Abstract
Patient: Female, 66
Final Diagnosis: Breast cancer metastasis in medication-related osteonecrosis of the jaw
Symptoms: —
Medication: —
Clinical Procedure: Clinical and radiological examination • surgical treatment
Specialty: Dentistry
Objective:
Rare co-existance of disease or pathology
Background:
Many authors have considered dental implants to be unrelated to increased risk of medication-related osteonecrosis of the jaw (MRONJ). Nevertheless, more recently, more cases of peri-implant MRONJ (PI-MRONJ) have been described, thus becoming a challenging health problem. Also, metastatic cancer deposits are not infrequently found at peri-implant sites and this may represent an additional complication for such treatments. We present the case of a breast cancer patient with PI-MRONJ, presenting a clinically and radiologically undetected metastasis within the necrotic bone, and highlight the necessity of an accurate histopathological analysis.
Case Report:
A 66-year-old female patient, who had received intravenous bisphosphonates for bone breast cancer metastases, came to our attention for a non-implant surgery-triggered PI-MRONJ. After surgical resection of the necrotic bone, conventional and immunohistochemical examinations were performed, which showed breast cancer deposits within the necrotic bone.
Conclusions:
Cancer patients with metastatic disease, who are undergoing bisphosphonate treatment, may develop unusual complications, including MRONJ, which is a site at risk for hosting additional metastatic deposits that may be clinically and radiologically overlooked. Such risk is increased by previous or concomitant implant procedures. Consequently, clinicians should be prudent when performing implant surgery in cancer patients with advanced-stage disease and consider the possible occurrence of peri-implant metastases while planning adequate treatments in such patients.
MeSH Keywords: Bisphosphonate-Associated Osteonecrosis of the Jaw, Breast Neoplasms, Dental Implants, Neoplasm Metastasis
Background
Medication-related osteonecrosis of the jaw (MRONJ) is a well-recognized severe complication of bisphosphonate (BPs) treatment in patients with osteoporosis or metastatic cancer. MRONJ is defined as the presence of exposed necrotic bone in the maxillofacial region, which does not heal within 8 weeks after clinical identification, occurring in patients undergoing BPs therapy who have not received radiotherapy to the jaws [1]. Although initially believed to be exclusively associated with bisphosphonates, MRONJ has been found in recent reports to be associated with additional drugs, especially the bone antiresorptive, denosumab [2–4]. The pathogenesis of MRONJ is generally linked to surgical procedures, even if spontaneous development of MRONJ has been reported [5]. Nevertheless, the role of dental implant procedures as MRONJ pathogenetic factors [1,6–10] is still unclear and the issues of whether BPs treatment should be an exclusion criterion for dental implants insertion, the mode of administration (intra-venous vs. oral) may influence the occurrence of complications [11], and whether oncologic patients are at higher risk for MRONJ than osteoporotic patients [11] are still matters of debate. Several studies have examined the occurrence of MRONJ after the placement of dental implants in patients undergoing oral BPs therapy, and many of them reported absence of MRONJ as a complication in hundreds of patients (Fugazzotto et al. [12], Grant et al. [8], Bell and Bell [7], and Jeffcoat [13]). Bell and Bell [7] concluded that patients under oral BPs show no greater risk of implant failure than untreated patients. Notably, no retrospective or prospective studies on the placement of dental implants in patients receiving intravenous BPs have been performed; nevertheless, some authors postulated dental implant placement was contraindicated in patients taking intravenous BPs but not in those taking oral BPs [1].
More recently, an increasing number of peri-implant MRONJs (PI-MRONJs) have been described (Bedogni et al. [11], Favia et al. [14], Lazarovici et al. [15], Kwon et al. [16], and Jacobsen et al. [9]). Also, PI-MRONJ has been classified into 2 types: implant surgery-triggered, when it develops within 6 months after implant surgery, suggesting that the surgical process may be a contributing factor, and non-implant surgery-triggered, if it develops 6 months or more after implant surgery, or when BPs administration started after implant placement and osteointegration [16].
