Abstract
Long chain polyunsaturated fatty acids (PUFAs) are important structural components of cellular membranes and are converted into eicosanoids which serve various biological roles. The most common dietary n-6 and n-3 PUFAs are linoleic acid and α-linoleic acid, respectively. These 18-carbon chain fatty acids undergo a series of desaturation and elongation steps to become the 20-carbon fatty acids arachidonic acid and eicosapentaenoic acid, respectively. Evidence from genome wide association studies has consistently demonstrated that plasma and tissue levels of the n-6 long-chain PUFA arachidonic acid and to a lesser extent the n-3 long-chain PUFA eicosapentaenoic acid, are strongly influenced by variation in fatty acid desaturase-1,-2, and elongation of very long chain fatty acid genes. Studies of functional variants in these genes, as well as studies in which desaturase activity has been indirectly estimated by fatty acid product-to -precursor ratios, have suggested that endogenous capacity to synthesize long-chain PUFAs may be associated with metabolic diseases such as diabetes mellitus. Interventional studies are starting to tease out the complicated relationship between dietary intakes of specific fatty acids, variation in desaturase and elongase genes and tissue levels of long chain PUFAs. Thus future studies of dietary PUFA interventions designed to reduce inflammatory and metabolic diseases will need to carefully consider how an individual's genetically-determined endogenous long-chain PUFA synthesis capacity might modify therapeutic response.
Keywords: Acids, polyunsaturated, fatty acid, delta 5 desaturase, fatty acid delta 6 desaturase, eicosanoids, diabetes mellitus
Introduction
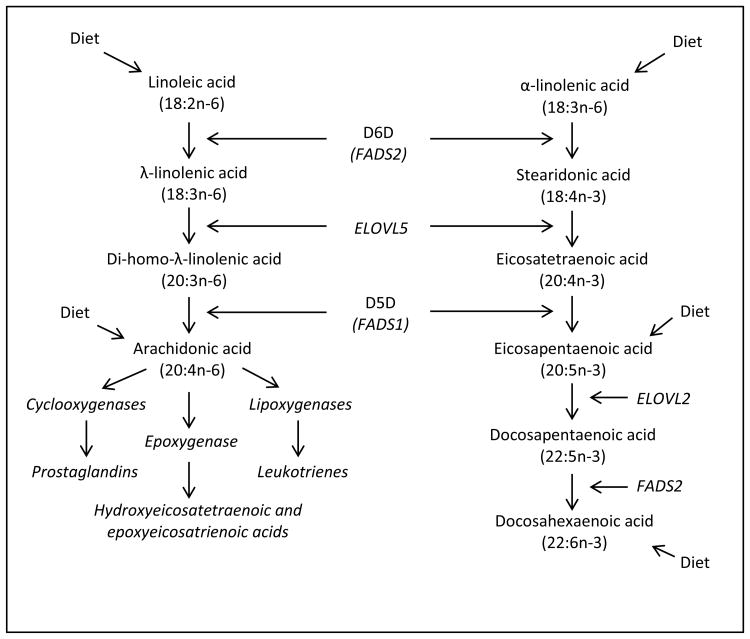
Long-chain polyunsaturated fatty acids (PUFA) are chains of 18 to 22 carbon atoms that contain two or more sequential double bonds. The two predominant classes of PUFAs are n-6 and n-3, categorized based on the position of the first double bond from the methyl end of the carbon chain (n- × nomenclature). In the Western diet, the n-6 PUFA linoleic acid (LA) (18:2n-6) accounts for 84-89% of total PUFA dietary intake [1]. LA is metabolized to arachidonic acid (ARA) (20:4n-6) through a series of desaturation and elongation reactions. The rate limiting step in this metabolic pathway is Δ6- desaturase (D6D, FADS2) which converts LA to γ-linolenic acid (GLA) (18:3n-6) [2]. GLA is then elongated to di-homo-γ-linolenic acid (DGLA) (20:3n-6) and then desaturated by Δ5-desaturase (D5D, FADS1) to ARA. Both the genes for D6D and D5D (Fatty Acid Desaturase (FADS)-2 and FADS1 respectively) exist in a cluster on chromosome 11 (11q12-q13.1) [3]. The elongase step involves Elongation of Very Long Chain Fatty Acid (ELOVL) 5 which is located on chromosome 6 (6p21.1) [4].
In the Western diet the most commonly consumed n-3 PUFA is α-linolenic acid (ALA) (18:3n3) which accounts for 9-11% of total PUFA intake. [1] ALA can be converted to eicosapentaenoic acid (EPA) (20:5n-3) through the same series of reactions. ALA is desaturated by D6D to stearidonic acid (SDA) (18:4n-3) then subsequently elongated to eicosatetraenoic acid (20:4n-3) and finally desaturated by D5D to EPA. Through an additional elongation (ELOVL2) and desaturation (D6D) EPA can be converted to docosahexaenoic acid (DHA) (22:6 n-3). While EPA can be produced in vivo from ALA, in humans, this process is extremely inefficient and most tissue EPA is derived from dietary consumption of fatty fish [5-7]. This inefficiency may result from the much higher levels of LA typically consumed in the Western diet. Stable isotope studies of ALA suggest that consuming 6.9% of dietary energy from ALA can result in increased production of endogenous EPA although DHA production is less influenced [8]. D6D has a greater affinity for ALA and is inhibited by EPA [9].
