Soft Tissue Sarcoma
Soft tissue sarcoma (STS) is diagnosed in approximately 15,000 patients yearly.1 The rarity of STS has historically made it difficult to study. However, over the last 30 years large clinical databases and tissue banks have led to a rapid increase in our understanding of STS.2 What was once thought to be a single disease entity is now known to represent an umbrella diagnosis describing almost 100 individual pathologies, each with a unique genomic underpinning (Table 1), progenitor cell, pattern of spread, prognosis (Figure 1), and sensitivity to adjuvant therapies. An understanding of these unique subtypes has allowed us to develop new diagnostic studies based on their associated genetic mutations (e.g., FISH for gene translocations or immunohistochemistry for aberrant protein expression as in Table 1), improving diagnosis, and in some instances leading to targeted therapies currently in clinical trials (e.g., imatinib for inhibition of activated c-Kit mutation in gastrointestinal stromal tumors). STS can occur in almost any region of the body, but is most common in the extremities (40%) and intra-abdominal and retroperitoneal regions (38%).2 Each of these locations is associated with a propensity to develop specific histologic subtypes (Figure 2).
Table 1.
Genomic alterations associated with common soft tissue sarcomas
| Histologic subtype | Genomic alteration |
|---|---|
| Well and dedifferentiated liposarcoma | 12q13-15 amplification (CDK4/MDM2 overexpression)57 |
| Myxoid/round cell liposarcoma | FUS-CHOP translocation58 |
| Synovial sarcoma | SYT-SSX translocation59 |
| Desmoid tumor | CTNNB1 mutation (nuclear β-catenin expression)60 |
| Solitary fibrous tumor | NAB2-STAT6 translocation (nuclear STAT6 expression)61,62 |
| eiomyosarcoma | Complex copy number alterations57 |
| Gastrointestinal stromal tumor | Activating C-KIT mutation63 |
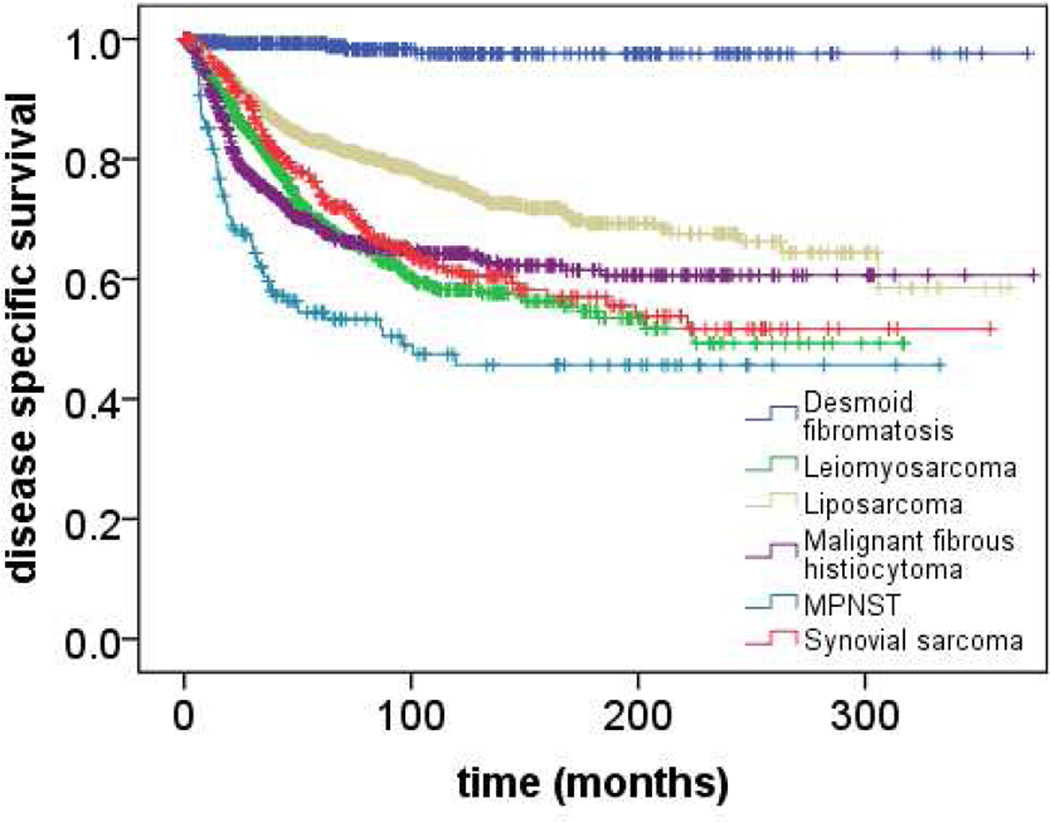
Figure 1.
Disease specific survival following surgical resection of primary soft tissue sarcoma (n=3840) between 1982 and 2014 at a single institution stratified by histologic subtype of primary soft tissue sarcomas.
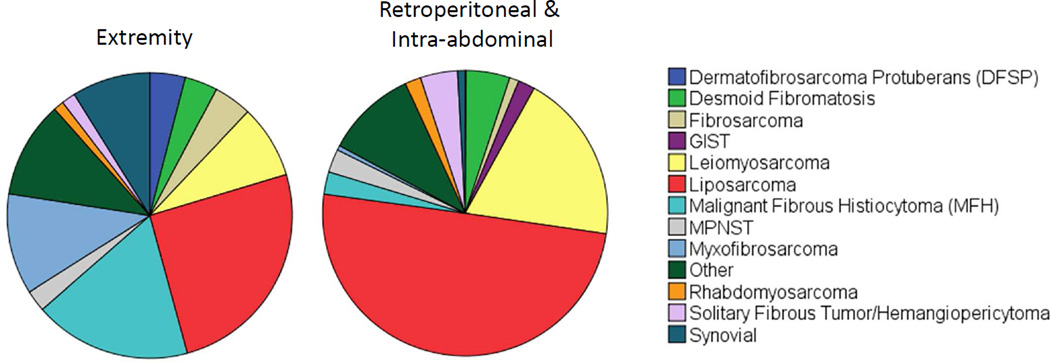
Figure 2.