Consequently, PI-MRONJ currently is considered an additional complication related to oral implants, along with nerve injury, bleeding, cortical plate perforation, sinus perforation, mandibular fracture, implant ingestion/aspiration, peri-implantitis, and mucositis [17–19].
Another exceptionally reported adverse event related to dental implants in oncologic patients is the localization of metastases around implant fixtures [20,21]. Diagnosis at an early stage in such instances is challenging, since their clinical and radiological appearance generally mimics unspecific peri-implant infections [22]. To date, only 4 cases of peri-implant metastases from breast and lung cancer have been reported [20–22].
In the current study, we present the case of a breast cancer patient affected by PI-MRONJ, in whom metastatic deposits were detected in the necrotic bone of MRONJ around dental implants, and highlight the role of accurate histopathological analyses in view of the lack of specific clinico-radiological signs, which could suggest the simultaneous occurrence of metastasis.
Case Report
A 66-year-old female patient was diagnosed with breast cancer in 2005 and was subsequently treated by chemotherapy, mastectomy, and radiotherapy. Also, due to the occurrence of bone metastases, she underwent 4 mg intravenous zoledronate/monthly for 33 months (from September 2009 to June 2012). On October 2013, the patient was referred to the Odontostomatology Unit of the University of Bari, Italy for intraoral necrotic bone exposure around 4 dental implants on the right anterior mandible, associated with pain, pus discharge, and paresthesia of the right inferior alveolar nerve (Figure 1). Rx OPT showed a poorly defined radiolucent area extending from the posterior right mandible to the premolar opposite region, which also included 4 dental implants, at 3.1, 4.1, 4.4 and 4.6 (Figure 2A). Three additional implants (at 1.6, 3.5, and 3.6) showed adequate osteointegration.
Figure 1.
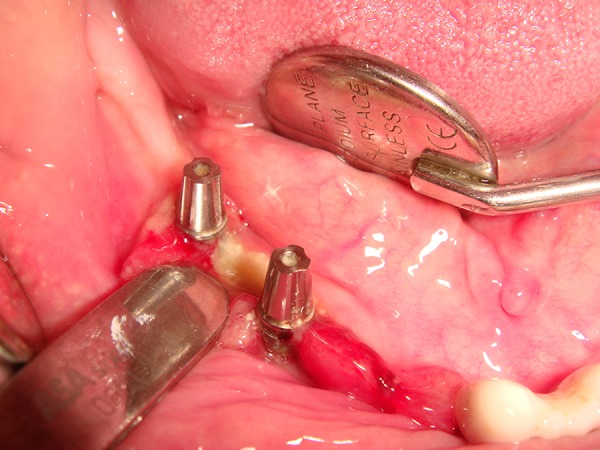
Intra-oral necrotic bone exposure around dental implants on the right mandible.
Figure 2.
