between conventional neurons was believed to be electrically mediated [Watts, 2011]. But as neuroscientific concepts imbued neuroendocrinology it became clear that neuroendocrine neurons functioned in basically the same way as conventional neurons in terms of the ionic basis of action potential propagation, vesicular release of chemical signals, etc. [Watts, 2011]. As the fine structure of neuroendocrine neurons has been determined with increasing detail, new facets of neuroendocrine communication have emerged. One example is the release of neuropeptides from the dendrites of neuroendocrine neurons, which was first identified by electron microscopy about 25 years ago [Pow & Morris, 1989]. Recent work from Stern and his colleagues now shows that dendritic release of vasopressin provides a way for magnocellular neuroendocrine neurons in the PVH to modify the activity of nearby pre-autonomic neurons thereby mediating integration between these two functionally distinct compartments [Son et al. 2013; Stern, 2014].
Conventional neural projections provide obvious ways for integrating the functions of different control systems. But as dendritic release and other forms of more spatially diffuse transmission [Fuxe et al., 2007] show, non-synaptic release mechanisms likely play a significant role in these integrative processes. How these types of chemical signalling work together with synaptic neurotransmission to effect neuroendocrine integration offers intriguing new ways to consider neuroendocrine motor control functions.
The Spatial and Hodological Organization of the Neuroendocrine Hypothalamus
We are still far from knowing how the neuroendocrine hypothalamus is organized with the clarity that is currently available for a system such as the hippocampus. The topological heterogeneity of neuroendocrine motor neurons (Fig. 4) along with great complexity of the connections into the neuroendocrine hypothalamus have hampered efforts to probe the detailed organization of the control networks.
Conventional tracing techniques have been used for the past 45 years to examine hypothalamic connectivity, and they have provided a very useful framework [Watts & Swanson, 2002]. But they do not have sufficient cell specificity to determine whether neuroendocrine control networks really are organized with the same principles as those controlling voluntary movement or particularly autonomic output, with which the neuroendocrine motor system may have close organizational relationships. The organization of premotor networks for GnRH neurons is better known than for other adenohypophysial releasing hormones. But details remain inadequate to describe the overall structure of these premotor networks, and how they fit into the larger integrative networks distributed throughout the brain that coordinate neuroendocrine and autonomic motor actions with motivated behaviors.
A major way forward for revealing these interactions comes from the ability to label single neuron populations with viruses that can drive the expression of FPs. These methods are now providing opportunities to characterize the hodology of phenotypically defined neuronal populations [Krashes et a., 2014; Sun et al., 2014] and should prove extremely useful for regions like the PVH that contain mixed populations of neuroendocrine neurons (Fig. 4).
4) CONCLUSION
The mid-1950s was a period of considerable debate about how the brain controlled the different parts of pituitary gland. Although evidence was already leaning heavily towards the view that the pituitary stalk contained the axons that transported oxytocin and vasopressin from the hypothalamus to the posterior pituitary (and most likely also transmitted the nerve impulses that released them), how signals from the hypothalamus controlled the adenohypophysis was still hotly contested. The experiments performed by Geoffrey Harris during the previous 8 years with John Green, Dora Jacobsohn, and others, continued to strengthen the neurohumoral transmission hypothesis first proposed by Hinsey and Markee almost twenty years earlier [Hinsey, 1937]. But it was a hypothesis that was still far from being universally accepted. And so it was into this debate that Harris contributed a short review in 1955 that presented a new framework for the neuroendocrine control of the adenohypophysis [Harris, 1955b]. The current article argues that Harris's framework was built solidly on the rapidly developing neuroanatomy and neurobiology of the time, and was perhaps an early sign that neuroendocrinology could dovetail with the then embryonic discipline of neuroscience. The huge amount of work on neuroendocrine anatomy in the 60 years since the publication of Harris's review has clearly vindicated his foresight about the structural bases of neuroendocrinology.