Distribution of common histologic subtypes of primary soft tissue sarcoma resected from extremity (n=3045) and retroperitoneal/intraabdominal (n=945) locations at a single institution between 1982 and 2014.
Risk factors associated with the development of STS are poorly understood. Trauma has been linked to the disease, but whether injury is the direct cause of tumor initiation is unclear. It is likely that in many cases trauma simply draws attention to a preexisting lesion. Radiation exposure has clearly been associated with development of aggressive STS with a poor prognosis.3 Radiation is closely linked with the development of tumors with complex chromosomal abnormalities and is a common cause of angiosarcoma of the breast when prescribed in the adjuvant setting following lumpectomy.4 Angiosarcoma of the extremity has also been observed in the context of chronic lymphedema (Stewart-Treves syndrome).5 Hereditary syndromes predispose patients to developing STS, particularly Li-Fraumeni syndrome (p53 mutation associated with malignant fibrous histiocytoma [MFH] and myxofibrosarcoma), Gardner’s syndrome (APC mutation associated with polyposis and desmoid tumor) and neurofibromatosis (NF1 mutation associated malignant peripheral nerve sheath tumors [MPNST]).6–8 Less common causes of STS are environmental exposures linked to the development of specific histologic subtypes of STS (e.g., hepatic angiosarcoma following vinyl chloride exposure).9
Diagnosis and Management of Extremity Sarcomas
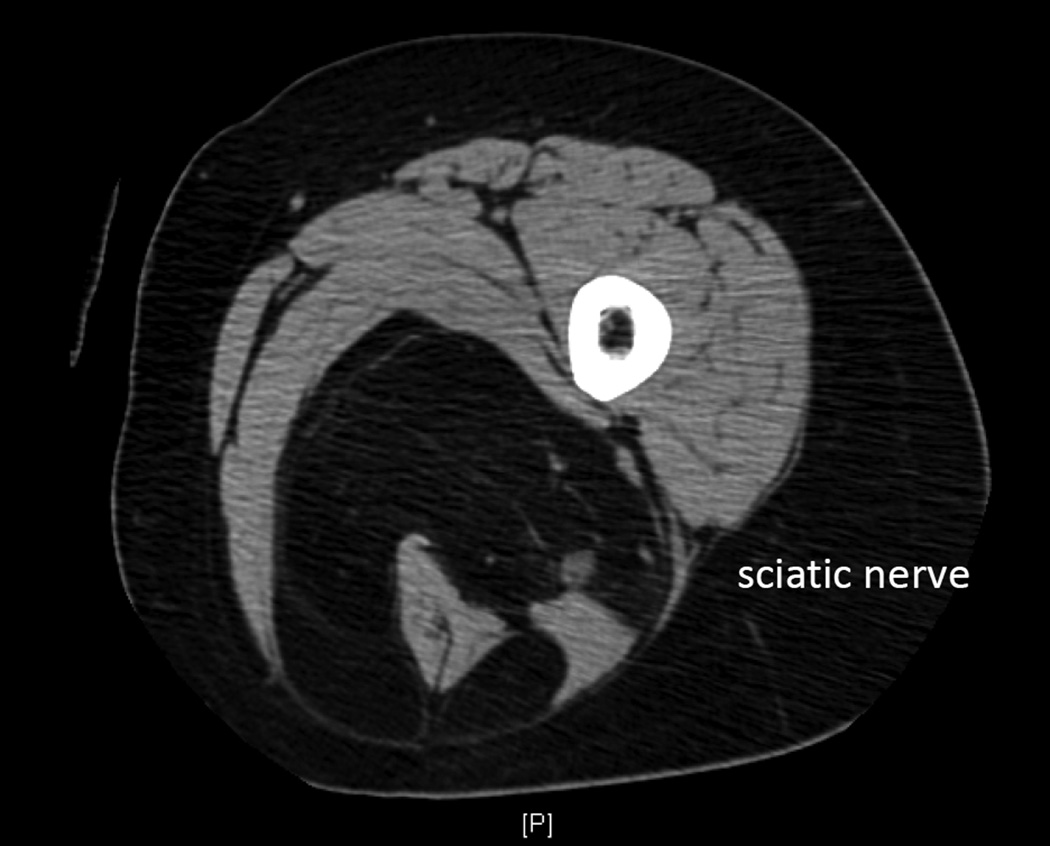
STS of the extremity generally presents as a painless mass. The lesion may appear rapidly or be associated with a prolonged history. Initial work-up includes cross-sectional imaging in all but the smallest superficial lesions (<3cm), which can be managed initially with both diagnostic and potentially therapeutic excision. MRI (magnetic resonance imaging) is somewhat more specific than CT and can be used to delineate the anatomy associated with a tumor (e.g., relation to neurovascular bundles).10 Carefully performed CT or MRI can be pathognomonic for well- and dedifferentiated liposarcoma and in many cases can be highly suggestive of histologic diagnosis (Figure 3).11
Figure 3.
Cross sectional imaging (CT) demonstrating a well-differentiated liposarcoma in the posterior compartment of the thigh, encasing the sciatic nerve and hamstring muscles. On both CT and MRI, well-differentiated liposarcoma has imaging characteristics almost identical to normal fat. Enhancing septations may be present and nodularity appears in the context of dedifferentiation.