Endogenous and exogenous sources of long chain PUFA
Humans cannot synthesize 18-carbon chain n-6 or n-3 PUFAs so all sources of LA and ALA are exogenous. Sources of LA include sunflower, corn, cottonseed, and safflower oils. ALA is commonly found in seed oils such as flax, rapeseed, and chia oils. Long-chain PUFAs such as ARA and EPA can come from both exogenous and endogenous sources. ARA is directly consumed through meat and egg products while EPA and DHA are the primary PUFAs in fatty fish.
Endogenous synthesis of ARA from LA may comprise a significant proportion of total body stores of ARA. Interestingly, in the largest study to date in which red blood cell (RBC) membrane phospholipid fatty acids were measured in 160,000 patients, Harris et al. reported that RBC membrane ARA changed very little with age despite finding age-related changes in LA, EPA, and DHA [4]. These very consistent concentrations of ARA over multiple age groups suggest a homeostatic mechanism. Compared with other PUFAs, older individuals tended to have lower LA and higher EPA and DHA RBC membrane content when compared to younger adults. A recent systematic review of interventional studies concluded that typical dietary intakes of LA, as well as marked increases or decrease of dietary LA, did not seem to affect tissue ARA levels [10]. Tracer studies have reported very low levels of synthesis of ARA from LA ranging from 0.2% to 0.6% [8, 11].
Despite studies suggesting a low rate of conversion of ARA from LA, multiple genetic studies have suggested variants in FADS1, and -2 make major contributions to tissue ARA levels. In a study conducted in kibbutz settlements in Israel of 80 families, the heritability estimated for RBC membrane concentrations were 0.60 ± 0.11 for ARA, 0.52 ± 0.11 for EPA, and 0.65 ± 0.09 for DHA, suggesting a major heritable component of variance in tissue levels of long chain PUFAs [12]. Correlations of RBC membrane ARA levels between spouses were estimated at 0.08 compared to 0.18 between parent-child and 0.41 between siblings. Similarly, genomic studies have established that variants in FADS1 strongly influence RBC membrane and plasma levels of ARA [13]. To date nine genome-wide association studies (GWAS) found a strong association between single nucleotide polymorphisms (SNPs) at the FADS gene cluster and PUFA levels [14-22] with 18 to 28% of the additive variance in tissue ARA levels explained by FADS genotypes [23, 24]. In a GWAS of the InCHIANTI study, Tanaka et al. investigated genetic factors associated with plasma PUFA levels and found a dose-response relationship between alleles at the rs174537 SNP in FADS1 and ARA levels (8.76% for GG, 7.39% for GT, 6.35% for TT, P-value <0.0001) [14]. This finding was replicated in the GOLDN cohort with a similar statistically significant association with ARA concentration [14]. This SNP tags haplotype blocks described by two prior studies that found strong associations between FADS1 gene variation and tissue levels of ARA [23, 24]. Additional GWAS studies have also associated variants in ELOVL2 with n-3 PUFA levels [17, 19-21] although these results have not been consistently demonstrated in other studies [25].
Although the FADS gene cluster has a well-described role in PUFA metabolism, recent studies have demonstrated that FADS may also have a role in saturated and monounsaturated synthesis via de novo lipogenesis. In a large GWAS including 8,961 European ancestry individuals from 5 population-based cohorts, variants in FADS1 and -2 were associated with higher plasma palmitoleic acid (16:1n-7) and oleic acid (18:1n-9) levels and lower stearic acid (18:0) levels[26]. Mechanisms behind these intriguing findings are unknown but suggest there may be an association between PUFA synthesis and de novo lipogenesis.
In order to determine whether variation in desaturase genes might influence desaturase activity several studies have investigated polymorphisms in FADS1 and 2, and indirectly calculated fatty acid desaturase product to precursor ratios. D6D activity is usually estimated by dividing the RBC membrane concentration of GLA by the concentration of LA and D5D activity is estimated by dividing the concentration of DGLA by ARA [27]. This method has been utilized in several observational studies, although in some cases the ratio is calculated by dividing ARA by LA, which considers desaturase activity and elongase activity. In a random sample of 2,066 participants from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study, Zietemann et al. found that variation at rs174546 was related to D6D and D5D activity and that the dietary ratio of n-6 to n-3 PUFA might modify the relationship between this variant and D5D activity [28]. An additional study also demonstrated that genetic variants in FADS are associated with indirectly calculated FADS activity [29].