Acknowledgments
Funding
Work from the author's laboratory described in this paper is provided by grant R01 NS029728 from the National Institute of Neurological Disorders and Stroke, the National Institutes of Health.
Footnotes
Declarations of Interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported herein.
REFERENCES
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann W. Über die neurosekretorische Verknüpfung von Hypothalamus und Neurohypophyse. Zeitschrift für Zellforschung und mikroskopische Anatomie. 1949;34:610–634. [PubMed] [Google Scholar]
- Barry J, Dubois MP, Poulain P. LRF producing cells of the mammalian hypothalamus. A fluorescent antibody study. Zeitschrift für Zellforschung und mikroskopische Anatomie. 1973;146:351–66. doi: 10.1007/BF02346227. [DOI] [PubMed] [Google Scholar]
- Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong HW. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. Journal of Comparative Neurology. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M, Sporns O, Swanson LW. Architecture of the cerebral cortical association connectome underlying cognition. Proceedings of the National Academy of Sciences U S A. 2015 doi: 10.1073/pnas.1504394112. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. Synaptic excitation and inhibition. Journal of Physiology. 1952;117(2):8. [PubMed] [Google Scholar]
- Brownstein MJ, Palkovits M, Saavedra JM, Bassiri RM, Utiger RD. Thyrotropin-releasing hormone in specific nuclei of rat brain. Science. 1974;185:267–9. doi: 10.1126/science.185.4147.267. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone neurons in the mouse using conditional viral tract tracing. Endocrinology. 2007;148:5884–90. 2153–7. doi: 10.1210/en.2007-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen CW, Montagnese C, Opacka-Juffry J. Coexistence of Gonadotrophin-Releasing Hormone and Galanin: Immunohisto-chemical and Functional Studies. Journal of Neuroendocrinology. 1990;2:107–11. doi: 10.1111/j.1365-2826.1990.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Christ J. Derivation and boundaries of the hypothalamus, with an atlas of the hypothalamic grisea. In: Haymaker W, Anderson E, Nauta WJH, editors. The Hypothalamus. Thomas; Springfield, Il: 1969. pp. 13–80. [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. ACTA Physiologica Scandinavica Supplement (Oxford) 1964;232:1–55. [PubMed] [Google Scholar]
- deGroot J. The rat hypothalamus in stereotaxic coordinates. Journal Comparative Neurology. 1959;113:389–400. doi: 10.1002/cne.901130304. 1959. [DOI] [PubMed] [Google Scholar]
- Dong HW. Allen Reference Atlas: A Digital Color Brain Atlas of the C57BL/6J Male Mouse. Wiley; New York: 2007. [Google Scholar]
- Du Vigneaud V. Hormones of the posterior pituitary gland: oxytocin and vasopressin. Harvey Lectures. 1954-1955;50:1–26. 1954-1955. [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Meister B, Hökfelt T, Melander T, Terenius L, Rökaeus A, Theodorsson-Norheim E, Dockray G, Edwardson J, Cuello C, et al. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Research. 1986;396:97–155. doi: 10.1016/s0006-8993(86)80192-5. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold potentials at motor nerve endings. Journal of Physiology. 1952;117:109–28. [PMC free article] [PubMed] [Google Scholar]
- Feldberg W, Harris GW. Distribution of histamine in the mucosa of the gastrointestinal tract of the dog. Journal of Physiology. 1953;120:352–64. doi: 10.1113/jphysiol.1953.sp004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W. The physiology of neuromuscular transmission and neuromuscular block. British Medical Journal. 1951 May 5;1(4713):967–76. doi: 10.1136/bmj.1.4713.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier D. The Functions of the Brain. Smith, Elder; London: 1876. [Google Scholar]
- Ferrier D. The Croonian Lectures on Cerebral Localisation. Smith, Elder; London: 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. The development of the releasing factor concept. Clinical Endocrinology (Oxford) 1976;5(Suppl):245S–260S. doi: 10.1111/j.1365-2265.1976.tb03833.x. [DOI] [PubMed] [Google Scholar]