When sarcoma histology cannot be clearly diagnosed by standard imaging as in the case of well- and dedifferentiated liposarcoma, core needle biopsy is performed to obtain a histologic diagnosis, which will assist in both surgical planning and discussions regarding the utility of neoadjuvant chemotherapy. Core needle biopsy is more accurate than is fine needle aspiration in its ability to define grade and histologic subtype and should be the default method for diagnosis; it will also allow the performance of specialized immunohistochemistry and cytogenetics analyses that define histology. In a study examining diagnosis based on 60 core needle biopsies as compared to that made on evaluation of corresponding surgical specimens, correct histologic diagnosis was made in 75% of cases; grade was accurately determined in ~90% of patients.12 Incisional biopsy is performed when core biopsy is not diagnostic and detailed information would change management recommendations.
Regardless of the method of biopsy – core, incisional or excisional biopsy of small lesions – the surgeon should plan carefully so that the tract can be easily resected at the time of definitive surgery. This generally requires that the biopsy incision be oriented along the length of the limb despite the fact that this may not result in the most cosmetically appealing scar as compared to a transversely designed incision. Re-excision following orientation of a biopsy scar transversely may preclude primary closure and necessitate placement of skin graft or soft tissue flap.
Once diagnosis is established, an extent of disease work-up should be completed. In high-grade tumors imaging of the chest is recommended, and a baseline CT (computed tomography) scan is generally performed. Chest X-ray may be utilized for patients with tumors that carry relatively low risk of metastasis (e.g., small, superficial sarcomas and low-grade lesions). There are rarely clinical indications to stage with PET (positron emission tomography) scan as many malignant soft tissue lesions are not FDG (fluorodeoxyglucose) avid, and other benign lesions (e.g., schwannoma) can be PET avid.
Surgical resection of soft tissue tumors in the extremity
Amputation was historically considered the standard of care for patients with STS of the extremity. The role of amputation was questioned in a classic randomized trial, however, comparing outcomes in 16 patients undergoing amputation with 23 treated with wide excision and adjuvant radiation (designed 2:1 randomization). Amputation essentially eliminated risk of local recurrence, but no difference in overall survival or disease specific survival was observed between the two groups. Despite what would be considered a seriously under-powered study by current criteria, the lack of difference in disease specific survival has been maintained over two decades. This reflects the fact that patients experiencing isolated local recurrence (in the absence of metastatic disease) could be salvaged with amputation and indicates limb-sparing surgery is a preferred alternative to amputation for the majority of patients with extremity sarcoma.13 In modern series from specialty centers, amputation is rarely employed for management of primary STS.
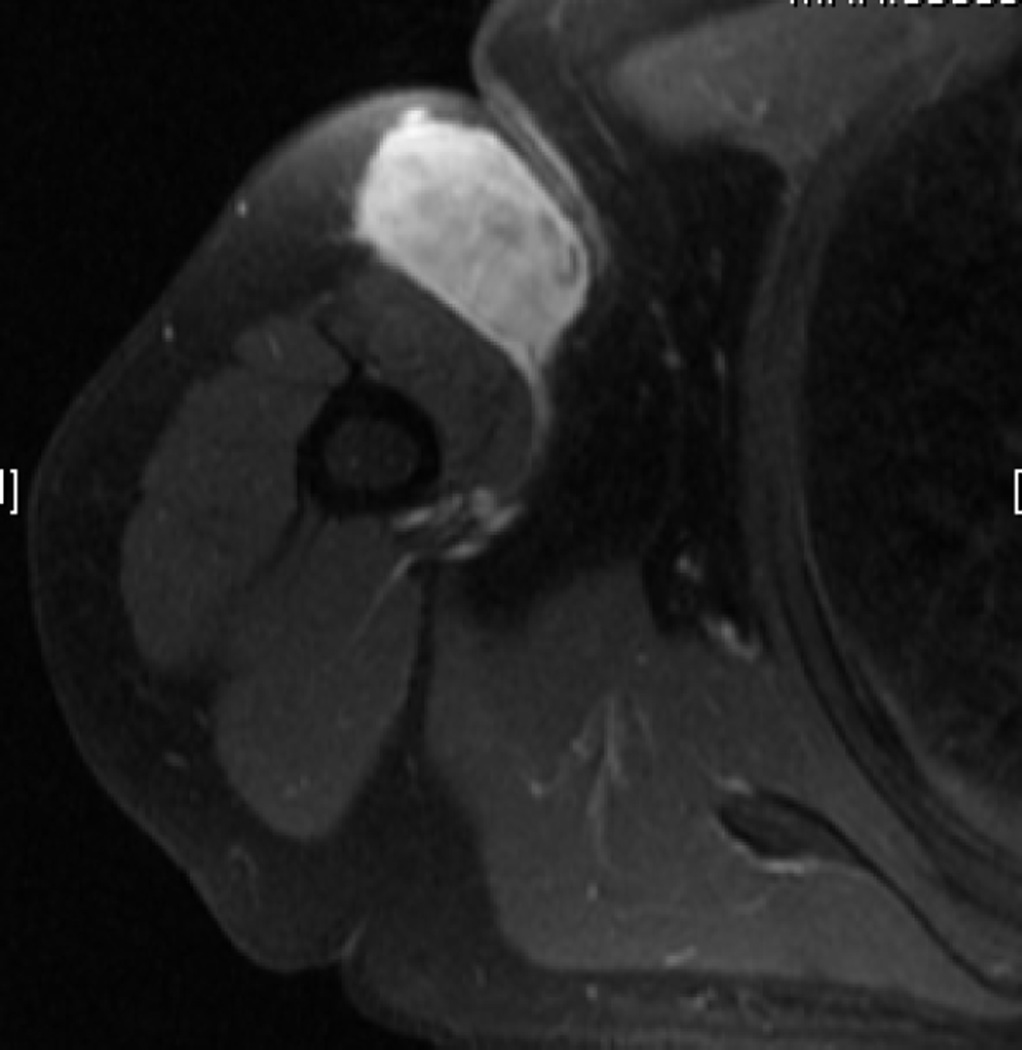
Initially, limb-sparing procedures were planned to include the entire muscular body (origin to insertion) containing a STS. It is now recognized that a 1cm margin circumferentially is adequate in most instances to achieve negative margins and minimize local recurrences. Specific consideration should be given to histologic subtypes with infiltrative borders, however. In the instance of myxofibrosarcoma, MRI demonstrates that infiltrative tails of microscopic tumor can grow far beyond the borders of the dominant tumor nodule (Fig 4).14 Surgical resection should be planned to encompass these tails as well as a margin of normal tissue surrounding them to ensure complete resection. It is likely that early reports demonstrating high rates of local recurrence following resection of myxofibrosarcoma reflect the fact that we did not previously understand the full extent of disease in many patients presenting with primary tumors.15
Figure 4.
