Abstract
Rationale
Preclinical literature indicates that allopregnanolone (ALLO), a neuroactive steroid metabolized from progesterone, may protect against drug abuse behaviors. It is important to understand how ALLO varies during smoking changes in clinical samples of with depressive symptoms (DS) given they are at high risk of smoking relapse.
Objectives
The purpose of this paper is to characterize changes in ALLO by menstrual phase during short-term smoking cessation among women with and without DS.
Methods
At screening, study participants (n=84) were classified as either having past or current DS (n=48) or not (n=36). In a controlled cross-over trial design, participants completed two testing weeks in the follicular (F; low ALLO) and luteal (L; high ALLO) menstrual phases. During each testing week, blood samples were collected during ad libitum smoking and on the fourth day of biochemically verified smoking abstinence.
Results
Participants were, on average, 30.1 ± 6.7 years old, smoked 12.6 ± 5.7 cigarettes per day and most (73%) were White. The change in ALLO during short-term smoking cessation varied significantly by menstrual phase such that it decreased by 10% in the follicular phase and increased by 31% in the luteal phase. There were no significant differences in ALLO levels by DS group.
Conclusion
In premenopausal women, ALLO levels varied by menstrual phase and smoking status, but not DS. Given that other research has indicated L phase is associated with improved smoking cessation outcomes, an increase in ALLO during short-term cessation in the L phase may protect against relapse whereas a decrease in ALLO, as observed in the F phase, may increase risk for relapse.
Keywords: Females, Nicotine, Abstinence, Depression, Hormones
Allopregnanolone (ALLO) is a stress-reducing neurosteriod that, based on preclinical studies, appears to have a protective role against drug abuse behaviors (Carroll & Anker, 2010). For instance, ALLO produces an inhibitory influence on the escalation of the self-administration of cocaine in rats (Anker et al, 2008). ALLO has also been shown to reduce reinstatement behavior in cocaine-seeking female rats, but not their male counterparts (Anker & Carroll, 2010). Information on the effect of ALLO on drug abuse behaviors in clinical samples, especially females, is scarce. Women are at high risk for smoking relapse (USDHHS, 2001; Perkins, 2001; Perkins & Scott, 2008), perhaps due to sex hormones and their metabolites (including ALLO). Substantial evidence from the preclinical literature indicates that sex hormones may either improve or exacerbate this sex difference as progesterone has been shown to be protective against drug self-administration whereas estradiol has been shown to facilitate drug self-administration (Anker & Carroll, 2011; Lynch & Sofuglu, 2011). The clinical literature, however, is mixed. Two studies have observed improved smoking cessation rates in luteal phase (i.e. high progesterone, low estradiol) in the absence of nicotine replacement therapy (Allen et al, 2008; Mazure et al, 2011), whereas two others observed improved cessation rates in the follicular phase using nicotine replacement therapy (Carpenter et al, 2008; Franklin et al, 2008). ALLO is a metabolite of progesterone that varies by menstrual phase such that it is the lowest in the follicular phase and peaks in the luteal phase (Genazzani et al, 1998). The role of ALLO in smoking cessation outcomes is unknown.
Women with depressive symptoms are at increased risk of smoking relapse (e.g. Borrelli et al, 1996; Nakajima & al’Absi, 2012). Interestingly, the dysregulation of ALLO has been implicated in a number of psychiatric conditions, particularly mood disorders (Girdler & Klatzkin, 2007). For example, compared to controls with no history of Major Depressive Disorder (MDD), women with either a history of or current MDD had lower levels of both naturally occurring endogenous ALLO, as well as lower ALLO produced following administration of exogenous progesterone (Girdler & Klatskin, 2007; Klatskin et al, 2006; Strohle et al, 2000). Increases in ALLO levels after successful treatment of MDD via selective serotonin reuptake inhibitors (SSRIs) have been observed (Birzniece et al, 2006). The same positive relationship may exist between ALLO and depressive symptoms as higher ALLO levels were associated with an improved sense of well-being in premenopausal women and less depressive illness during the postpartum period (Nappi et al, 2001; Wang et al, 1996). Additional evidence comes from the preclinical literature where models of depression (such as social isolation) have been shown to reduce ALLO levels (Porcu, 2003; Roselli et al, 2011). However, Andreen and colleagues (2006) concluded that the association between ALLO and negative mood symptoms is not a linear relationship; rather, a U-shape curve between ALLO and negative mood may exist. Specifically, in postmenopausal women treated with exogenous progesterone, ALLO levels in the ‘mid-range’ (0.57–0.76 ng/mL or 1.5–2.0 nmol/L) had the highest negative mood scores, whereas women with lower (<0.57 ng/mL or <1.5 nmol/L) and higher (>0.76 ng/mL or >2.0 nmol/L) ALLO levels had lower levels of negative mood. In sum, these data suggest that depressive symptoms and ALLO may have either an inverse linear relationship (i.e., higher ALLO, lower depressive symptoms) or there may be an ideal therapeutic range - either way, ALLO and depressive symptoms appear to be related.