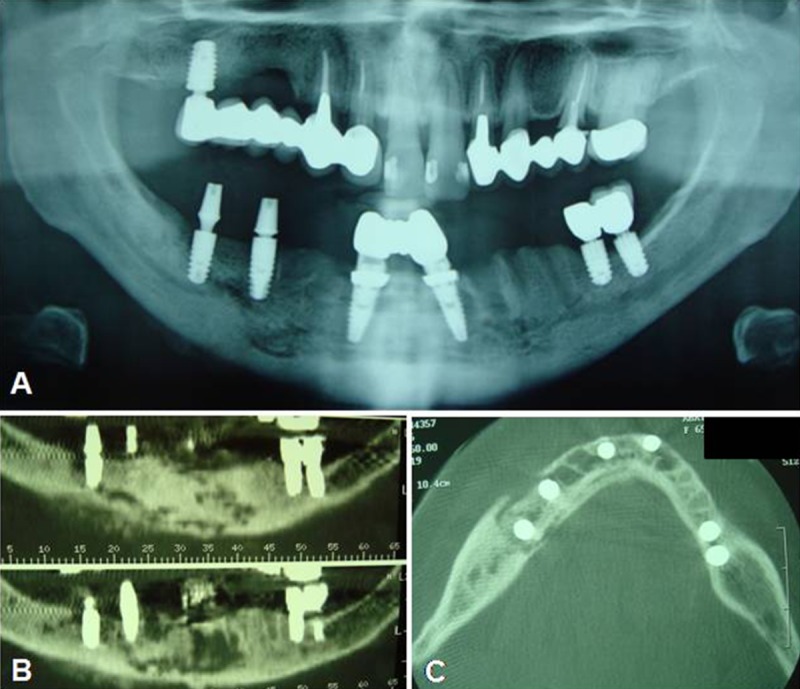
(A) Rx OPT showing a poorly-defined radiolucent area including 4 dental implants, located at 3.1, 4.1, 4.4, and 4.6; (B) and (C) CT scans showing an osteolytic lesion involving both dental implants and the inferior alveolar nerve.
Multislice spiral computed tomography (CT) showed mandibular osteolytic lesions, which involved the implants located at 3.1, 4.1, 4.4, and 4.6 and the inferior alveolar nerve, causing paresthesia (Figure 2B, 2C).
The patient’s clinical history revealed that all the dental implants had been placed during 2008, more than 6 months before the start of BPs therapy, and appeared radiologically well-osteointegrated at the beginning of BPs administration, thus indicating a non-implant surgery-triggered PI-MRONJ. Cycles of antibiotics were administered, consisting of a combination of ceftriaxone (1 g once a day i.m.) and metronidazole (500 mg twice a day per os) for 8 days, followed by 10 days of interruption after each cycle. Subsequently, under general anesthesia, surgical resection of the necrotic bone was performed, consisting of mandibular partial resection from 4.8 to 3.5, and removal of the 4 involved dental implants. The depth of resection was pinpointed by bleeding evaluation of residual bone tissues. Piezoelectric tools were used on the resection margins to remove residual damaged bone tissues, and a sterile gel formulation of sodium hyaluronate and amino acids Gly-Pro-Leu-Lys was placed into the bone defect, allowing for faster bone regeneration and healing at the surgical site.
The whole surgical samples were promptly fixed in neutral-buffered formalin for 48 h and then divided into 2 parts, 1 of wich was sent to the Pathological Anatomy Unit of the University of Bari, decalcified in formic acid (5% in distilled water) for 24 h, embedded in paraffin, sectioned at 4-µm thickness, and stained with hematoxylin-eosin (H&E). Additional sections collected on positively-charged slides were used for the immunohistochemical stains with wide-spectrum cytokeratins (clone AE1/AE3, dilution 1:20; Dako), cytokeratin 7 (clone OVTL 12/30, dilution 1:100; Dako), and estrogen receptors (clone 1D5, dilution 1:20; Dako), using a polymer-based (EnVision Flex, Dako) detection system with an automated immunostainer (Autostainer, Dako). Finally, the sections were lightly counter-stained with hematoxylin and coverslipped. Appropriate negative controls, obtained by substituting the primary antibodies with pre-immune serum, and positive controls (breast cancer) were included in the procedure.
The other part of the each sample was sent to the Pathological Anatomy Unit of the University of Chieti, dehydrated in alcohol, and embedded in a glycol-methacrylate resin. After polymerization, the specimen was sectioned longitudinally with high-precision diamond disk at about 150 µm and ground down to about 30 µm. The slides were stained with acid fuchsin and toluidine blue.