T2-weighted images from cross-sectional MRI using gadolinium contrast and evaluating a primary myxofibrosarcoma of the right upper arm. An enhancing ‘tail sign’ is demonstrated by the arrow.
As in the case of myxofibrosarcoma, dermatofibrosarcoma protuberans (DFSP) has historically been associated with high rates of local recurrence and multiple resections may be required to remove all microscopic disease from the tumor bed. This is almost certainly a reflection that, in this disease, tendrils of microscopic tumor can extend radially from the dominant mass. Such information initially led many surgeons to plan resection with 4cm margins to reduce rates of local recurrence. A recent review of surgical and pathologic specimens suggests that rates of reoperation are reasonable if 2cm margins are planned at the time of surgical resection. Eighty-four percent of DFSP patients underwent complete microscopic resection at the time of initial procedure when tumors were removed with 2cm margins.16
Even when a standard 1cm margin is recommended, the extent of resection is generally limited by an adjacent neurovascular bundle, which often abuts deep tumors. In the instance that the tumor pushes on the bundle without encasing the structure, the tumor can be removed with the fibrous sheath encasing the bundle in attempt to gain a negative margin. In almost all instances, the structures of the neurovascular bundle can be isolated above the tumor and protected as the tumor is retracted from the surgical bed. Rarely, the neurovascular bundle is encased by the tumor. If this is observed in the context of a low-grade tumor, the lesion can be bi-valved to preserve major nerves and arteries, and sacrifice of the bundle be reserved for the context of tumor recurrence. Of interest in all sarcomas, is that a microscopic positive margin following complete gross resection does not uniformly result in subsequent local recurrence, so resection of an adjacent artery or nerve to obtain an R0 versus R1 resection is not indicated in the context of a primary lesion.
When a high-grade tumor surrounds or cannot be separated from major vascular structures to obtain a complete gross resection (e.g., a leiomyosarcoma arising from a major vein), the vascular bundle is sacrificed (often with the associated nerve). Arterial resections may require vascular bypass. Attempts at venous bypass generally fail, and in the event of significant swelling, compression and elevation will temporize symptoms until collateral vessels develop. Resection of perineal or sciatic nerves can generally be managed with an ankle orthotic though patients should be counseled regarding the sequelae of paresthesia on the dorsum of the foot resulting in unrecognized injuries if not examined on a regular basis.17 Femoral nerve resection requires bracing of the knee to prevent long-term joint damage and falls related to buckling at the joint; such falls can contribute to an increased rate of fracture after limb-preserving procedures.18 Resection of one of three of the major upper extremity nerves preserves opposition in the hand despite development of classically defined deficits.
Adjuvant therapy for sarcoma of the extremity
Adjuvant radiation and chemotherapy both play a role in the management of STS. When recommending multimodality therapy to a patient, the relative risk to benefit ratio of the treatment should be carefully considered in the context of results available from published clinical trials. Most trials have been performed on a heterogeneous subset of STS, but each specific histologic subtype carries unique propensity for local and distant recurrence, which can modulate a generalized algorithm for prescription of radiation and chemotherapy. Nomograms predicting histology-specific rates of local recurrence and disease specific survival are widely available and can assist the clinician in counseling patients.19–22 Tumors more likely to recur locally may benefit from more frequent prescription of adjuvant radiation while tumors commonly associated with distal metastases may require more aggressive recommendations regarding systemic treatment. Sarcomas with low rates of local and distal recurrence may be best managed with surgery alone.
