Abstract
Fc engineering can modulate the Fc–FcγR interaction and thus enhance the potency of Abs that target membrane-bound Ags, but it has not been applied to Abs that target soluble Ags. In this study, we revealed a previously unknown function of inhibitory FcγRII in vivo and, using an Ab that binds to Ag pH dependently, demonstrated that the function can be exploited to target soluble Ag. Because pH-dependent Ab dissociates Ag in acidic endosome, its Ag clearance from circulation reflects the cellular uptake rate of Ag/Ab complexes. In vivo studies showed that FcγR but not neonatal FcR contributes to Ag clearance by the pH-dependent Ab, and when Fc binding to mouse FcγRII and III was increased, Ag clearance was markedly accelerated in wild-type mice and FcR γ-chain knockout mice, but the effect was diminished in FcγRII knockout mice. This demonstrates that mouse FcγRII efficiently promotes Ab uptake into the cell and its subsequent recycling back to the cell surface. Furthermore, when a human IgG1 Fc variant with selectively increased binding to human FcγRIIb was tested in human FcγRIIb transgenic mice, Ag clearance was accelerated without compromising the Ab half-life. Taken together, inhibitory FcγRIIb was found to play a prominent role in the cellular uptake of monomeric Ag/Ab immune complexes in vivo, and when the Fc of a pH-dependent Ab was engineered to selectively enhance human FcγRIIb binding, the Ab could accelerate soluble Ag clearance from circulation. We assume such a function would enhance the therapeutic potency of Abs that target soluble Ags.
Introduction
Immunoglobulin G has a unique interaction with FcγRs through its Fc region. Because FcγRs are involved in various functions of IgG, Fc engineering to increase FcγR binding has been applied to various Ab therapeutics to enhance their therapeutic potency (1, 2). For example, increasing the binding to human (h)FcγRIIIa or hFcγRIIa has enhanced the ability of Abs that target tumor cells to induce cellular cytotoxicity or phagocytosis. Moreover, increasing the binding to hFcγRIIb has enhanced the agonistic activity of Abs targeting the TNFR superfamily (3). Although these are examples of how Ab engineering significantly contributed to improving the therapeutic potency of Abs that target membrane-bound Ag, disease-relevant target Ags for a therapeutic Ab also include soluble Ags, such as cytokines and soluble receptors. Nevertheless, Fc engineering to modulate the interaction of Fc with FcγR has so far only been applied to Abs that target membrane-bound Ags.
Recently, we reported recycling Ab, an Ab with a novel modality that accelerates the clearance of targeted Ag in vivo by binding to the Ag at neutral pH and dissociating the Ag in acidic pH (4). This pH-dependent binding property of recycling Ab enables the Ab to bind to Ag in plasma and, after the Ab/Ag immune complex has been taken up into the cell, dissociate the Ag in the acidic endosome (Supplemental Fig. 3B). Because the dissociated Ag is transferred to the lysosome and degraded, the Ag clearance is accelerated and free Ab without the Ag is recycled back to plasma. This is in sharp contrast to the action of a conventional Ab, which continues to bind the Ag in the acidic endosome and thereby prevents soluble Ag from being degraded (Supplemental Fig. 3A) and causes the Ag to accumulate in circulation (5–10).
Recycling Ab can accelerate Ag clearance by dissociating the Ag in acidic endosome, but first the Ag/Ab immune complex must be taken up into the endosome. It has long been said that a large to midsize multivalent immune complex is internalized and cleared by hepatic FcγR via multivalent binding and crosslinking of the Fc to FcγR. In contrast, a monomeric immune complex containing a single Fc, that is, a complex of 1:1 or 1:2 formed by one Ab with one or two Ags, is not internalized by FcγR, because the monovalent interaction between Fc and FcγR is weak (11–15). Moreover, studies have shown that FcγR does not affect the clearance of Ab itself, which suggests that FcγR does not contribute to the internalization and clearance of monomeric immune complex in vivo (16, 17). Thus, we previously assumed that the cellular uptake of monomeric immune complexes by recycling Ab was mediated by nonspecific uptake or pinocytosis, not by FcγR-dependent uptake.
We previously reported that the intracellular uptake of a monomeric immune complex of pH-dependent Ab with human soluble (hs)IL-6R could be accelerated by enhancing the neonatal FcR (FcRn) binding at neutral pH, but the innate mechanism of intracellular uptake of the monomeric immune complex was not studied in detail (4, 18). In this study, to investigate the innate uptake pathway, we took advantage of a specific property of pH-dependent Ab to examine the intracellular uptake of immune complexes; namely, that Ag clearance from circulation by pH-dependent Ab in vivo equates to the cellular uptake rate of a complex. Because our studies in wild-type mice revealed an unexpected contribution of FcγR to the uptake and Ag clearance even in the case of a monomeric immune complex, we extended the study to investigate whether engineering the Fc to increase the binding affinity to FcγR would enhance Ag clearance in wild-type and various FcγR knockout mice and, furthermore, we sought to confirm that when the Fc is engineered to selectively increase the binding to specific human FcγR, the therapeutic potential of pH-dependent binding Abs against soluble Ags can be enhanced.