Overall, the histopathological analysis of the decalcified samples showed areas of extensive bone necrosis, without residual osteocytes/osteoblasts, with large and empty Haversian canals, consistent reduction of blood vessels, abundant inflammatory cell infiltration (mainly neutrophils, plasma cells, monocytes, and lymphocytes), and several basophilic bacterial colonies interspersed with necrotic debris. The medullary spaces were mostly replaced by fibrous connective tissue, including inflammatory cells (Figure 3A). About 5% of the analyzed tissue samples also included small clusters of neoplastic cells (Figure 3B), consistent with metastatic breast cancer based on wide-spectrum cytokeratins, cytokeratin 7, and estrogen receptor immunoreactivity (Figure 3C).
Figure 3.
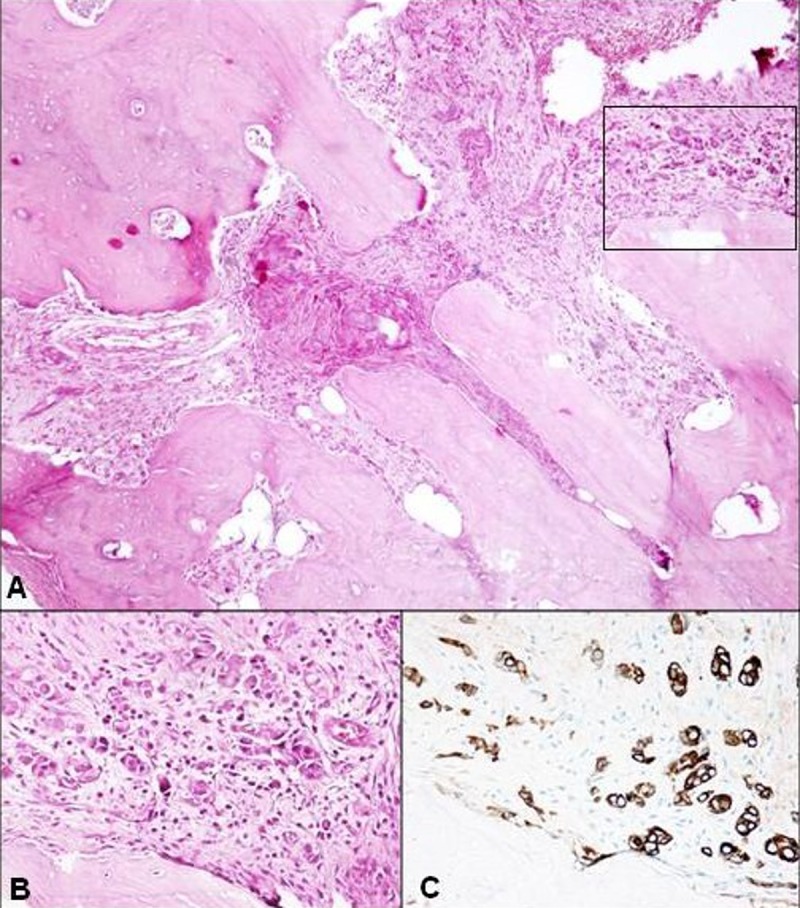
(A) Panoramic view showing poorly-vascularized osteonic and newly-formed bone; the medullary spaces are replaced by fibrous connective tissue, including inflammatory cells and (upper right) neoplastic cells. (Hematoxylin-Eosin; ×40). (B) At higher magnification, the neoplastic cells are seen as being grouped in small clusters or dispersed in the fibrous stroma where inflammatory cells are also present. (Hematoxylin-Eosin; ×100). (C) The neoplastic epithelial cells demonstrate strong immunoreactivity for cytokeratin 7 (×200).
Undecalcified samples, consisting of bone tissues and implant fixture, showed bone necrosis around the implant and the adjacent alveolar bone, empty Haversian system without cellular component, and remarkable inflammatory infiltration (Figure 4).
Figure 4.