For example, a randomized trial examining the role of adjuvant radiation therapy after surgical resection has been performed in a cohort of 50 patients.23 It is clear that radiation decreases the risk of local recurrence in STSs and could be considered as a standard therapy prescribed to patients following surgical resection; over 30% of patients managed with surgery alone developed a local recurrence, whereas less than 5% of those receiving adjuvant radiation recurred locally (p=0.0028). Further analysis revealed, however, that no difference in overall survival was observed between patients treated with adjuvant radiation and those treated with surgery alone. Similar results were observed in a study examining the use of brachytherapy as an adjuvant to surgery in the management of STS. Patients with both high and low grade STS (n=164) were randomized to receive surgery with or without brachytherapy. Again, local recurrence was reduced in patients treated with brachytherapy (89% 5 year actuarial local control rates as compared to 66% in those managed with surgery alone, p=0.04), but no difference in distant recurrence (76 vs 83%, respectively; p=0.60) or disease specific survival (84 vs81%; p=0.65) were observed.24
Based on these trials, adjuvant or neoadjuvant radiation is generally prescribed to patients with large, high grade sarcomas with significant risk of local recurrence. Given, however, the long-term complications of radiation (e.g., secondary malignancies and joint fibrosis), the fact that radiation does not improve disease specific survival, and the low baseline risk of local recurrence in low-grade tumors and small, high-grade lesions (<5cm), many physicians will generally recommend against adjuvant radiation in patients with these subtypes of sarcoma. This is based, in part, on subgroup analyses published as part of the randomized trial by Pisters et al., which included 45 patients with low-grade STS among the cohort of patients randomized to receive adjuvant brachytherapy or surgery alone. At median follow-up of 67 months, local recurrence rates in patients with these tumors were similar (27% versus 22%) regardless of whether they received radiation; the benefit of adjuvant therapy was limited to patients with high grade tumors.24,25 Again, while a small study, the result was independent of margin status and has been maintained even with follow-up of over 20 years. Subsequently, a prospective trial examining outcomes in 74 patients with small extremity tumors (T1 tumors, ≤5cm regardless of grade) was reported and suggests these low risk tumors can be treated with surgery alone. Ten year local recurrence rates of only 10.6% were observed when treatment was complete microscopic resection (R0 resection) was performed and no radiation was administered.26 When wide margins are obtained, radiation can be deferred in this patient population as well.
Radiation can be administered in the adjuvant or neoadjuvant setting. The risks and benefits of each option have been carefully delineated in a study, which randomized 94 patients to received 50Gy preoperatively as compared to 66Gy in the postoperative setting.27 There was no difference in rates of local recurrence or long-term survival between the two cohorts of patients (p=0.71); however, significant differences in complications were observed. In patients treated with preoperative therapy, wound complications necessitated secondary operations and prolonged drainage in 35% of patients (as compared to 18% of those receiving adjuvant radiation), particularly those with lower extremity tumors. Patients receiving radiation in the postoperative period had high rates of joint fibrosis (48 versus 32% grade 2 or greater in long-term follow-up).28 Given these findings, our practice has been to consider radiation in the neoadjuvant setting when tumors are adjacent to a major joint and in cases where surgical resection will require soft tissue reconstruction for closure to avoid radiation of the flap. In general, to minimize surgical complications most patients are prescribed adjuvant radiation therapy. In addition to joint fibrosis and wound infection, careful consideration should be paid to the long-term risk of bone fracture (~5%).29 This complication may be even more common in the context of tumors that are resected with the periosteum.30 In this instance, patients with osteopenia or cortical thinning should be evaluated for prophylactic rodding if adjuvant radiation will be required.
Decisions regarding the ideal candidates for adjuvant or neoadjuvant chemotherapy are more difficult than are those regarding radiation. At many referral centers adjuvant chemotherapy is not recommended to patients with soft tissues sarcoma outside of those with Ewing’s sarcoma and rhabdomyosarcoma, which have particularly poor outcomes.31 This is based on data from a large number of randomized clinical trials examining patient outcomes that, in most cases, demonstrate no difference in outcome has been observed between patients receiving doxorubicin-based therapies and those receiving no systemic treatment.32 The most recent of these trials has been performed under the auspices of the EORTC.33 However, while no difference in overall survival was observed in the trial itself, a meta-analysis of clinical trials examining outcomes in 2,145 patients receiving doxorubicin-based therapy and included in the group’s report did show that the hazard ratio describing overall survival in patients receiving chemotherapy as compared to those who were observed was significant (0.86); patients may experience a small benefit if receiving adjuvant chemotherapy. In subgroup analysis of the trial participants, trends toward improved survival were observed in those patients with extremity tumors or high-grade tumors who received adjuvant therapy, leading most in the field to hypothesize that patients with these lesions are those who have the greatest potential to benefit from adjuvant chemotherapy and to selectively administer therapy to patients with these high-risk STS.
In addition to these data, retrospective series have been used by some clinicians to justify administration of adjuvant or neoadjuvant chemotherapy in the context of select tumors associated with poor outcomes. For example, in a retrospective review of 356 patients with large, high-grade STSs, patients with >10cm, high-grade tumors who were treated with neoadjuvant doxorubicin and ifosfamide had improved disease specific survival at three years as compared to those patients who did not receive therapy (83% versus 62%, respectively).34 Criticism regarding the utility of clinical trials has also been raised since most trials include a heterogeneous group of patients with a wide range of histologic subtypes of STS each with different chemosensitivity profiles, and retrospective analyses seeking to evaluate the role of chemotherapy in specific histologic subtypes of STS have also been reported to identify select patients who may benefit from adjuvant treatment. For example, in the context of synovial sarcoma and round cell liposarcoma, outcomes were better than predicted in patients receiving adjuvant treatment as compared to local therapy alone even in the context of smaller lesions (<7cm).22,35 Patients with chemoresistant subtypes of sarcoma, such as dedifferentiated liposarcoma have classically not been prescribed neoadjuvant chemotherapy by clinicians who believe in selected use of adjuvant therapy, although this pattern may alter as targeted therapies for these tumors are developed and clinical trials examining their activity become available.
Because of significant heterogeneity between histologic subtypes and individual tumors, as well as the unclear role of adjuvant therapy in improving survival, it has been our practice, when considering prescription of neoadjuvant chemotherapy to patients with high-risk STS of the extremity, to administer the drug in the neoadjuvant setting. This allows the clinician to assess interval response to the drugs as opposed to committing to a full course of therapy in a cohort of patients who may have variable responses. Patients whose disease progresses through initial cycles of chemotherapy are not prescribed a prolonged course of systemic therapy but instead proceed to resection and radiation treatment.