- Fox SR, Smith MS. Changes in the pulsatile pattern of luteinizing hormone secretion during the rat estrous cycle. Endocrinology. 1985;116:1485–1492. doi: 10.1210/endo-116-4-1485. [DOI] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos GT. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 1996. [Google Scholar]
- Fuxe K, Dahlström A, Höistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, et al. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Research Reviews. 2007:5517–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Gee CE, Chen CL Roberts JL, Thompson R, Watson SJ. Identification of proopiomelanocortin neurones in rat hypothalamus by in situ cDNA-mRNA hybridization. Nature. 1983;306:374–6. doi: 10.1038/306374a0. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–18. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J D, Harris G W. The neurovascular link between the neural lobe and adenohypophysis. Journal of Endocrinology. 1947;5:136–46. doi: 10.1677/joe.0.0050136. [DOI] [PubMed] [Google Scholar]
- Green JD, Harris G W. Observation of the hypophysio-portal vessels of the living rat. Journal of Physiology. 1949;108:359–61. [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. Journal of Comparative Neurology. 1997;384:142–164. [PubMed] [Google Scholar]
- Halasz B, Pupp L, Uhlarik S. Hypophysiotrophic area in the hypothalamus. Journal of Endocrinology. 1962;25:147–54. doi: 10.1677/joe.0.0250147. [DOI] [PubMed] [Google Scholar]
- Harris GW. Oestrous rhythm, pseudopregnancy and the pituitary stalk in the rat. Journal of Physiology. 1950;111:347–60. doi: 10.1113/jphysiol.1950.sp004484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Neural control of the pituitary gland. I. The neurohypophysis. British Medical Journal. 1951a Sep 8;2(4731):559–64. doi: 10.1136/bmj.2.4731.559. 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Neural control of the pituitary gland. II. The adenohypophysis, with special reference to the secretion of A.C.T.H. British Medical Journal. 1951b Sep 15;2(4732):627–34. doi: 10.1136/bmj.2.4732.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Neural Control of The Pituitary Gland. Monographs of the Physiological Society”. Edward Arnold; London: 1955a. p. 298. [Google Scholar]
- Harris GW. The function of the pituitary stalk. Bulletin of the Johns Hopkins Hospital. 1955b;97:358–75. [PubMed] [Google Scholar]
- Harris GW. Humours and hormones. Journal of Endocrinology. 1972;53:2–23. [PubMed] [Google Scholar]
- Harris GW, Fortier C. The regulation of anterior pituitary function, with special reference to the secretion of adrenocorticotrophic hormone.. 4th Annual Report on Stress; Montreal: Acta Inc; 1954. pp. 106–126. [Google Scholar]
- Harris GW, Jacobsohn D. Proliferative capacity of the hypophysial portal vessels. Nature. 1950;165:854. doi: 10.1038/165854a0. [DOI] [PubMed] [Google Scholar]
- Harris GW, Jacobsohn D. Functional grafts of the anterior pituitary gland. Proceedings of the Royal Society of London B Biological Sciences. 1952;139:263–76. doi: 10.1098/rspb.1952.0011. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. Journal of Neuroscience. 2009;29:13684–90. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR, Simonian SX, Skynner MJ, Sim JA. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Molecular and Cellular Endocrinology. 2001;185:185–94. doi: 10.1016/s0303-7207(01)00618-9. [DOI] [PubMed] [Google Scholar]
- Hinsey JC. The relationship of the nervous system to ovulation and other phenomena of the female reproductive tract. Cold Spring Harbor Symposia on Quantitative Biology. 1937;5:269–279. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;117:500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger KM, Kolp L A, Strobl FJ, Veldhuis JD. Evaluation of LH secretory dynamics during the rat proestrous LH surge. American Journal of Physiology. 1999;276:R219–R225. doi: 10.1152/ajpregu.1999.276.1.R219. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Everitt B, Meister B, Melander T, Schalling M, Johansson O, Lundberg JM, Hulting AL, Werner S, Cuello C, et al. Neurons with multiple messengers with special reference in neuroendocrine systems. Recent Progress in Hormone Research. 1986;42:1–70. doi: 10.1016/b978-0-12-571142-5.50005-7. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Wittmann G, Turi GF, Liposits Z, Fekete C. Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology. 2005;146:341–7. doi: 10.1210/en.2004-0856. [DOI] [PubMed] [Google Scholar]