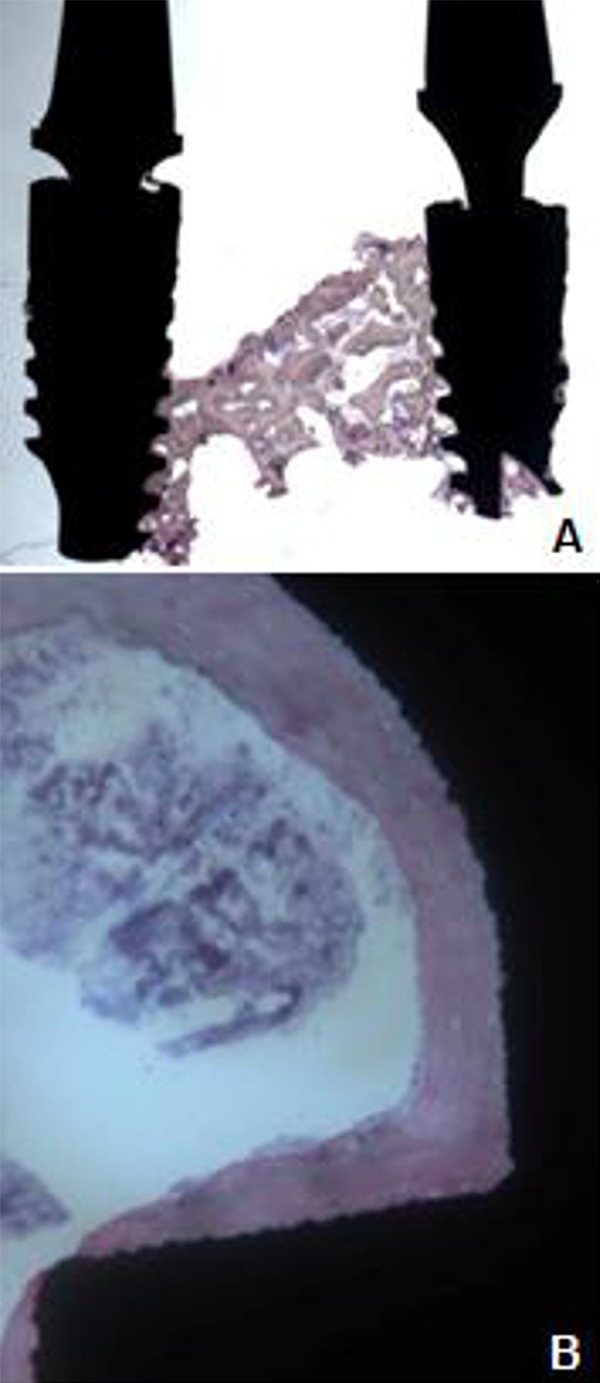
Undecalcified tissue samples with extensive bone necrosis around implant fixtures.
Following the surgical treatment, wounds healed without complications, and after 12 months no signs of recurrence of MRONJ were detectable (Figure 5). Rehabilitation with a removable prosthesis (Figure 6) for functional and aesthetic reasons was chosen, with good stabilization of the surgical sites. In view of the poor oral hygiene of the patient, with increased risk for subsequent infectious complications, strict follow-up was undertaken on a monthly basis for the first 6 months, and every 3 months thereafter, the patient having remained free of additional events for 18 months.
Figure 5.
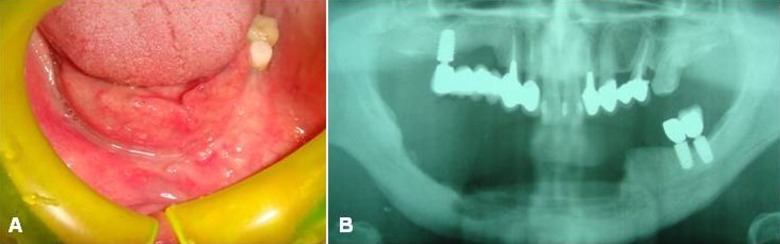
Clinical (A) and radiological (B) complete healing of the surgical wound without recurrence (at 11-month follow-up).
Figure 6.