Prognosis and management of recurrent disease
Prognosis in patients presenting with STS of the extremity is highly variable and dependent on a range of clinicopathologic factors. Among these are tumor histology, size, grade, and site (upper versus lower extremity versus trunk). Unlike in most cancers, it is rare that a specialist would treat patients or predict outcome based on tumor stage. This is generally because the current AJCC staging systems do not incorporate data regarding histologic subtype, which can dictate both local and distal recurrence risk. For example, high-grade myxofibrosarcoma rarely recurs distantly, but instead is prone to local recurrence so that patients presenting with this disease have significantly better rates of disease specific survival when presenting with large, high-grade tumors as compared to patients who present with similarly sized undifferentiated pleomorphic sarcomas. Perhaps a better method for predicting survival are clinical nomograms which incorporate multiple risk factors into a single model.20 Many have been validated in multi-institutional studies and can be accessed online, and ranges have been developed to model outcomes in specific sarcoma subtypes.36–38
In general, high-grade tumors tend to recur relatively quickly with over half of recurrences being observed in the first two years and almost all of the remaining recurrences happening in the first five years.34 For this reason and because at least 40% of patients with tumors over 5cm in diameter will recur, our routine has been to follow these patients every four months for the first two years after surgery, every six months until five years postoperatively and then yearly after that point. Low-grade tumors may have smaller rates of recurrence, however these recurrences may recur late after surgery; in fact half recur more than five years postoperatively.39 We would generally follow these patients every six months to a year for the first five years after surgery, but as with high-grade lesions, follow them indefinitely.
Patients routinely undergo physical exam to survey for recurrence, and imaging of the chest is obtained for all patients whose tumors carry metastatic potential. Abdominal and pelvic CT is completed for patients with a history of myxoid/round cell liposarcoma as metastases to the retroperitoneal fat pads can occur. The extremity itself is imaged if the tumor is deep and recurrence would not be detected by exam. This imaging may be in the form of ultrasound or CT, but most commonly MRI, which can detect subtle differences between postoperative change and recurrence. This is particularly helpful in the context of tumors such as myxofibrosarcoma where infiltrative components may not be well visualized by modalities other than MRI.
Distant metastases are generally managed with systemic therapy but solitary or oligo- (≤3) metastases that occur after a long disease free interval may be resected with curative intent.40 The treatment paradigm for local recurrences is more complex. Tumors may be surgically resected using principles similar to those employed in management of primary lesions. The entire surgical bed should be re-excised when possible. If limb-sparing surgery is not feasible or disease is widespread, amputation may be considered. If radiation were not employed in management of the primary tumor, it is generally prescribed (except in the context of low-grade indolent lesions such as atypical lipomatous tumors/well-differentiated liposarcoma). In the context of rapidly recurring (>5cm lesions recurring less than 16 months after initial resection), high-grade tumors, outcomes are particularly poor (4 year disease specific survival of 18%) and neoadjuvant chemotherapy should be considered before planning complex surgical resections or committing a patient to amputation.41
Management of abdominal and retroperitoneal tumors
Primary retroperitoneal sarcomas generally present as large lesions. The tumors can remain undetected for a prolonged period of time as patients may feel they are simply gaining weight. Abdominal distention may lead to diagnosis or the lesions may be identified incidentally when patients undergo scans for an unrelated symptom. Imaging can show a bland or heterogeneous tumor in the retroperitoneum, mesentery or adjacent to a loop of bowel, and is often highly suggestive of histology. For this reason, unlike in the context of extremity tumors, retroperitoneal and intraabdominal tumors evaluated at high volume sarcoma treatment centers can often be treated without biopsy. Approximately 70% of retroperitoneal tumors are either well- and dedifferentiated liposarcomas or leiomyosarcomas. Liposarcomas are almost always associated with abnormal appearing fat, which appears associated with an infiltrative process and in the context of dedifferentiated tumors have one or more solid, enhancing masses. Heterogeneous lesions in the retroperitoneum are often leiomyosarcoma; those located in the abdomen and associated with the small bowel may be leiomyosarcomas but often represent gastrointestinal stromal cell tumors (GIST). The differential diagnosis of retroperitoneal and intraabdominal masses can be broad, and if there is any concern for other processes (e.g., desmoid tumor, schwannoma, metastatic testicular cancer, and lymphoma), core needle biopsy under image guidance or via endoscopic approach should be performed.
The treatment algorithm for primary retroperitoneal tumors is relatively straightforward. Surgical resection is the mainstay of therapy. The tumors are resected with a margin of normal bowel when arising from the visceral wall or with the entire retroperitoneal fat pad when localized to the retroperitoneum. A more extensive resection has been advocated by some authors who argue that routine removal of uninvolved but adjacent organs should minimize rates of local recurrence in patients with these diseases, and as local recurrence can often be life limiting due to associated bowel obstruction that this will translate into improved patient survival.42,43 Such a strategy is highly debated in the field.44 There is no prospective data to support this position and many retroperitoneal tumors are adjacent to the major abdominal vessels. This plane becomes the closest margin of resection and cannot be addressed by resection of additional uninvolved organs. In addition, it is rare that adjacent organs are directly involved or invaded in the context of primary disease so that our approach has been to remove the colon or kidney only when this is the case.45 In most instances, the colon can be separated from the tumor leaving a margin of the posterior mesenteric sheath with the tumor and the kidney preserved by resecting the renal capsule to provide an adequate margin.