Overall, the literature suggests sex hormones and depressive symptoms play an important role in smoking behavior in women, especially during attempted smoking cessation; however, the causal factors involved in this process have yet to be identified. While ALLO varies dramatically by menstrual phase (Genazzani et al, 1998), very little information is available regarding the interaction between depressive symptoms and change of ALLO by menstrual phase among smokers, particularly during smoking cessation. Although it is plausible that women who have depressive symptoms may have an inability to recover from the stressful experience of smoking cessation via lack of an appropriate ALLO response, this information is lacking in the literature. Therefore, in this controlled cross-over study design where a sample of women with and without depressive symptoms were recruited and completed testing periods in the follicular and luteal menstrual phases our aims were to: (1) determine the ALLO levels by menstrual phase during ad libitum smoking and smoking abstinence, and (2) investigate the role of depressive symptoms on ALLO levels by menstrual phase during short-term smoking cessation. We hypothesized that ALLO levels would be higher in the luteal menstrual phase during both ad libitum smoking and smoking cessation. Further, regardless of menstrual phase, women who have depressive symptoms would have both lower absolute levels of ALLO, as well as a blunted menstrual phase variation in ALLO during short-term smoking cessation, versus women with no depressive symptoms.
Method
Participants
Women between the ages of 18–40 were recruited via mass media advertising to participate in a controlled cross-over study to examine the relationship of depressive symptoms and menstrual phase on short-term smoking cessation. Eligibility was initially assessed over the telephone followed by an in-person screening visit. To be eligible, women had to smoke at least five cigarettes per day for at least 12 months, have regular menstrual cycles for at least the past six months, and no major physical or mental health problems in the past six months. Exclusionary items included the use of exogenous hormones or psychotropic medications, and recent (< 3 months) pregnancy or breast feeding. Of the 225 participants who met eligibility criteria and were enrolled, 121 participants completed the parent study protocol (results forthcoming). This report is a secondary data analysis of participants (n=84) who had additional blood samples available for analysis of ALLO (i.e. those who had two or more missed blood draws and/or an insufficient volume of blood was collected were not included).
Procedure
All procedures were approved by the human subjects committee at the University of Minnesota. At the in-person screening visit (conducted during the early follicular phase), participants were stratified into one of two groups – past or current depressive symptoms (PCDS) and no depressive symptoms (NDS). Using the Composite International Diagnostic Interview (CIDI) Computer Assisted Interview (Wittchen et al, 1991) to assess the DSM-IV criteria (APA, 1994) and the Patient Health Questionnaire-9 (PHQ9; Spitzer et al, 1999, the PCDS group was defined as meeting at least one of the following criteria: (1) lifetime presence of depressed mood or loss of interest/pleasure for at least 14 consecutive days, (2) lifetime presence of four or more DSM-IV behavioral symptoms, and/or (3) a score of at least five, indicating mild depressive, on the PHQ9 symptoms (Kroenke et al, 2001). Those who met DSM-IV criteria for Premenstrual Dysphoric Disorder (PMDD) or Major Depressive Disorder (MDD) within the last six months were excluded and referred for treatment. The NDS group was defined as not meeting any of the criteria for the PCDS group.