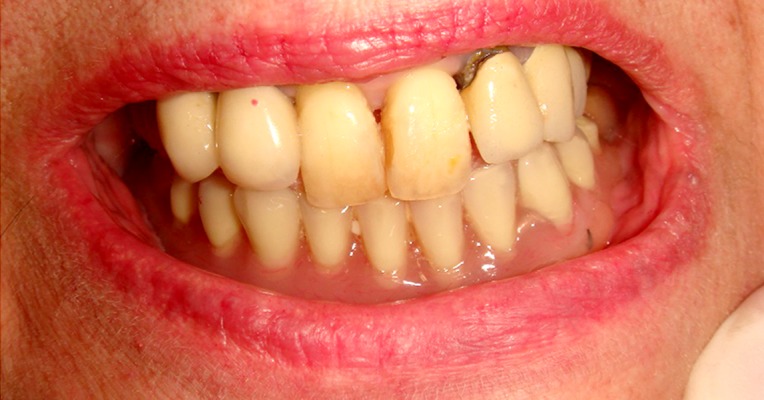
Prosthetic rehabilitation with removable prosthesis.
This study was performed in accordance to the principles of the Declaration of Helsinki and was approved by our institution ethics committee (Study no. 4599, Prot. 1528/C.E.); the patient released informed consent for diagnostic and therapeutic procedures and possible use of the biologic samples for research purposes.
Discussion
PI-MRONJ has recently been identified as a challenging problem for many dental practitioners, consisting in the development of osteonecrosis around dental implants. In the past, many studies considered that dental implants were unrelated to increased risk for MRONJ development, especially in patients taking oral BPs [6–8]; nevertheless, over the last few years, an increasing number of PI-MRONJs have been reported [11,14–16]. In particular, some authors considered BPs treatment as a contraindication to oral implants [23], and not only the surgical insertion of a dental implants (implant surgery-triggered MRONJ), but also the mere presence of inserted implants (non implant surgery-triggered MRONJ) were identified as risk factors for the development of osteonecrosis [9].
Peri-implant metastases are additional implant-related adverse events, which have been rarely reported in the literature [22–24], breast cancer being the most common primary site in women, and lung and prostate the most common in men [25,26]. To date, the pathogenesis of the metastatic process in the jawbones, and particularly around dental implants, is still unclear. One theory is that, in view of the abundance of bone marrow in the mandibular premolar and molar regions, reduced blood flow rates facilitate the entrapment of metastatic cells [27]. Local factors, such as trauma or surgical procedures, could further increase such a process, due to the entrapment of tumor cells during clot formation in fresh wounds and to host-generated growth factors locally released by regenerating tissues, which would stimulate hosting and proliferation of malignant tumor cells. Chronic inflammation also promotes local metastasis because circulating tumor cells may be entrapped within the rich capillary network of chronically inflamed tissues [28,29]. The diagnosis of such events at an early stage may be challenging, since their clinical appearance mimics non-specific peri-implant infections, with pain, bleeding and swelling. Moreover, radiographic findings such as peri-implant osteolysis are not clearly distinguishable from conventional peri-implantitis [22]. Even PET-CT may not allow proper diagnosis of peri-implant metastases because MRONJ-related inflammation and tissue remodeling may be responsible for persistent areas of contrast uptake.
In the current study, we report on a patient affected by PIMRONJ and breast cancer metastasis, in whom dental implants had been placed more than 6 months before BPs therapy was started, thus suggesting a non implant surgery-triggered MRONJ. Consequently, surgical removal of necrotic bone and involved implants was performed, with complete healing of the lesion and no evidence of recurrence after prolonged follow-up, notwithstanding poor oral hygiene. The latter certainly might predispose to subsequent additional infectious complications, thus suggesting strict and prolonged follow-up to possibly reduce such risk.