Estimated desaturase activity can be influenced by diet, particularly PUFA intake [27]. Vessby et al. conducted a randomized controlled, three-month dietary intervention comparing a diet containing butter fat (n = 17) with a diet containing monounsaturated fats (n = 17) [30]. Both diets contained 37% total energy from fats, with 17% energy from saturated fats (butter fat) versus 23% energy from MUFA in the comparison diet. In each diet participants were also randomized to receive fish oil capsules (2.4 g EPA and DHA) or placebo. There was no change in estimated D6D and D5D ratios in either serum cholesterol esters or phospholipids over the course of the study in either arm; however, participants randomized to fish oil had a decreased estimated D6D activity and an increased estimated D5D activity. These results are somewhat different from an 8-week crossover trial in 17 women comparing a high-fat diet (40% fat), a low-fat diet (20% fat) and a low-fat diet with the addition of 3% energy from n-3 PUFA (ALA, EPA, and DHA). The ratio of saturated to MUFA to PUFA was 1:1:1 in the high fat and the low fat diets [31]. In this study, the high-fat diet reduced D6D and increased D5D activity from baseline while the low-fat diet only increased D5D activity [31]. The low-fat with n-3 fatty acids diet had no effect on indirectly calculated desaturase activity which is intriguing as animal studies suggest that n-3 LC PUFAs inhibit D6D [32]. In this study, the total daily amount of EPA and DHA was 1.45 g and somewhat lower than the Vessby trial.
In addition, variants in desaturase and elongase genes might interact with dietary fats to influence PUFA tissue levels. In a secondary analysis of the Modulation of Atherosclerosis Risk by increasing dose of N-3 fatty Acids (MARINA) study, variants in ELOVL2 significantly influenced participant responses to n-3 supplementation [33]. This study found that minor alleles in three SNPs in ELOVL2 were associated with lower baseline levels of DHA but higher post-treatment levels of EPA and DHA compared to homozygotes for the major allele. This interaction was only seen in the high dose group (1.8 g EPA/DHA per day). Gillingham et al. compared a diet enriched with flaxseed oil or high-oleic acid canola oil to a Western diet in a randomized crossover clinical trial [34]. Although carriers of FADS variant minor alleles had lower EPA levels, they also had a marked increase in EPA levels in response to increasing ALA intake. Thus, a complicated relationship exists between intake of dietary PUFAs, genetic variation in desaturase and elongase enzymes, and tissue levels of long chain PUFAs, which has not been fully elucidated.
Lifestyle factors might also influence estimated desaturase activities [35]. In a cross-sectional analysis of 1,782 participants of the EPIC-Potsdam study estimated desaturase activities were calculated from RBC membrane phospholipids to discover dietary and lifestyle factors which might be associated with estimated activities [36]. Body mass index and waist-to-hip ratio were only weakly correlated with desaturase activity (positively correlated for D6D and negatively correlated for D5D). Alcohol use was positively correlated to D6D activity but only explained a small percentage of the variance (1.52%).
Arachidonic Acid Derived Eicosanoids
Understanding the contributions of endogenous long chain PUFA synthesis to tissue ARA stores could have important clinical implications given the biological function of ARA-derived eicosanoids and their strong role in many common diseases [37, 38]. ARA is released from the cellular membranes through the action of phospholipase A2. Free ARA can be converted into various series of 2, 4 prostaglandins (PG), leukotrienes (LT) and thromboxanes (TXs). In the cyclooxygenase (COX) pathway, ARA is enzymatically converted to PGG2 which is further reduced to PGH2. PGH2 is then metabolized via specific PG synthases into various bioactive lipid molecules including PGE2, PGD2, PGF2α, PGI2 and TXA2. These molecules then exert their cellular functions through binding to cell surface receptors of the 7 transmembrane G-coupled rhodopsin-type receptors. Some of the cellular functions of these ARA-derived eicosanoids include: inducing inflammation and promoting tumor angiogenesis (PGE2) inhibiting platelet aggregation and vasodilation (PGI2), and promoting platelet aggregation and vasoconstriction (TXA2).
EPA is converted to eicosanoids through the same enzymatic pathways as ARA but produces series-3 prostanoids that have less inflammatory actions due to lower receptor affinities when compared to ARA derived series-2 prostanoids [39, 40]. Several studies have demonstrated that increased consumption of marine fatty fish results in an increased phospholipid membrane proportion of EPA and a concomitant decreased in ARA [41-43].
Data on whether dietary changes in PUFA might impact the production of eicosanoids-derived from ARA has been inconsistent. In a randomized crossover trial including 17 postmenopausal women who consumed an 8-week high-fat diet, low-fat diet and a low-fat diet enriched with n-3 PUFAs, urinary prostaglandin E metabolite and 11-dehydro-thromboxane B2 concentrations were greater in the high-fat group compared to the low-fat group [44]. Interestingly plasma ARA increased with the low fat diet and decreased with the high fat diet. Clinical trials have demonstrated increased eicosanoid production with increased dietary n-6 PUFAs [45], decreased eicosanoid production with n-3 supplementation [46] or even increased concentrations of prostaglandin-F2α and thromboxane B2 with n-3 supplementation [47].
Although the effect of COX-2 (PTGS2) inhibitors on eicosanoid production, particularly PGE2 is well described [48], several studies have suggested that inhibition of D6D might also impact PGE2 production. He at al. found that reducing D6D activity through either RNAi knockdown or a selective D6D inhibitor reduced production of PGD2, PGE2, 12-HETE and 15-HETE by up to 80-95% in B16 melanoma cell lines [49]. They found a similar but lower reduction in these ARA-derived eicosanoids in LLC lung cancer cell lines.