After stratification, order of menstrual phase testing was randomly assigned. Participants were randomized to begin testing in the follicular phase followed by testing in the luteal (F-L) phase or vice versa (L-F). The six-day testing week started on the day after the onset of menses for F phase and two days after ovulation (determined with urine Luteinizing Hormone described in Allen et al, 2008) for the L phase. If schedule conflicts occurred, the entire testing week was shifted by one day in either direction. Each testing week included daily clinic visits for six consecutive days. On testing Days 1 and 2, participants were smoking ad libitum. At midnight on testing Day 2, participants quit smoking and remained abstinent for the next four days. Smoking status was confirmed via carbon monoxide breathalyzer (<5 ppm indicating abstinence), salivary cotinine samples (<15 ng/mL indicating abstinence), and serum nicotine samples (< 2 ng/ml indicating abstinence; Jarvis et al, 1987; Benowitz et al, 2008). Blood samples were collected at each clinic visit and analyzed for progesterone and estradiol for retrospective menstrual phase confirmation. Upon completion of the testing week, participants resumed ad libitum smoking for the 1.5 menstrual cycles (or approximately six weeks depending on cycle length) until they arrived at the alternate menstrual phase. Identical data collection procedures were then completed (Figure 1).
Figure 1.
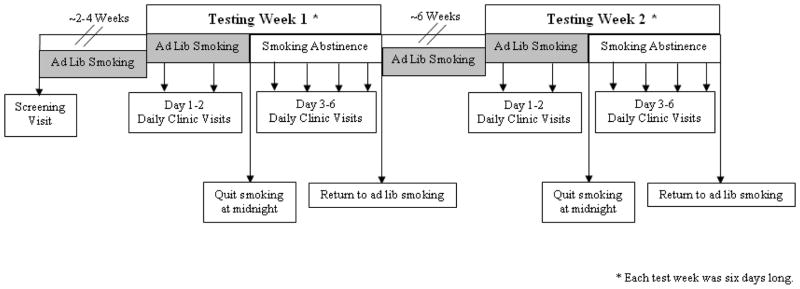
Study Protocol
A total of four blood samples to measure ALLO levels were collected. These samples were collected on Days 2 (ad libitum smoking) and 6 (fourth day of smoking abstinence) during each testing week. The University of California, San Diego measured concentrations of ALLO in batches of serum samples and ether extracted plasma samples via an in-house 3H-radioimmunoassay (Purdy et al, 1990). Intraassay and interassay coefficient of variations were approximately 5% and 8%, respectively. Sensitivity is about 200 pg/mL, with a standard range of 0.2 to 50.0 ng/mL (Alan Turken, personal communication, 5/31/12).
Data Analysis
Descriptive statistics were calculated for demographics and smoking behavior variables including mean and standard deviation for continuous variables, and percent for categorical variables. The “cigarettes per day” variable was log transformed given the distribution was non-normal. Differences by depressive symptoms groups in demographic and smoking behavior variables were assessed by t-tests and Chi-square tests. Paired t-tests were used to compare differences in ALLO levels by phase and smoking status. Multiple regression models were used to assess differences in absolute levels of ALLO during ad libitum smoking and smoking abstinence by menstrual phase. A random coefficient model with fixed linear time (i.e. menstrual cycle day) and an order effect was used to investigate the difference in ALLO levels by depressive symptoms status during the different menstrual phase and smoking status conditions. In subsequent analyses, a depressive symptoms group and time (i.e. menstrual cycle day) interaction variable was included in the model, and cigarettes smoked per day was assessed as a possible confounder using hierarchical regression. P-values less than 0.05 were deemed statistically significant. No adjustments for multiple comparisons were made. SAS V9.1.3 (SAS Institute, Cary, NC) was used for the analyses.
Results
Study Sample
A total of 121 study participants completed the parent study, but 37 were excluded from the present study due to a lack of available blood samples (81%), inability to remain abstinent during the testing week (14%), and having hormone levels (progesterone, estradiol) inconsistent with menstrual phase of testing (8%). Of the remaining 84 participants, 36 (43%) were stratified into the NDS group and 48 (57%) into the PCDS group. The PCDS group consisted of those with a history of MDD (n=17; 35%), subclinical depressive symptoms (n=6; 13%), and/or a PHQ9 score of five or more (n=43; 90%). Compared to those in the NDS group, those stratified into the PCDS group had significantly higher PHQ9 scores, indicating a higher level of depressive symptoms (1.8±1.4 vs. 11.7±6.2, p<0.001, respectively). Participants were, on average, 30.1 (± 6.7) years old, 73% were White and 36% had a high school education or less. On average, they smoked 12.6 (± 5.7) cigarettes per day. There were no significant differences in demographics or smoking behavior by depressive symptoms group (Table 1).