- Hu MH, Li XF, McCausland B, Li SY, Gresham R, Kinsey-Jones JS, Gardiner JV, Sam AH, Bloom SR, Poston L, Lightman SL, Murphy KG, O'Byrne KT. Relative importance of the arcuate and anteroventral periventricular kisspeptin neurons in control of puberty and reproductive function in female rats. Endocrinology. 2015 Apr;15:en20141655. doi: 10.1210/en.2014-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Research. 2010;1364:35–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Koller KJ, Wolff RS, Warden MK, Zoeller RT. Thyroid hormones regulate levels of thyrotropin-releasing-hormone mRNA in the paraventricular nucleus. Proceedings of the National Academy of Sciences U S A. 1987;84:7329–33. doi: 10.1073/pnas.84.20.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–42. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg WJS. The hypothalamus of the albino rat. Journal of Comparative Neurology. 1932;55:19–89. [Google Scholar]
- Le Gros Clark WE. The topography and homologies of the hypothalamic nuclei in man. Journal of Anatomy. 1936;70:203–214. [PMC free article] [PubMed] [Google Scholar]
- Lechan RM, Jackson IM. Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology. 1982;111:55–65. doi: 10.1210/endo-111-1-55. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Research. 2010;1364:90–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardelli J, Barry J, Dubois MP. Mise en evidence par immunofluorescence d'un constituant immunologiquement apparente au LH-RH dans l'hypothalamus et l'eminence mediale chez les Mammiferes. Comptes Rendus de l'Académie des Sciences. 1973;276:2043–46. [PubMed] [Google Scholar]
- Lightman SL, Young WS., 3rd Corticotrophin-releasing factor, vasopressin and proopiomelanocortin mRNA responses to stress and opiates in the rat. Journal of Physiology. 1988;403:511–23. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean DB, Jackson IM. Molecular biology and regulation of the hypothalamic hormones. Baillieres Clinical Endocrinology and Metabolism. 1988;2:835–68. doi: 10.1016/s0950-351x(88)80021-1. [DOI] [PubMed] [Google Scholar]
- Markee JE, Everett JW, Sawyer CH. The relationship of the nervous system to the release of gonadotrophin and the regulation of the sex cycle. Recent Progress in Hormone Research. 1952;8:139–163. [Google Scholar]
- Nauta WJ, Gygax PA. Silver impregnation of degenerating axons in the central nervous system: a modified technic. Stain Technology. 1954;29:91–3. doi: 10.3109/10520295409115448. [DOI] [PubMed] [Google Scholar]
- Nikitovitch-Winer M, Everett JW. Resumption of gonadotrophic function in pituitary grafts following retransplantation from kidney to median eminence. Nature. 1957;180:1434–5. doi: 10.1038/1801434a0. [DOI] [PubMed] [Google Scholar]
- Palade GE, Palay SL. Electron microscope observations of intemeuronal and neuromuscular synapses. Anatomical Record. 1954;118:335. [Google Scholar]
- Palay SL. VII. The preoptic–hypophysial pathway in fishes. Journal of Comparative Neurology. 1945;82:129–143. [Google Scholar]
- Palkovits M, Arimura A, Brownstein M, Schally AV, Saavedra JM. Luteinizing hormone-releasing hormone (LH-RH) content of the hypothalamic nuclei in rat. Endocrinology. 1974;95:554–8. doi: 10.1210/endo-95-2-554. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Arimura A, Sato H, Schally V, Kizer JS. Somatostatin content of the hypothalamic ventromedial and arcuate nuclei and the circumventricular organs in the rat. Brain Research. 1976;109:430–4. doi: 10.1016/0006-8993(76)90549-7. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Vale W. Corticotropin releasing factor (CRF) immunoreactivity in hypothalamic and extrahypothalamic nuclei of sheep brain. Neuroendocrinology. 1983;37:302–5. doi: 10.1159/000123564. [DOI] [PubMed] [Google Scholar]