Desaturase Activity and Intermediate Endpoints
Both genetic variation in FADS and alterations in indirectly calculated desaturase activity have been found to be associated with intermediate endpoints of disease. Recently published GWAS studies have found FADS variants associated with total cholesterol [50, 51], LDL cholesterol [50-52], HDL-cholesterol [50, 51, 53, 54], and triglyceride levels [50, 51, 53, 54]. FADS polymorphisms may not only impact absolute concentrations of certain blood lipids but also have other effects. Solakivi et al. genotyped 58 healthy volunteers for the FADS2 deletion variant rs3834458 located in the promoter region of D6D and measured oxidation of LDL and HDL2 cholesterol in response to CU2+ induced oxidation [55]. In homozygote carriers of the deletion, plasma total EPA and ARA were reduced. In addition the peroxidizability index of LDL cholesterol was markedly reduced. Stancakova et al. found that the FADS1 variant rs174550 was associated with increased concentrations of very large and large HDL particles [56]. Additional variants in this gene cluster have also been found to be associated with HDL particle size, although which variants are determinants of particle size and which are associated by linkage disequilibrium is unresolved [57].
The complex interaction between diet and genetic variation in endogenous long chain PUFA synthesis has also been investigated. Several studies have suggested that genetic variation in FADS appears to interact with dietary PUFA and possibly statin medications to influence serum lipids [58-60]. Interactions have been reported between variants within the FADS gene cluster, diet, and blood lipids. Within the Doetinchem Cohort Study, the C allele in rs174546 (FADS1) was associated with higher HDL cholesterol only in individuals with high intake of n-6 PUFAs (P for interaction = 0.02) [60]. Hellstrand et al. found no evidence of an interaction between the C allele in rs174547, HDL cholesterol, and intake of n-6 PUFAs however only carriers of the C allele had higher HDL cholesterol levels with increasing ALA to LA ratio. [61] These two studies suggest that diet and desaturase genotype may influence HDL levels but the direction of such an effect is still uncertain. To date no studies have investigated interactions between variants within the FADS gene cluster, dietary PUFAs, and glycemic traits, an area requiring future study. Fewer studies have investigated variants in elongase genes but noteworthy a recent population-based myocardial infarction case-control study investigated the association of seven ELOVL polymorphisms located in ELOVL2, ELOVL4 and ELOVL5 was unable to find any replicating association between any variants and serum lipids [25].
Although limited investigations utilizing genetic markers have been conducted, the effect of indirectly assessed desaturase activity has been investigated for inflammatory markers. In a study of Japanese school children, increased D6D and decreased D5D activity were correlated with CRP level [62]. This association was only seen for boys and not girls, which might suggest hormone interactions. In adults, Martinelli et al. found an increasing ratio of ARA to LA was associated with increased high-sensitivity C-reactive protein (hsCRP) [63]. Additionally, hsCRP concentrations increased with FADS alleles associated with the AA/LA ratio. Hong followed 122 non-obese healthy men for 3 years to determine the impact of variants in FADS on markers of oxidative stress. After 3 years, men with the rs17457 T allele had lower serum ARA, lower indirectly assessed D5D activity, and lower urinary 8-epi-prostaglandin F2α, a marker of oxidative stress [64]. In general these studies suggest that decreasing D5D activity and increasing D6D activity might be associated with increased inflammation, although the evidence is still limited. In addition, lower D5D appeared to be associated with reduced oxidative stress.
Desaturase Activity and Metabolic Risk Factors
The observation that diabetes and insulin resistance may be associated with defects in n-6 PUFA metabolism has been reported in several studies using varied approaches [35, 65-88]. To date, three GWAS studies have reported associations between FADS variants and fasting glucose or metabolic syndrome [89-91]. In cross-sectional studies, insulin resistance, as determined by euglycemic clamp studies or the homeostatic model assessment for insulin resistance (HOMA-IR) have consistently demonstrated that D6D activity is positively correlated with insulin resistance while D5D activity is negatively correlated [66, 68, 69, 73, 74, 88]. In longitudinal studies, indirectly calculated D6D activity is associated with worsening hyperglycemia based on oral-glucose tolerance testing [92].
In the Uppsala Longitudinal Study of Adult Men, high activity of D6D was associated with an increased risk of developing metabolic syndrome (OR=1.35, 95% CI 1.10-1.65) while higher activity of D5D was associated with a decreased risk (OR = 0.71, 95% CI 0.57-0.87).[72] Another prospective study found the risk of developing diabetes to be increased with greater D6D activity (OR = 1.31, 95% CI 0.92-1.88) and reduced with increasing D5D activity (OR = 0.83; 95% CI 0.60-1.14) [67]. These results were not statistically significant as the study included 450 participants and was limited in power. To date, five prospective cohort studies have associated decreased D5D and increased D6D activity with development of insulin resistance [72, 92-95].