Table 1.
Demographics, Smoking Behavior & Depressive Symptoms Score by Depressive Symptoms Status (n=84)
| All (n=84) | No Depressive Symptoms (n=36) | Past or Current Depressive Symptoms (n=48) | T/Χ2 (p) | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 30.1 ± 6.7 | 29.9 ± 7.0 | 30.2 ± 6.6 | 0.18 (0.860) |
| Race (% White) | 73% | 67% | 77% | 3.03 (0.388) |
| Education (% ≤ High School) | 86% | 83% | 88% | 2.86 (0.586) |
| Smoking Behavior | ||||
| Cigarettes/Day | 12.6 ± 5.7 | 13.2 ± 5.5 | 12.1 ± 5.9 | 0.88 (0.380) |
| Time to First Cigarette (minutes) | 38.2 ± 37.8 | 38.6 ± 38.6 | 37.9 ± 37.5 | 0.09 (0.930) |
| PHQ9 (Depressive Symptoms) Score | 8.3 ± 6.9 | 1.8 ± 1.4 | 11.7 ± 6.2 | 6.90 (<0.001) |
ALLO Levels by Menstrual Phase & Smoking Status
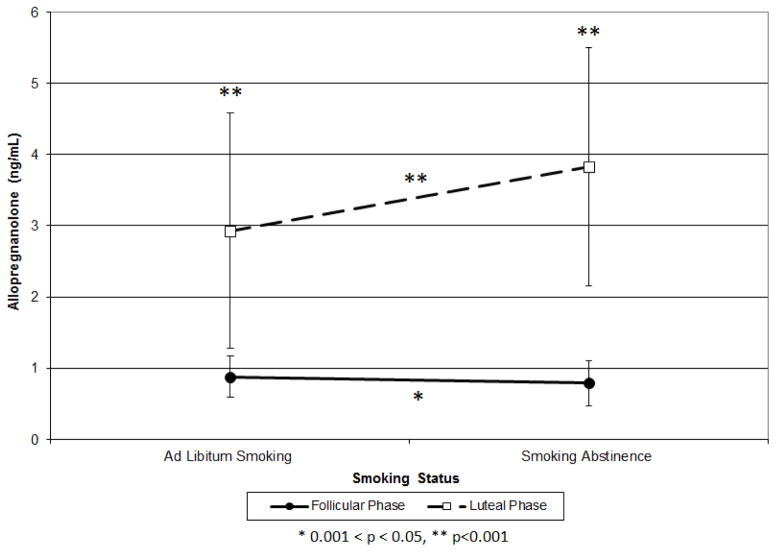
During the F phase, the average ALLO level decreased by 10% from ad libitum smoking (0.88±0.29 ng/mL) to smoking abstinence (0.79±0.32 ng/mL; t=2.03, p=0.047; Figure 2). Conversely, during the L phase, ALLO levels increased by 31% from ad libitum smoking (2.93±1.65 ng/mL) to smoking abstinence (3.83±1.67 ng/mL; t=4.71, p<0.001). During both ad libitum smoking and smoking abstinence, L phase ALLO levels were significantly higher than their corresponding F phase levels (t=10.67, p<0.001; t=16.00, p<0.0001; respectively).
Figure 2.
Allopregnanolone Levels by Menstrual Phase and Smoking Status
ALLO by Depressive Symptoms Status
Although time (i.e. menstrual cycle day) was a significant independent predictor (f=192.37, p<0.001) of absolute ALLO level, depressive symptoms status (f=0.05, p=0.831) did not predict ALLO level or the change in ALLO over time (i.e. menstrual cycle day; f=0.64, p=0.424). These results remained unchanged when controlling for cigarettes/day.