- Paton WDM. Central and synaptic transmission in the nervous system; pharmacological aspects. Annual Review of Physiology. 1958;20:431–70. doi: 10.1146/annurev.ph.20.030158.002243. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1982. [Google Scholar]
- Piet R, de Croft S, Liu X, Herbison AE. Electrical properties of kisspeptin neurons and their regulation of GnRH neurons. Frontiers in Neuroendocrinology. 2015;35:15–27. doi: 10.1016/j.yfrne.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Rho JH, Swanson LW. A morphometric analysis of functionally defined subpopulations of neurons in the paraventricular nucleus of the rat with observations on the effects of colchicine. Journal of Neuroscience. 1989;9:1375–88. doi: 10.1523/JNEUROSCI.09-04-01375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioch D M, Wislocki G B, O'Leary J L. A précis of preoptic, hypothalamic and hypophysial terminology with atlas. Research Publications of the Association for Research in Nervous and Mental Disease. 1940;20:3–30. [Google Scholar]
- Roberts JL, Seeburg PH, Shine J, Herbert E, Baxter JD, Goodman HM. Corticotropin and beta-endorphin: construction and analysis of recombinant DNA complementary to mRNA for the common precursor. Proceedings of the National Academy of Sciences U S A. 1979;76:2153–7. doi: 10.1073/pnas.76.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;26:461–3. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Rivier J, Vale WW. The distribution of growth-hormone-releasing factor (GRF) immunoreactivity in the central nervous system of the rat: an immunohistochemical study using antisera directed against rat hypothalamic GRF. Journal of Comparative Neurology. 1985;237:100–15. doi: 10.1002/cne.902370108. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proceedings of the National Academy of Sciences U S A. 1984;81:1883–7. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Federation Proceedings. 1985;44:221–7. [PubMed] [Google Scholar]
- Sawyer CH, Markee J E, Townsend B F. Cholinergic and adrenergic components in the neurohumoral control of the release of LH in the rabbit. Endocrinology. 1949;44:18–37. doi: 10.1210/endo-44-1-18. [DOI] [PubMed] [Google Scholar]
- Sayers G, Redgate ES, Royce PC. Hypothalamus, adenohypophysis and adrenal cortex. Annual Review of Physiology. 1958;20:243–74. doi: 10.1146/annurev.ph.20.030158.001331. [DOI] [PubMed] [Google Scholar]
- Scharrer E, Scharrer B. Secretory cells within the hypothalamus. In The Hypothalamus and Central Levels of Autonomic Function. Research Publications of the Association for Research in Nervous and Mental Disease. 1940;20:170–194. [Google Scholar]
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology. 1998;139:2629–35. doi: 10.1210/endo.139.5.6000. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. Journal of Comparative Neurology. 2009;516:423–41. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. Journal of Neuroscience. 1999;19:5955–66. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–49. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. Journal of Neuroscience. 1999;19:2037–50. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE. Neuroendocrine-Autonomic Integration in the PVN: Novel Roles for Dendritically Released Neuropeptides. Journal of Neuroendocrinology. 2014 Dec 26; doi: 10.1111/jne.12252. 2014 doi: 10.1111/jne.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Reports. 2014;7:269–80. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–9. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. Journal of Comparative Neurology. 1989;285:413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Neuropeptides—New vistas on synaptic transmitters. Trends in Neuroscience. 1983;6:294–295. [Google Scholar]
- Swanson LW. Biochemical switching in hypothalamic circuits mediating responses to stress. Progress in Brain Research. 1991;87:181–200. doi: 10.1016/s0079-6123(08)63052-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain, edn 1. First Edition Elsevier; New York: 1992. [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain, edn 3. Elsevier; New York: 2003. [Google Scholar]