In the European Prospective Investigation into Cancer and Nutrition study, high activity of D6D was associated with an increased risk of type 2 diabetes (T2D)(OR=2.46, 95% CI 1.67-3.63) while higher activity of D5D was associated with decreased risk (OR = 0.46, 95% CI 0.31-0.70) [94]. This study was notable in that the investigators confirmed these results using a Mendelian randomization approach using the SNP rs174546 as the instrumental variable, strongly supporting a causal relationship. In this study, genetically determined low D6D activity was associated with a reduced risk for diabetes while lower D5D activity was associated with increased risk. Additional evidence supporting a causal association between polymorphisms in FADS and D6D/D5D activity comes from expression quantitative trait locus (eQTL) mapping studies. Westra at al. conducted an eQTL meta-analysis which included a discovery set of 5,311 and a replication set of 2,775 non-transformed peripheral blood samples pooled from 7 studies [96]. These investigators found a SNP in the 3′ UTR region of FADS1 (rs174546) to effect expression of FADS1, FADS2 and TMEM258. This SNP has been found to be associated with LDL cholesterol levels [50-52] and interestingly this variant was also associated with LDLR gene expression levels suggesting additional downstream pathway effects of fatty acid desaturase polymorphisms.
Genetic variation in FADS genes may also modify the effects of n-3 PUFAs on fasting glucose. In a 6-week study of fish oil supplementation, a significant interaction was seen between supplement and FADS genotype on fasting glucose levels and HOMA-IR [97]. Participants took 5 g/d of fish oil and were genotyped for 18 SNPs in FADS1,-2. These investigators found a significant interaction between n-3 PUFA and fasting glucose based on rs482548 genotype.
While most prior studies of diabetes have focused on T2D in adults there is also evidence to suggest genetic variation in FADS might be associated with pancreatic beta cell dysfunction and type I diabetes. In the Diabetes Autoimmunity Study in the Young (DAISY) study 2,547 children at increased risk for type I diabetes, determine either through the detection of diabetes susceptibility alleles measured in cord blood or having a first-degree relative with type I diabetes were enrolled and followed with serial measurements for serum autoantibodies to insulin, glutamic acid decarboxylase, and insulinoma antigen 2. In this case-cohort study SNPs in FADS1, 2 (rs174556, rs174570, rs174583) significantly interacted with ALA intake, reducing the risk of islet autoimmunity [98].
Fatty Acid Desaturase Genetic Variants in CAD
The relationship of genetic variants of the FADS gene cluster to cardiovascular disease has been less clear. Baylin et al. found no association between FADS promoter variant rs3834458 which was related to EPA, ALA, and ARA levels in adipose tissue and plasma and myocardial infarction risk in a population-based case-control study conducted in Costa Rica [99]. In the Verona Heart Study, FADS haplotypes were associated with a higher desaturase activity and cardiovascular risk [63]. In a case-control study of coronary artery disease (CAD) in a Chinese Han population, rs174460 was found to be associated with CAD risk and increased D6D activity [100], and Qin et al. found rs174556 to be associated with CAD in Chinese Han [101]. In a case-cohort design from the prospective CAREMA study, estimated D5D activity, derived from measures of plasma cholesterol esters, was inversely associated with CAD risk [102]. In sub-analyses, participants with high baseline D5D activity estimates along with a high D5D activity genotype had a marked reduction in CAD risk compared to individuals with the lowest D5D activity and low activity genotype (HR = 0.35, 95% CI 0.15-0.81).
Conclusions
It is becoming increasingly clear that capacity to endogenously synthesize long-chain PUFAs, whether measured through genetic markers or fatty acid product-to-precursor ratios, is associated with metabolic diseases, with consistent effects noted for diabetes mellitus risk. It is possible that this association is mediated through the production of inflammatory eicosanoids; however additional studies are needed. Prior studies of PUFA-based dietary interventions on inflammatory mediators and disease outcomes have produced mixed results and this may stem from a failure to account for factors which might influence endogenous long-chain PUFA synthesis. A deeper understanding of the complicated relationship between dietary PUFAs and desaturase and elongase activity in vivo could help lead to better designed clinical studies and more patient-specific PUFA-based dietary interventions.
Figure 1. Endogenous Production of Polyunsaturated Fatty Acids.