Discussion
In this controlled cross-over study in which women without depressive symptoms (n=36) and women with past or current depressive symptoms (n=48) completed two testing weeks during the follicular and luteal menstrual phases, including ad libitum smoking followed by four days of smoking abstinence, we observed three noteworthy findings. First, the luteal phase had significantly higher levels of allopregnanolone compared to the follicular phase, regardless of smoking status. Second, there was a significant menstrual phase difference in the change in allopregnanolone during smoking cessation compared to ad libitum smoking, such that it decreased in the follicular phase and increased in the luteal phase. Third, there were no significant differences between the women with and without depressive symptoms in the absolute or the change in allopregnanolone levels by menstrual phase and smoking status.
As expected based on prior research (Genazzani et al, 1998), allopregnanolone levels were significantly lower in the follicular phase as compared to the luteal phase. However, we found a novel finding with a significant 10% decrease in the follicular phase and a significant 31% increase in the luteal phase of allopregnanolone during short-term smoking cessation compared to ad libitum smoking. Since changes were observed in allopregnanolone during the luteal phase from approximately menstrual cycle day 17 to 21, the significant increase may be attributed to the typical fluctuation of allopregnanolone levels during the course of the menstrual cycle as it typically increases dramatically from ovulation (i.e. approximately menstrual cycle day 14) until later in the luteal phase (i.e. approximately menstrual cycle day 22; Genazzani et al, 1998; Nyberg et al, 2007). However, the observed decrease in allopregnanolone during the follicular phase is not as easily explained as allopregnanolone levels characteristically remain flat during this phase (Genazzani et al, 1998; Nyberg et al, 2007). However, our observations extend the findings of a recently published study by Childs and colleagues (2010) in which nonsmokers were exposed to an acute stressor, women in the luteal phase had a greater stress-induced allopregnanolone response compared to women in the follicular phase. Another explanation of these results may be that there is more progesterone, allopregnanolone’s precursor, available in the luteal phase than the follicular phase. This may allow for additional production of allopregnanolone in response to stress. The luteal phase has been associated with improved smoking cessation outcomes in the absence of nicotine replacement (Franklin & Allen, 2009). This may be due, in part, to the increase in allopregnanolone during this luteal phase. Allopregnanolone has been shown to be associated with the reduction of drug abuse behaviors, such as reduction of self-administration and a reduction of reinstatement behavior, in a number of preclinical studies, especially in females (Anker et al, 2008; Anker & Carroll, 2010). Thus, given that allopregnanolone is thought to have a protective effect against drug abuse behaviors (Bowen et al, 1999; Grant et al, 1997; Sinnott et al, 2002), an increase in allopregnanolone during smoking cessation during the luteal phase, regardless of mechanism of action, may protect against relapse whereas a drop in the follicular phase, as observed in this study, may increase risk for relapse.
Contrary to our hypotheses, we did not identify a significant difference in allopregnanolone based on depressive symptom status as previously observed (Girdler & Klatzkin, 2007). This observation appears to conflict with a recent study reporting that women with a history of MDD had a blunted allopregnanolone levels after exposure to an acute stressor compared to those without a history of MDD (Klatzkin et al, 2006), which may relate to lower or depressed production of allopregnanolone. There were a number of methodological differences between our study and Klatzkin’s including the inclusion of smokers in the study sample (100% versus < 14%, respectively) and the use of a more long-term stressor (e.g. smoking cessation) versus an acute stressor. Both of these factors may impact allopregnanolone production (Childs et al, 2010; Marx et al, 2006). Further, Klatzkin and colleagues compared those with a history of MDD to those without, whereas we compared any past or current depressive symptoms (i.e. history of MDD, or history or current subclinical depressive symptoms) to those without any depressive symptoms. While, our results remain unchanged in ad hoc analyses that compared history of MDD versus those without a history of MDD and those with current depressive symptoms versus those without (data not shown), these conflicting results may be due to the heterogeneous nature of our past or current depressive symptoms group. Therefore, additional research is needed to further explore allopregnanolone response to stress in women with and without a history of MDD, as well as those with and without current depressive symptoms. Given the effect of depressive symptoms on risk for smoking relapse and the potential for allopregnanolone to protect against smoking relapse, elucidating this relationship in women who smoke is of particular interest.