- Szentágothai J, Flerko B, Mess B, Halasz B. Hypothalamic control of the anterior pituitary: an experimental-morphological study. Akadémiai Kiadó; Budapest: 1968. The trophic dependence of the anterior pituitary on the diencephalon; the hypophysiotrophic area of the hypothalamus. pp. 110–155. [Google Scholar]
- Tanimura SM, Sanchez-Watts G, Watts AG. Peptide gene activation, secretion, and steroid feedback during stimulation of rat neuroendocrine corticotropin-releasing hormone neurons. Endocrinology. 1998;139:3822–9. doi: 10.1210/endo.139.9.6191. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Research Brain Research Reviews. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Vogt ML. Geoffrey Wingfield Harris, 1913-1971. Biographical Memoirs of Fellows of the Royal Society. 1972;18:309–29. doi: 10.1098/rsbm.1972.0010. [DOI] [PubMed] [Google Scholar]
- Wamsteeker Cusulin JI, Füzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the Hypothalamus of Crh-IRES-Cre Mutant Mice. PLoS ONE. 2013;8(5):e64943. doi: 10.1371/journal.pone.0064943. doi:10.1371/journal.pone.0064943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Frontiers in Neuroendocrinology. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Frontiers in Neuroendocrinology. 2005;26:109–30. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Watts AG. Structure and function in the conceptual development of mammalian neuroendocrinology between 1920 and 1965. Brain Research Reviews. 2011;66:174–204. doi: 10.1016/j.brainresrev.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Watts AG, Khan AM. Identifying links in the chain: the dynamic coupling of catecholamines, peptide synthesis, and peptide release in hypothalamic neuroendocrine neurons. Advances in Pharmacology. 2013;68:421–44. doi: 10.1016/B978-0-12-411512-5.00020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Physiological regulation of peptide messenger RNA colocalization in rat hypothalamic paraventricular medial parvicellular neurons. Journal of Comparative Neurology. 1995;352:501–14. doi: 10.1002/cne.903520403. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. Journal of Neuroscience. 2002;22:6282–9. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Diurnal variations in the content of preprocorticotropin-releasing hormone messenger ribonucleic acids in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–8. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Eds Hal Pashler & Randy Gallistell. John Wiley & Sons; 2002. Anatomy of Motivational Systems. In Stevens Handbook of Experimental Psychology, Volume 3, Learning, Motivation, and Emotion edn 3. pp563-632. [Google Scholar]
- Wetsel WC, Valença MM, Merchenthaler I, Liposits Z, López FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proceedings of the National Academy of Sciences U S A. 1992;89:4149–53. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Iacangelo A, Luo XZ, King C, Duncan K, Ginns EI. Transgenic expression of green fluorescent protein in mouse oxytocin neurones. Journal of Neuroendocrinology. 1999;11:935–9. doi: 10.1046/j.1365-2826.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Mezey E, Siegel RE. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neuroscience Letters. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- Yue C, Ponzio TA, Fields RL, Gainer H. Oxytocin and vasopressin gene expression and RNA splicing patterns in the rat supraoptic nucleus. Physiological Genomics. 2008;35:231–42. doi: 10.1152/physiolgenomics.90218.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Hintiryan H, Gou L, Song MY, Bay M, Bienkowski MS, Foster NN, Yamashita S, Bowman I, Toga AW, Dong HW. Neural networks of the mouse neocortex. Cell. 2014;156:1096–111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Young WS., 3rd Changes in cellular levels of messenger ribonucleic acid encoding gonadotropin-releasing hormone in the anterior hypothalamus of female rats during the estrous cycle. Endocrinology. 1988;123:1688–9. doi: 10.1210/endo-123-3-1688. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. Control of pituitary function. Nature. 1956;178:442–43. [Google Scholar]