Materials and Methods
Ethics statement
Animal studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals at Chugai Pharmaceutical Co. under the approval of the company’s Institutional Animal Care and Use Committee. The company is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (http://www.aaalac.org).
Generation of anti–IL-6R Abs with increased binding affinity to mFcγRs at neutral pH
A pH-dependent binding Ab against hsIL-6R (PH-IgG1) was generated from a non-pH-dependent hsIL-6R binding Ab (NPH-IgG1), as previously described (4). To increase the binding affinity to mouse FcγRs at neutral pH, various Fc-engineered variants were generated by site-directed mutagenesis of hIgG1 and mouse (m)IgG1. Effective mutations were identified and combined to generate Fc variants with increased binding affinity to FcγRs at neutral pH. The generated variants were assessed for their binding affinity to recombinant mFcγRs (19) at pH 7.4 using Biacore T200 (GE Healthcare). The interaction of each variant with FcγRs was monitored using Biacore instruments (GE Healthcare), as previously described (20). Ab variants were captured on a CM5 sensor chip (GE Healthcare) on which protein A/G (Thermo Scientific) had been immobilized, and FcγRs were then injected. The binding of each variant to each FcγR was normalized by the amount of Ab captured on the sensor chip and was expressed as a percentage of that of IgG1. Kinetic analysis was performed by global fitting of binding data with a 1:1 Langmuir binding model using Biacore evaluation software (GE Healthcare). Fc variants with the desired affinity to mFcγRs were identified. Abs against hsIL-6R with pH-dependent Ag binding and their Fc variants were expressed transiently using HEK293 cells and purified by protein A.
Animals
C57BL/6J mice (wild-type mice) were purchased from Charles River Laboratories and hFcRn transgenic (Tg) mice were licensed from The Jackson Laboratory (supplier’s reference, B6.Cg-Fcgrttm1DcrTg(FCGRT)32Dcr/DcrJ). C57BL/6J mice deficient in γ-chain subunits of the FcγRI, FcγRIII, and FceRI receptors (mFcR γ-chain knockout mice; supplier’s reference, FcR γ-chain−/−, B6.129P2-Fcer1gtm1Rav) and FcγRII knockout mice (supplier’s reference, Fcgr2−/−, B6.129S4-Fcgr2btm1TtK) were purchased from Taconic, and FcγRIII knockout mice (supplier’s reference, Fcgr3−/−, B6.129P2-Fcgr3tm1Sjv/J) were purchased from The Jackson Laboratory.
Generation of hFcγRIIb Tg mice
A hFcγRIIb expression vector was constructed by modifying a bacterial artificial chromosome genomic library clone that contains all the exons of the human FcγRIIb gene with ∼30-kbp upstream and downstream regions. The hFcγRIIb vector was microinjected into the pronuclei of fertilized oocytes of C57BL/6N (C57BL/6NCrj, Charles River Laboratories) mice. Expression of hFcγRIIb in the transgenic mice was analyzed by RT-PCR and flow cytometry.
In vivo study of single doses of Abs in a steady-state model of hFcRn Tg mice, wild-type mice, and mFcγR knockout mice
An infusion pump (Alzet) filled with 92.8 μg/ml hsIL-6R was implanted under the skin on the back of wild-type mice or hFcRn Tg mice (21) to prepare a mouse model with a constant plasma concentration of hsIL-6R. Monoclonal anti-mouse CD4 Ab GK1.5 (22) was administered by i.v. injection to inhibit the production of mouse Ab against hsIL-6R by depleting CD4+ T cells. Abs against hsIL-6R were administered at 1 mg/kg to wild-type mice or hFcRn Tg mice with or without a single i.v. injection of 1 g/kg IVIG (CSL Behring) to mimic endogenous hIgG. Plasma anti–hsIL-6R Ab concentration in the presence of hIgG was determined using an anti-idiotype Ab coated on ELISA 96-well plates, and detected by hsIL-6R, biotinylated anti–hIL-6R Ab (R&D Systems), and streptavidin–poly-HRP80 (Stereospecific Detection Technologies) using peroxidase substrate. Plasma total hsIL-6R and Ab concentrations in the absence of hIgG were determined as previously described (4).
In vivo study of single doses of Abs in wild-type mice and an hFcγRIIb Tg mouse coinjection model
In a coinjection model, wild-type mice or hFcγRIIb Tg mice were i.v. given single doses of 50 μg/kg hsIL-6R and 1 mg/kg anti–IL-6R Abs. Plasma total hsIL-6R and Ab concentration in the absence of hIgG were determined as previously described (4).