Following histopathological examination of both calcified and undecalcified surgical samples, a “frozen-type” MRONJ [16–30] was diagnosed, showing extensive bone necrosis around the implants and the adjacent alveolar bone, with remarkable inflammatory infiltration. Conventional histological and immunohistochemical analyses also highlighted small clusters of neoplastic cells, consistent breast cancer metastasis of minimal extension (5% of the analyzed sample), and peripherally located rather distant from the implant site. We speculate such morphological features of the lesion may indicate that osteonecrosis occurred before neoplastic cell colonization and, PI-MRONJ-associated chronic inflammation may have been responsible for neoplastic cell colonization.
Conclusions
PI-MRONJ nowadays is a well-documented, though infrequent, complication of implant procedures in patients undergoing BPs therapy. In this context, special attention should be paid to cancer patients, in view of the possible concomitant occurrence of metastases at peri-implant sites, which may be clinicoradiologically overlooked.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References:
- 1.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons: Position paper on bisphosphonate-related osteonecrosis of the jaws - 2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Katsarelis H, Shah NP, Dhariwal DK, Pazianas M. Infection and medication-related osteonecrosis of the jaw. J Dent Res. 2015;94:534–39. doi: 10.1177/0022034515572021. [DOI] [PubMed] [Google Scholar]
- 3.Oh KC, Moon HS, Lee JH, et al. Effects of alendronate on the peri-implant bone in rats. Oral Dis. 2015;21:248–56. doi: 10.1111/odi.12258. [DOI] [PubMed] [Google Scholar]
- 4.Liddelow G, Klineberg I. Patient-related risk factors for implant therapy. A critique of pertinent literature. Aust Dent J. 2011;56:417–26. doi: 10.1111/j.1834-7819.2011.01367.x. [DOI] [PubMed] [Google Scholar]
- 5.Lam DK, Sándor GK, Holmes HI, et al. A review of bisphosphonate-associated osteonecrosis of the jaws and its management. J Can Dent Assoc. 2007;73:417–22. [PubMed] [Google Scholar]
- 6.Koka S, Babu NMS, Norell A. Survival of dental implants in post-menopausal bisphosphonate users. J Prosthodont Res. 2010;54:108–11. doi: 10.1016/j.jpor.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Bell BM, Bell RE. Oral bisphosphonates and dental implants: a retrospective study. J Oral Maxillofac Surg. 2008;66:1022–24. doi: 10.1016/j.joms.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Grant BT, Amenedo C, Freeman K, Kraut RA. Outcomes of placing dental implants in patients taking oral bisphosphonates: a review of 115 cases. J Oral Maxillofac Surg. 2008;66:223–30. doi: 10.1016/j.joms.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen C, Metzler P, Rössle M, et al. Osteopathology induced by bisphosphonates and dental implants: clinical observations. Clin Oral Invest. 2013;17:167–75. doi: 10.1007/s00784-012-0708-2. [DOI] [PubMed] [Google Scholar]
- 10.Goss A, Bartold M, Sambrook P, Hawker P. The nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in dental implant patients: a South Australian case series. J Oral Maxillofac Surg. 2010;68:337–43. doi: 10.1016/j.joms.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Bedogni A, Bettini G, Totola A, et al. Oral bisphosphonate-associated osteonecrosis of the jaw after implant surgery: a case report and literature review. J Oral Maxillofac Surg. 2010;68:1662–66. doi: 10.1016/j.joms.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 12.Fugazzotto PA, Lightfoot WS, Jaffin R, Kumar A. Implant placement with or without simultaneous tooth extraction in patients taking oral bisphosphonates: Postoperative healing, early followup, and the incidence of complications in two private practices. J Periodontol. 2007;78:1664–69. doi: 10.1902/jop.2007.060514. [DOI] [PubMed] [Google Scholar]