Acknowledgments
Supported by the National Cancer Institute (grants CA143288 and CA160938)
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kris-Etherton PM, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1 Suppl):179S–88S. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–76. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 3.Marquardt A, et al. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000;66(2):175–83. doi: 10.1006/geno.2000.6196. [DOI] [PubMed] [Google Scholar]
- 4•.Harris WS, et al. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids. 2013;88(4):257–63. doi: 10.1016/j.plefa.2012.12.004. This study is the largest study to date that has measured erythrocyte membrane phospholipid fatty acids and described age- and gender-based norms. [DOI] [PubMed] [Google Scholar]
- 5.Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004;7(2):137–44. doi: 10.1097/00075197-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 7.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32(4):619–34. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 8.Hussein N, et al. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46(2):269–80. doi: 10.1194/jlr.M400225-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–44. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 10.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demmelmair H, et al. Comparison of bolus versus fractionated oral applications of [13C]-linoleic acid in humans. Eur J Clin Invest. 1999;29(7):603–9. doi: 10.1046/j.1365-2362.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre RN, et al. Familial aggregation of red blood cell membrane fatty acid composition: the Kibbutzim Family Study. Metabolism. 2008;57(5):662–8. doi: 10.1016/j.metabol.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Malerba G, et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43(4):289–99. doi: 10.1007/s11745-008-3158-5. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5(1):e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gieger C, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008;4(11):e1000282. doi: 10.1371/journal.pgen.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illig T, et al. A genome-wide perspective of genetic variation in human metabolism. Nat Genet. 2010;42(2):137–41. doi: 10.1038/ng.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhre K. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477(7362):54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kettunen J, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44(3):269–76. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demirkan A, et al. Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS Genet. 2012;8(2):e1002490. doi: 10.1371/journal.pgen.1002490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye M, et al. Novel Loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 2012;8(8):e1002907. doi: 10.1371/journal.pgen.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaitre RN. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7(7):e1002193. doi: 10.1371/journal.pgen.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong MG, et al. A genome-wide assessment of variability in human serum metabolism. Hum Mutat. 2013;34(3):515–24. doi: 10.1002/humu.22267. [DOI] [PubMed] [Google Scholar]
- 23.Rzehak P, et al. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 ( FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2009;101(1):20–6. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 24.Schaeffer L, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15(11):1745–56. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 25.Aslibekyan S, et al. Genetic variation in fatty acid elongases is not associated with intermediate cardiovascular phenotypes or myocardial infarction. Eur J Clin Nutr. 2012;66(3):353–9. doi: 10.1038/ejcn.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Wu JH, et al. Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway: results from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium. Circ Cardiovasc Genet. 2013;6(2):171–83. doi: 10.1161/CIRCGENETICS.112.964619. Very interesting study which identified common variants in genes associated with polyunsaturated fatty acid metabolisms to be associated with the de novolipogenesis pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merino DM, Ma DW, Mutch DM. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010;9:63. doi: 10.1186/1476-511X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zietemann V, et al. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Br J Nutr. 2010;104(12):1748–59. doi: 10.1017/S0007114510002916. [DOI] [PubMed] [Google Scholar]
- 29.Bokor S, et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res. 2010;51(8):2325–33. doi: 10.1194/jlr.M006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vessby B, et al. Indices of fatty acid desaturase activity in healthy human subjects: effects of different types of dietary fat. Br J Nutr. 2013;110(5):871–9. doi: 10.1017/S0007114512005934. [DOI] [PubMed] [Google Scholar]
- 31•.Raatz SK, et al. Total dietary fat and fatty acid content modifies plasma phospholipid fatty acids, desaturase activity indices, and urinary prostaglandin E in women. Nutr Res. 2012;32(1):1–7. doi: 10.1016/j.nutres.2011.12.006. Clinical trial that suggests that dietary fat content might influence desaturase activity and that low fat diets with or without fish oil may reduce prostaglandin E2 production. [DOI] [PubMed] [Google Scholar]
- 32.Garg ML, et al. Delta 6-desaturase activity in liver microsomes of rats fed diets enriched with cholesterol and/or omega 3 fatty acids. Biochem J. 1988;249(2):351–6. doi: 10.1042/bj2490351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alsaleh A, et al. ELOVL2 gene polymorphisms are associated with increases in plasma eicosapentaenoic and docosahexaenoic acid proportions after fish oil supplement. Genes Nutr. 2014;9(1):362. doi: 10.1007/s12263-013-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillingham LG, et al. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr. 2013;97(1):195–207. doi: 10.3945/ajcn.112.043117. [DOI] [PubMed] [Google Scholar]