While this study has several strengths including the controlled cross-over study design, which limits bias and confounding, along with the detailed measurement of smoking status and allopregnanolone levels, it also has some limitations. First, our study sample included women who did not want to quit smoking permanently. Because our study participants knew they could return to smoking within a matter of days, it is possible that the allopregnanolone response to smoking cessation may be different in women who are intending to abstain from smoking indefinitely. Second, we did not include a sample of non-smokers. Given allopregnanolone has been shown to be correlated with cotinine levels in male smokers (Marx et al 2006), it is possible that smoking may effect allopregnanolone production. Therefore, it is unknown how much of the observed changes in allopregnanolone were due to smoking cessation itself, due to the natural fluctuation of allopregnanolone over the course of the menstrual cycle, or both. Third, we were not able to assess the effect of other Axis I disorders (such as anxiety) or other drugs of abuse (such as alcohol) on allopregnanolone levels; these factors may have influenced our results (e.g. Torrest & Ortega, 2003; Zheng, 2009). Lastly, we have a relatively small sample size, which limits our power to detect smaller differences in allopregnanolone levels. Despite these limitations this study fills a current void in the literature by being the first to assess menstrual phase differences in allopregnanolone levels in women with and without depressive symptoms during short-term smoking cessation.
In conclusion, this study observed significant increases in allopregnanolone levels from follicular to luteal phase in a sample of premenopausal smokers. During short-term smoking cessation, allopregnanolone levels changed significantly within each menstrual phase such that there was a significant decrease during the follicular phase and a significant increase in the luteal phase. These findings did not vary between women with and without depressive symptoms. Additional research is needed to verify this observation in a sample of premenopausal women who are attempting to quit smoking permanently and explore the effect of allopregnanolone on risk for relapse during attempted smoking cessation.
Acknowledgments
We would like to thank our research staff – Lindsay Farnsworth, Kathryn Resner, Sara Paradise, Nicole Tosun, Jennifer Widenmier, and Danielle Young – for their dedication to participant recruitment and follow-up, as well as data collection, entry and management. Additional thanks go to Dr. Darin Erickson and Dr. Bernard Harlow for their statistical expertise and editorial comments. We would also like to acknowledge Dr. Richard Hauger and Alan Turken at the University of California, San Diego for analyzing the allopregnanolone samples.
Funding
This project was funded by National Institute on Drug Abuse (NIDA) Grants R01-DA08075 and R36-DA032539, and the J.B. Hawley Award (School of Public Health, University of Minnesota). Additional support comes from Grant Number 1UL1RR033183 from the National Center for Research Resources (NCRR) and by Grant Number 8UL1TR000114-02 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) to the University of Minnesota Clinical and Translational Science Institute (CTSI). Dr. al’Absi was supported by R01DA016351 and R01DA027232. The University of Minnesota CTSI is part of a national Clinical and Translational Science Award (CTSA) consortium created to accelerate laboratory discoveries into treatments for patients Its contents are solely the responsibility of the authors, and do not necessarily represent the official views of CTSI, NIDA, NCRR, NCATS or NIH.
Footnotes
Conflicts of Interest
None
Contributor Information
Alicia M. Allen, Email: alle0299@umn.edu, Department of Family Medicine & Community Health, Medical School, University of Minnesota, 717 Delaware Street SE, Room 256, Minneapolis, MN 55414, Phone: 612-624-0896
Mustafa al’Absi, Department of Behavioral Sciences, Medical School, University of Minnesota, Duluth, 1035 University Drive, 236 SMed, D601A, Duluth, MN 55812
Harry Lando, Department of Epidemiology & Community Health, School of Public Health, University of Minnesota, 1300 South 2nd Street, 300 WBOB, Minneapolis, MN 55454
Dorothy Hatsukami, Department of Psychiatry, Medical School, University of Minnesota, 717 Delaware Street SE, Room 256, Minneapolis, MN 55414
Sharon S. Allen, Department of Family Medicine & Community Health, Medical School, University of Minnesota, 420 Delaware Street SE, Room A682, Minneapolis, MN 55455
References
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Nyberg S, Backstrom T. Allopregnanolone concentration and mood -- A biomodal association in postmenopausal women treated with oral progesterone. Psychopharmacology. 2006;187:209–221. doi: 10.1007/s00213-006-0417-0. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug and Alcohol Dependence. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, Biochemistry and behavior. 2008;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychological Association; 1994. Print. [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology. 2008;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. American Journal of Preventive Medicine. 1996;12:378–387. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus of endogenous neuroactive steroids: Effect of ethanol training dose and dosing procedure. Journal of Pharmacology and Experimental Therapeutics. 1999;289:405–411. doi: 10.1016/j.pbb.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Backstrom T, Johansson IM, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Research Reviews. 2006;51:212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Saladin ME, Leinbach AS, LaRowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: A pilot feasibility study. Journal of Women’s Health. 2008;17:293–301. doi: 10.1089/jwh.2007.0415. [DOI] [PubMed] [Google Scholar]