Adjuvant chemotherapy has not been shown to provide any benefit in the context of abdominal and visceral tumors except for patients undergoing surgery for GIST. In that instance, adjuvant imatinib can be prescribed for high risk lesions to prevent recurrence; these management algorithms have been extensively reviewed elsewhere.46 While neoadjuvant radiation is prescribed at many institutions in the context of retroperitoneal sarcomas, this is another point of contention with many in the field of sarcoma oncology. Prospective trials suggest that neoadjuvant radiation in conjunction with surgery produces low rates of local recurrence; however a direct comparison with surgery alone has not been performed.47 Those who believe surgery alone is sufficient to optimize outcomes argue that neoadjuvant radiation may make surgery more difficult to perform and may increase the rate of peri-operative complications. Several randomized clinical trials sought to examine the role of radiation in retroperitoneal sarcomas, but to date most have failed to accrue sufficient numbers of patients to be powered to answer the question. A phase III trial has recently been opened by the EORTC and long-term rates of abdominal recurrence and disease specific survival will be compared between groups undergoing surgical resection and surgical resection after administration of external beam radiation.48 Results could have significant impact on our treatment algorithms, though the studies are not stratified by histologic subtype and many practitioners will argue that this compromises our ability to understand the role radiation will play in determining outcome.
Long-term outcomes after resection of retroperitoneal sarcomas and high risk intraabdominal lesions are generally poor. Imatinib may be able to control rates of local recurrence following resection of high risk GIST, but over 50% of liposarcoma patients recur during the first five years following surgical resection of a primary tumor. Unlike after resection of extremity lesions, recurrence can be late with over 80% of patients developing additional disease during long-term follow-up.49 This is particularly true of patients with well-differentiated liposarcoma when many of the recurrences are detected over three years after surgery. For these reasons, we have advocated for long-term imaging follow-up of patients to detect recurrence, which can potentially be managed surgically or with novel therapies. Patients diagnosed with high-grade disease undergo cross-sectional imaging every four months for two years after surgery, every six months between two and five years postoperatively and yearly for an indefinite period of time. Patients with low-grade disease are followed every six months for five years and then yearly.
Treatment of recurrent retroperitoneal sarcomas can be difficult. Even after complete resection, the tumor almost always recurs and management must be thought of in terms of a chronic disease. The clinician constantly balances the risk of surgery, systemic therapy and observation. Surgery is routinely employed in the context of recurrence, but should be considered in the same context as metastasectomy for pulmonary metastases. The goal should be complete gross resection to ensure any chance of prolonged disease free intervals. Unfortunately even in selective series, complete gross resection rates decline with each succeeding operation. A simple algorithm is that complete resection rates of over 80% at initial operation decline to 60, 40, 20, and 10% with each succeeding operation.
Incomplete gross resection may improve symptoms and prolong asymptomatic periods, but will not be curative. Prolonged disease free intervals can be observed in patients with a limited number of sites of disease and late recurrences, but multifocality is associated with poor outcomes.50 A recent review of patients presenting with recurrence after complete resection of a liposarcoma demonstrates that rate of recurrence is also an important prognostic factor. Patients whose tumors recur at a rate of greater than one centimeter per month after complete gross resection do poorly. Even after complete removal of all visible disease, outcomes are comparable to those observed in patients who are managed with systemic therapy alone; disease specific survival following surgical resection is just 13 months.51 Ongoing research seeks to determine the genomic events that are associated with poor surgical outcomes in an effort to identify those patients best suited for surgery and to inform potential novel therapeutics. One such event, copy number loss on chromosome 19 is associated with rapid recurrence and death from disease.52
When patients present with rapid recurrence or poor prognostic factors, systemic treatments should be considered. Liposarcoma may respond to gemcitabine-based chemotherapy or traditional doxorubicin-based regimens. Similar protocols have been shown to be efficacious in the context of recurrent or metastatic leiomyosarcoma.53 More recently, target therapeutics have been examined to determine their role in management of retroperitoneal tumors. Leiomyosarcoma responds to trabectadin.54 Well- and dedifferentiate liposarcoma specifically is characterized by amplification of the chromosome 12 genes CDK4 and MDM2. Inhibitors of MDM2 have been examined in clinical trials and are associated with significant rates of grade 3 toxicities and few responses.55 CDK4 inhibitors may trigger response and prolong progression free survival by over four months.56
Summary and Conclusions
STSs remain a divergent group of diseases with variable presentation by site and histology. Molecular diagnosis is increasingly utilized, defining tumors (e.g., synovial sarcoma and solitary fibrous tumors) when presentation is atypical. Accurate diagnosis is essential as multi-modality management protocols and surgical plan can differ significantly depending on histologic subtype. Surgical resection remains the primary modality of potentially curative treatment. Adjuvant radiation and chemotherapy can be used selectively, though with limited survival benefit. Molecular targets appear to be the most promising new approach to identify novel means to inhibit growth of these tumors and prevent death related to disease. A wide range of active research programs are rapidly advancing our understanding of a panel of diseases, which differ significantly in their clinical behavior. This is allowing us to continually improve and optimize therapy in individual patients.
Key Points.
Soft tissue sarcomas (STSs) remain a divergent group of diseases with variable presentation by site and histology.
Molecular diagnosis is increasingly utilized, defining tumors (e.g., synovial sarcoma and solitary fibrous tumors) when presentation is atypical.
Accurate diagnosis is essential as multi-modality management protocols and surgical plan can differ significantly depending on histologic subtype.
Surgical resection remains the primary modality of potentially curative treatment.