- 13.Jeffcoat MK. Safety of oral bisphosphonates: Controlled studies on alveolar bone. Int J Oral Maxillofac Implants. 2006;21:349–53. [PubMed] [Google Scholar]
- 14.Favia G, Piattelli A, Sportelli P, et al. Osteonecrosis of the posterior mandible after implant insertion: a clinical and histological case report. Clin Implant Dent Relat Res. 2011;13:58–63. doi: 10.1111/j.1708-8208.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 15.Lazarovici TS, Yahalom R, Taicher S, et al. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010;68:790–96. doi: 10.1016/j.joms.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Kwon TG, Lee CO, Park JW, Choi SY, et al. Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clin Oral Implants Res. 2012;25:632–40. doi: 10.1111/clr.12088. [DOI] [PubMed] [Google Scholar]
- 17.Camargo IB, Van Sickels JE. Surgical complications after implant placement. Dent Clin North Am. 2015;59:57–72. doi: 10.1016/j.cden.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Misch K, Wang HL. Implant surgery complications: etiology and treatment. Implant Dentistry. 2008;17:159–68. doi: 10.1097/ID.0b013e3181752f61. [DOI] [PubMed] [Google Scholar]
- 19.Smeets R, Henningsen A, Jung O, et al. Definition, etiology, prevention and treatment of peri-implantitis – a review. Head Face Med. 2014;10:34. doi: 10.1186/1746-160X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orhan K, Bayndr H, Aksoy S, et al. Numb chin syndrome as a manifestation of possible breast cancer metastasis around dental implants. J Craniofac Surg. 2011;22:942–45. doi: 10.1097/SCS.0b013e31820fe1af. [DOI] [PubMed] [Google Scholar]
- 21.Tseng HS, Chen LS, Kuo SJ, et al. Tumor characteristics of breast cancer in predicting axillary lymph node metastasis. Med Sci Monit. 2014;20:1155–61. doi: 10.12659/MSM.890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfammatter C, Lindenmüller IH, Lugli A, et al. Metastases and primary tumors around dental implants: A literature review and case report of peri-implant pulmonary metastasis. Quintessence Int. 2012;43:563–70. [PubMed] [Google Scholar]
- 23.Scully C, Madrid C, Bagan J. Dental endosseous implants in patients on bisphosphonate therapy. Implant Dentistry. 2006;15:212–18. doi: 10.1097/01.id.0000236120.22719.02. [DOI] [PubMed] [Google Scholar]
- 24.Hirshberg A, Berger R, Allon I, Kaplan I. Metastatic tumors to the jaws and mouth. Head and Neck Pathol. 2014;8:463–74. doi: 10.1007/s12105-014-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishinari Y, Kashiwaba M, Umemura A, et al. Pulmonary hilar lymph node metastasis of breast cancer induced bronchopleural fistula and superior vena cavasyndrome. Am J Case Rep. 2014;15:492–95. doi: 10.12659/AJCR.892159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andree C, Schmidt VJ, Munder BI, et al. Detecting of breast cancer metastasis by means of regional lymph node sampling during autologous breast-reconstruction – a screening of 519 consecutive patients. Med Sci Monit. 2012;18(10):CR605–10. doi: 10.12659/MSM.883486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankar S. Dental pulp metastases and pan-osseous mandibular involvement with mammary adenocarcinoma. Br J Oral Maxillofac Surg. 1984;22:455–61. doi: 10.1016/0266-4356(84)90053-6. [DOI] [PubMed] [Google Scholar]
- 28.Dib LL, Soares AL, Sandoval RL, Nannmark U. Breast metastasis around dental implants: a case report. Clin Implant Dent Relat Res. 2007;9:112–15. doi: 10.1111/j.1708-8208.2007.00033.x. [DOI] [PubMed] [Google Scholar]
- 29.Favia G. Manifestazioni oro-facciali di Malattie Sistemiche e Generalizzate. 2nd ed. Fasano di Puglia: Schena Editor; 2005. [in Italian] [Google Scholar]
- 30.Favia G, Pilolli GP, Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009;45:406–13. doi: 10.1016/j.bone.2009.05.008. [DOI] [PubMed] [Google Scholar]