- Childs E, Dlugos A, DeWit H. Cardiovascular, hormonal and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010;47:550–559. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Allen SS. Influence of menstrual cycle phase on smoking cessation treatment outcome: A hypothesis regarding the discordant findings in the literature. Addiction. 2009:1941–1942. doi: 10.1111/j.1360-0443.2009.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortion N, O’Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: A retrospective analysis. Journal of Women’s Health. 2008;17:287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Benardi F, Casarosa E, Salvestroni C, Tonetti A, et al. Circulating levels of allopregnanolone in humans: Gender, age and endocrine influences. Journal of Clinical Endocrinology and Metabolism. 1998;83:2099–2103. doi: 10.1210/jc.83.6.2099. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: Implications for depressive disorders. Pharmacology & Therapeutics. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3 alpha-hydroxy-5 alcpha-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys. Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77:1435–1445. doi: 10.2105/AJPH.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow LA, Light KC, Pederson CA, Girdler SS. Histories of depression, allopregnanolone response to stress, and premenstrual symptoms in women. Biology Psychology. 2006;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of Progesterone in Nicotine Addiction: Evidence form initiation to relapse. Experimental and Clinical Psychoparmacology. 2010;18:451–461. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, et al. Neuroactive steroids, negative affect and nicotine dependence severity in male smokers. Psychopharmacology. 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open-label trial of bupropion. Drug & Alcohol Dependence. 2011;114:68–72. doi: 10.1016/j.drugalcdep.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M. Predictors of smoking relapse in men and women: A prospective examination. Psychology of Addictive Behavior. 2012;26:633–637. doi: 10.1037/a0027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues. Obstetrics & Gynecology. 2001;97:77–80. doi: 10.1016/S0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Backstrom T, Zingmark E, Purdy R, Poromaa IS. Allopregnanolone decrease with symptom improvement during placebo and gonadotropin-releasing hormone agonist treatment in women with severe premenstrual syndrome. Gynecological Endocrinology. 2007;23:257–266. doi: 10.1080/09513590701253511. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research. 2008;10:1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women: Special considerations. CNS Drug. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacology Biochemistry & Behavior. 2003;74:683–690. doi: 10.1016/S0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Rao PN, et al. Radioimmunoassay of 3-alpha-hydroxy-5-alpha-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128X(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Fin TJ, Ronnekleiv-Kelly SM, Tanchuck MA, Kaufman KR, Finn Deboarh A. Localization of brain 5-alpha-reductase meseenger RNA in mice selectively bred for high chronic alcohol withdrawal severity. Alcohol. 2011;45:763–772. doi: 10.1016/j.alcohol.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Mark GP, Finn DA. Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacology, Biochemistry and Behavior. 2002;72:923–929. doi: 10.1016/S0091-3057(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroneke K, Williams JB. Validation and utility of a self-report version of the PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Health Disorders, Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I. Action of progesterone and progesterone metabolites in menstrual-cycle-related disorders. Headache. 2008;48:S90–S98. doi: 10.1111/j.1526-4610.2008.01201.x. [DOI] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) Women and Smoking: A report of the Surgeon General. Public Health Service, Office of the Surgeon General; Rockville, Maryland: 2001. http://www.cdc.gov/tobacco/data_statistics/sgr/2001/complete_report/index.htm. [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences of the United States of America. 1998;17:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Backstrom T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: Study on serum pregnenolone, prenenolone sulfate, 5 alpha-pregnenlone-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. Journal of Clinical Endocrinology and Metabolism. 1996;81:1076–1082. doi: 10.1210/jc.81.3.1076. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The multicenter WHO/ADAMHA Field Trials. British Journal of Psychiatry. 1991;159:645–653. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neurosteroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Progress in Neurobiology. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]