- 35.Warensjo E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16(2):128–36. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Schiller K, et al. Associated factors of estimated desaturase activity in the EPIC-Potsdam study. Nutr Metab Cardiovasc Dis. 2014;24(5):503–10. doi: 10.1016/j.numecd.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Greene ER, et al. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011;96(1-4):27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Gennaro A, Haeggstrom JZ. The leukotrienes: immune-modulating lipid mediators of disease. Adv Immunol. 2012;116:51–92. doi: 10.1016/B978-0-12-394300-2.00002-8. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61(3):345–58. doi: 10.1079/pns2002166. [DOI] [PubMed] [Google Scholar]
- 40.Wada M, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282(31):22254–66. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 41.Chilton FH, et al. Dietary n-3 fatty acid effects on neutrophil lipid composition and mediator production. Influence of duration and dosage. J Clin Invest. 1993;91(1):115–22. doi: 10.1172/JCI116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibney MJ, Hunter B. The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. 1993;47(4):255–9. [PubMed] [Google Scholar]
- 43.Healy DA, et al. Effect of low-to-moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35(7):763–8. doi: 10.1007/s11745-000-0583-1. [DOI] [PubMed] [Google Scholar]
- 44.Young LR, et al. Effect of dietary fat and omega-3 fatty acids on urinary eicosanoids and sex hormone concentrations in postmenopausal women: a randomized controlled feeding trial. Nutr Cancer. 2011;63(6):930–9. doi: 10.1080/01635581.2011.589957. [DOI] [PubMed] [Google Scholar]
- 45.Blair IA, et al. Dietary modification of omega 6 fatty acid intake and its effect on urinary eicosanoid excretion. Am J Clin Nutr. 1993;57(2):154–60. doi: 10.1093/ajcn/57.2.154. [DOI] [PubMed] [Google Scholar]
- 46.Ferretti A, et al. Effect of fish oil supplementation on the excretion of the major metabolite of prostaglandin E in healthy male subjects. Lipids. 1991;26(7):500–3. doi: 10.1007/BF02536593. [DOI] [PubMed] [Google Scholar]
- 47.Zulyniak MA, et al. Fish oil supplementation alters circulating eicosanoid concentrations in young healthy men. Metabolism. 2013;62(8):1107–13. doi: 10.1016/j.metabol.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He C, et al. Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS One. 2012;7(10):e47567. doi: 10.1371/journal.pone.0047567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Global Lipids Genetics, C et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teslovich TM, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabatti C, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41(1):35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kathiresan S, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterworth DM, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;30(11):2264–76. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solakivi T, et al. Delta-6-desaturase gene polymorphism is associated with lipoprotein oxidation in vitro. Lipids Health Dis. 2013;12:80. doi: 10.1186/1476-511X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stancakova A, et al. Effects of 34 risk loci for type 2 diabetes or hyperglycemia on lipoprotein subclasses and their composition in 6,580 nondiabetic Finnish men. Diabetes. 2011;60(5):1608–16. doi: 10.2337/db10-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chasman DI, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5(11):e1000730. doi: 10.1371/journal.pgen.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reardon HT, et al. Insertion-deletions in a FADS2 intron 1 conserved regulatory locus control expression of fatty acid desaturases 1 and 2 and modulate response to simvastatin. Prostaglandins Leukot Essent Fatty Acids. 2012;87(1):25–33. doi: 10.1016/j.plefa.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barber MJ, et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS One. 2010;5(3):e9763. doi: 10.1371/journal.pone.0009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y, et al. Dietary n-3 and n-6 polyunsaturated fatty acid intake interacts with FADS1 genetic variation to affect total and HDL-cholesterol concentrations in the Doetinchem Cohort Study. Am J Clin Nutr. 2010;92(1):258–65. doi: 10.3945/ajcn.2009.29130. [DOI] [PubMed] [Google Scholar]
- 61.Hellstrand S. Intake levels of dietary long-chain PUFAs modify the association between genetic variation in FADS and LDL-C. J Lipid Res. 2012;53(6):1183–89. doi: 10.1194/jlr.P023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito E, et al. Abdominal adiposity is associated with fatty acid desaturase activity in boys: implications for C-reactive protein and insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2013;88(4):307–11. doi: 10.1016/j.plefa.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Martinelli N, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008;88(4):941–9. doi: 10.1093/ajcn/88.4.941. [DOI] [PubMed] [Google Scholar]
- 64.Hong SH, et al. Association of polymorphisms in FADS gene with age-related changes in serum phospholipid polyunsaturated fatty acids and oxidative stress markers in middle-aged nonobese men. Clin Interv Aging. 2013;8:585–96. doi: 10.2147/CIA.S42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corpeleijn E, et al. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: the SLIM study. Diabetologia. 2006;49(10):2392–401. doi: 10.1007/s00125-006-0383-4. [DOI] [PubMed] [Google Scholar]
- 66.Enriquez YR, et al. Fatty acid composition of erythrocyte phospholipids is related to insulin levels, secretion and resistance in obese type 2 diabetics on Metformin. Clin Chim Acta. 2004;346(2):145–52. doi: 10.1016/j.cccn.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 67.Krachler B, et al. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–10. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Murakami K, et al. Lower estimates of delta-5 desaturase and elongase activity are related to adverse profiles for several metabolic risk factors in young Japanese women. Nutr Res. 2008;28(12):816–24. doi: 10.1016/j.nutres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Sartore G, et al. Desaturase activities and metabolic control in type 2 diabetes. Prostaglandins Leukot Essent Fatty Acids. 2008;79(1-2):55–8. doi: 10.1016/j.plefa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Vessby B. Desaturation and elongation of Fatty acids and insulin action. Ann N Y Acad Sci. 2002;967:183–95. doi: 10.1111/j.1749-6632.2002.tb04275.x. [DOI] [PubMed] [Google Scholar]