Adjuvant radiation and chemotherapy can be used selectively, though with limited survival benefit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have nothing to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–421. doi: 10.1097/SLA.0000000000000869. discussion 21–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064–2069. doi: 10.1200/JCO.2009.25.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781–788. doi: 10.1097/01.pas.0000126055.33916.0b. [DOI] [PubMed] [Google Scholar]
- 5.Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema; a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64–81. doi: 10.1002/1097-0142(194805)1:1<64::aid-cncr2820010105>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Li FP, Fraumeni JF., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Annals of internal medicine. 1969;71:747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- 7.Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. American journal of human genetics. 1953;5:139–147. [PMC free article] [PubMed] [Google Scholar]
- 8.LaFemina J, Qin LX, Moraco NH, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013;20:66–72. doi: 10.1245/s10434-012-2573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Block JB. Angiosarcoma of the liver following vinyl chloride exposure. JAMA. 1974;229:53–54. [PubMed] [Google Scholar]
- 10.Panicek DM, Gatsonis C, Rosenthal DI, et al. CT and MR imaging in the local staging of primary malignant musculoskeletal neoplasms: Report of the Radiology Diagnostic Oncology Group. Radiology. 1997;202:237–246. doi: 10.1148/radiology.202.1.8988217. [DOI] [PubMed] [Google Scholar]
- 11.Peterson JJ, Kransdorf MJ, Bancroft LW, O'Connor MI. Malignant fatty tumors: classification, clinical course, imaging appearance and treatment. Skeletal radiology. 2003;32:493–503. doi: 10.1007/s00256-003-0647-8. [DOI] [PubMed] [Google Scholar]
- 12.Heslin MJ, Lewis JJ, Woodruff JM, Brennan MF. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Surg Oncol. 1997;4:425–431. doi: 10.1007/BF02305557. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skeletal radiology. 2013;42:809–818. doi: 10.1007/s00256-012-1563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. Clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20:391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Farma JM, Ammori JB, Zager JS, et al. Dermatofibrosarcoma protuberans: how wide should we resect? Ann Surg Oncol. 2010;17:2112–2128. doi: 10.1245/s10434-010-1046-8. [DOI] [PubMed] [Google Scholar]
- 17.Brooks AD, Gold JS, Graham D, et al. Resection of the sciatic, peroneal, or tibial nerves: assessment of functional status. Ann Surg Oncol. 2002;9:41–47. doi: 10.1245/aso.2002.9.1.41. [DOI] [PubMed] [Google Scholar]
- 18.Jones KB, Ferguson PC, Deheshi B, et al. Complete femoral nerve resection with soft tissue sarcoma: functional outcomes. Ann Surg Oncol. 2010;17:401–406. doi: 10.1245/s10434-009-0745-5. [DOI] [PubMed] [Google Scholar]
- 19.Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 21.Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246:105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 24.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 25.Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol. 1994;12:1150–1155. doi: 10.1200/JCO.1994.12.6.1150. [DOI] [PubMed] [Google Scholar]
- 26.Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–681. doi: 10.1097/SLA.0b013e318155a9ae. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 28.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Alektiar KM, Brennan MF, Singer S. Influence of site on the therapeutic ratio of adjuvant radiotherapy in soft-tissue sarcoma of the extremity. International journal of radiation oncology, biology, physics. 2005;63:202–208. doi: 10.1016/j.ijrobp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Lin PP, Schupak KD, Boland PJ, Brennan MF, Healey JH. Pathologic femoral fracture after periosteal excision and radiation for the treatment of soft tissue sarcoma. Cancer. 1998;82:2356–2365. [PubMed] [Google Scholar]
- 31.Esnaola NF, Rubin BP, Baldini EH, et al. Response to chemotherapy and predictors of survival in adult rhabdomyosarcoma. Ann Surg. 2001;234:215–223. doi: 10.1097/00000658-200108000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 33.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. The lancet oncology. 2012;13:1045–1054. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 34.Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–1672. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 35.Eilber FC, Eilber FR, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–695. doi: 10.1097/01.sla.0000141710.74073.0d. discussion 95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eilber FC, Brennan MF, Eilber FR, Dry SM, Singer S, Kattan MW. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101:2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 37.Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg. 2013;258:347–353. doi: 10.1097/SLA.0b013e31828c8a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canter RJ, Qin LX, Maki RG, Brennan MF, Ladanyi M, Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canter RJ, Qin LX, Ferrone CR, Maki RG, Singer S, Brennan MF. Why do patients with low-grade soft tissue sarcoma die? Ann Surg Oncol. 2008;15:3550–3560. doi: 10.1245/s10434-008-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temple LK, Brennan MF. The role of pulmonary metastasectomy in soft tissue sarcoma. Semin Thorac Cardiovasc Surg. 2002;14:35–44. doi: 10.1053/stcs.2002.31892. [DOI] [PubMed] [Google Scholar]
- 41.Eilber FC, Brennan MF, Riedel E, Alektiar KM, Antonescu CR, Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Ann Surg Oncol. 2005;12:228–236. doi: 10.1245/ASO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 42.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 43.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 44.Pisters PW. Resection of some -- but not all -- clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]
- 45.Russo P, Kim Y, Ravindran S, Huang W, Brennan MF. Nephrectomy during operative management of retroperitoneal sarcoma. Ann Surg Oncol. 1997;4:421–424. doi: 10.1007/BF02305556. [DOI] [PubMed] [Google Scholar]
- 46.Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annual review of medicine. 2012;63:247–258. doi: 10.1146/annurev-med-043010-091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 48.A phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with Retroperitoneal sarcomas (RPS) - STRASS.
- 49.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. doi: 10.1097/01.sla.0000086542.11899.38. discussion 70–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009;249:137–142. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]
- 51.Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009;250:977–982. doi: 10.1097/sla.0b013e3181b2468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crago AM, Socci ND, Decarolis P, et al. Copy Number Losses Define Subgroups of Dedifferentiated Liposarcoma with Poor Prognosis and Genomic Instability. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007;25:2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 54.Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol. 2013;24:1703–1709. doi: 10.1093/annonc/mds659. [DOI] [PubMed] [Google Scholar]
- 55.Ray-Coquard I, Blay JY, Italiano A, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. The lancet oncology. 2012;13:1133–1140. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 56.Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 59.Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 60.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- 61.Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]