- 71.Warensjo E, et al. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr Metab Cardiovasc Dis. 2008;18(10):683–90. doi: 10.1016/j.numecd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48(10):1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 73.Warensjo E, et al. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou YE, et al. Decreased activity of desaturase 5 in association with obesity and insulin resistance aggravates declining long-chain n-3 fatty acid status in Cree undergoing dietary transition. Br J Nutr. 2009;102(6):888–94. doi: 10.1017/S0007114509301609. [DOI] [PubMed] [Google Scholar]
- 75.Nigam A, et al. Relationship between n-3 and n-6 plasma fatty acid levels and insulin resistance in coronary patients with and without metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19(4):264–70. doi: 10.1016/j.numecd.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Riserus U. Fatty acids and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2008;11(2):100–5. doi: 10.1097/MCO.0b013e3282f52708. [DOI] [PubMed] [Google Scholar]
- 77.Wang L, et al. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78(1):91–8. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 78.Berry EM. Dietary fatty acids in the management of diabetes mellitus. Am J Clin Nutr. 1997;66(4 Suppl):991S–997S. doi: 10.1093/ajcn/66.4.991S. [DOI] [PubMed] [Google Scholar]
- 79.Horrobin DF. Fatty acid metabolism in health and disease: the role of delta-6-desaturase. Am J Clin Nutr. 1993;57(5 Suppl):732S–736S. doi: 10.1093/ajcn/57.5.732S. discussion 736S-737S. [DOI] [PubMed] [Google Scholar]
- 80.Aro A. Fatty acid composition of serum lipids: is this marker of fat intake still relevant for identifying metabolic and cardiovascular disorders? Nutr Metab Cardiovasc Dis. 2003;13(5):253–5. doi: 10.1016/s0939-4753(03)80028-5. [DOI] [PubMed] [Google Scholar]
- 81.Truong H, et al. Does genetic variation in the Delta6-desaturase promoter modify the association between alpha-linolenic acid and the prevalence of metabolic syndrome? Am J Clin Nutr. 2009;89(3):920–5. doi: 10.3945/ajcn.2008.27107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steffen LM, et al. Serum phospholipid and cholesteryl ester fatty acids and estimated desaturase activities are related to overweight and cardiovascular risk factors in adolescents. Int J Obes (Lond) 2008;32(8):1297–304. doi: 10.1038/ijo.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sjogren P, et al. Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia. 2008;51(2):328–35. doi: 10.1007/s00125-007-0876-9. [DOI] [PubMed] [Google Scholar]
- 84.Warensjo E, et al. Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. Am J Clin Nutr. 2006;84(2):442–8. doi: 10.1093/ajcn/84.1.442. [DOI] [PubMed] [Google Scholar]
- 85.Das UN. A defect in the activity of Delta6 and Delta5 desaturases may be a factor predisposing to the development of insulin resistance syndrome. Prostaglandins Leukot Essent Fatty Acids. 2005;72(5):343–50. doi: 10.1016/j.plefa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Lovejoy JC, et al. Relationship of dietary fat and serum cholesterol ester and phospholipid fatty acids to markers of insulin resistance in men and women with a range of glucose tolerance. Metabolism. 2001;50(1):86–92. doi: 10.1053/meta.2001.19440. [DOI] [PubMed] [Google Scholar]
- 87.Lewis-Barned NJ, et al. Plasma cholesteryl ester fatty acid composition, insulin sensitivity, the menopause and hormone replacement therapy. J Endocrinol. 2000;165(3):649–55. doi: 10.1677/joe.0.1650649. [DOI] [PubMed] [Google Scholar]
- 88.Pan DA, et al. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J Clin Invest. 1995;96(6):2802–8. doi: 10.1172/JCI118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zabaneh D, Balding DJ. A genome-wide association study of the metabolic syndrome in Indian Asian men. PLoS One. 2010;5(8):e11961. doi: 10.1371/journal.pone.0011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manning AK. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–69. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahendran Y, et al. Association of erythrocyte membrane fatty acids with changes in glycemia and risk of type 2 diabetes. Am J Clin Nutr. 2014;99(1):79–85. doi: 10.3945/ajcn.113.069740. [DOI] [PubMed] [Google Scholar]
- 93.Hodge AM, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr. 2007;86(1):189–97. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 94••.Kroger J, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93(1):127–42. doi: 10.3945/ajcn.110.005447. This study finds an association between calculated desaturase activites and diabetes risks using product-to-precursor ratios but is notable for including a Mendelian Randomization approach to futher support the causal relationship between desaturase activity and diabetes mellitus risk. [DOI] [PubMed] [Google Scholar]
- 95.Patel PS, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. 2010;92(5):1214–22. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 96.Westra H. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–43. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cormier H, et al. Polymorphisms in Fatty Acid Desaturase (FADS) Gene Cluster: Effects on Glycemic Controls Following an Omega-3 Polyunsaturated Fatty Acids (PUFA) Supplementation. Genes (Basel) 2013;4(3):485–98. doi: 10.3390/genes4030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Norris JM, et al. Erythrocyte membrane docosapentaenoic acid levels are associated with islet autoimmunity: the Diabetes Autoimmunity Study in the Young. Diabetologia. 2014;57(2):295–304. doi: 10.1007/s00125-013-3106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baylin A, et al. alpha-Linolenic acid, Delta6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am J Clin Nutr. 2007;85(2):554–60. doi: 10.1093/ajcn/85.2.554. [DOI] [PubMed] [Google Scholar]
- 100.Li SW, et al. FADS gene polymorphisms confer the risk of coronary artery disease in a Chinese Han population through the altered desaturase activities: based on high-resolution melting analysis. PLoS One. 2013;8(1):e55869. doi: 10.1371/journal.pone.0055869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qin L, et al. A case-control study between the gene polymorphisms of polyunsaturated fatty acids metabolic rate-limiting enzymes and coronary artery disease in a Chinese Han population. Prostaglandins Leukot Essent Fatty Acids. 2011;85(6):329–33. doi: 10.1016/j.plefa.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y, et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS One. 2012;7(7):e41681. doi: 10.1371/journal.pone.0041681. [DOI] [PMC free article] [PubMed] [Google Scholar]