Results
Uptake mediated by FcγR, not FcRn, contributes to Ag clearance by a pH-dependent IgG1 Ab in mice
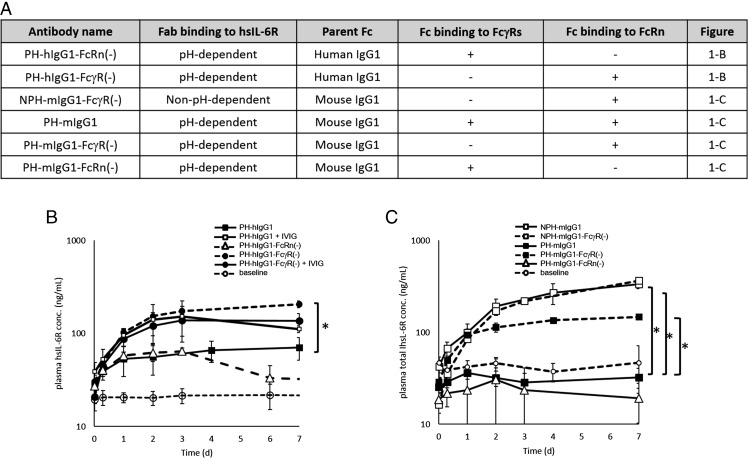
To elucidate whether native IgG1 uses a cellular uptake pathway other than nonspecific pinocytosis in vivo, we first evaluated the effect of an excess amount of IVIG on the clearance of Ags by PH-hIgG1 in an hFcRn Tg mouse steady-state model. Characteristics of Abs used in this study are summarized in Fig. 1A. Injection of 1 g/kg IVIG resulted in higher accumulation of Ags after an injection of PH-hIgG1 (Fig. 1B), which indicates that IVIG competes with a monomeric immune complex of PH-hIgG1 for intracellular uptake.
FIGURE 1.
FcγR but not FcRn contributes to the Ag clearance of a pH-dependent binding Ab. (A) Ab variants used in (B) and (C) are described. (B and C) Effect of Abs on the total hsIL-6R plasma concentration was evaluated in a steady-state model using hFcRn Tg mice or wild-type mice. Steady-state plasma concentration of ∼20 ng/ml hsIL-6R was maintained using an infusion pump filled with hsIL-6R solution. The time profiles of total hsIL-6R plasma concentration are shown. (B) PH-hIgG1 (▪), PH-hIgG1-FcRn(−) (△), and PH-hIgG1-FcγR(−) (● with dashed line) were i.v. administered to hFcRn Tg mice as single doses of 1 mg/kg, and PH-hIgG1 (□) and PH-hIgG1-FcγR(−) (▪ with solid line) were i.v. administered to hFcRn Tg mice as single doses of 1 mg/kg together with 1 g/kg IVIG. Plasma hsIL-6R concentration without Ab was set as baseline (○). An asterisk indicates a statistically different level of hsIL-6R between PH-hIgG1 and PH-hIgG1-FcγR(−) on day 7. (C) NPH-mIgG1 (□ with solid line), NPH-mIgG1-FcγR(−) (□ with dashed line), PH-mIgG1 (▪ with solid line), PH-mIgG1-FcγR(−) (▪ with solid line), and PH-mIgG1-FcRn(−) (△ with solid line) were i.v. administered as single doses of 1 mg/kg. An asterisk indicates statistically different levels of hsIL-6R between PH-mIgG1 and NPH-mIgG1, NPH-mIgG1-FcγR(−), or PH-mIgG1-FcγR(−) on day 7. Each datum point represents the mean ± SD (n = 3 each). Statistical significance was determined by a Dunnett test. *p < 0.05.
Because IVIG binds to both hFcRn and mFcγRs expressed in hFcRn Tg mice, IVIG can compete with either hFcRn- or mFcγR-mediated uptake of an immune complex formed by PH-hIgG1. Therefore, we investigated whether hFcRn and/or mFcγR contributes to the Ag clearance by PH-hIgG1. To test the contribution of hFcRn, we generated a variant of PH-hIgG1 in which hFcRn binding is abrogated [PH-hIgG1-FcRn(−)]. Injection of PH-hIgG1-FcRn(−) to hFcRn Tg mice exhibited an Ag accumulation level similar to PH-hIgG1, which demonstrates that hFcRn does not contribute to the uptake of a monomeric immune complex of PH-hIgG1 (Fig. 1B). Next, we generated a variant of PH-hIgG1 in which mFcγR binding is abrogated [PH-hIgG1-FcγR(−)] and injected it into hFcRn Tg mice. Ag accumulation with the PH-hIgG1-FcγR(−) Ab was increased over that of PH-hIgG1 and was similar to that of PH-hIgG1 in the presence of IVIG, but was not itself affected by IVIG (Fig. 1B). These results demonstrate that mFcγR contributes to the intracellular uptake of monomeric immune complexes but hFcRn does not, and that an excess amount of IVIG inhibits mFcγR-mediated internalization by competing for mFcγR binding.
Next, we injected steady-state normal mice with five different Ab variants (Fig. 1C): a pH-dependent anti–hsIL-6R Ab with mIgG1 (PH-mIgG1); a non–pH-dependent anti–hsIL-6R Ab with mIgG1 (NPH-mIgG1); a pH-dependent anti–hsIL-6R Ab with engineered mIgG1, in which mFcγR binding is abrogated [PH-mIgG1-FcγR(−)]; a non–pH-dependent anti–hsIL-6R Ab with engineered mIgG1, in which mFcγR binding is abrogated [NPH-mIgG1-FcγR(−)]; and a pH-dependent anti–hsIL-6R Ab with engineered mIgG1, in which mFcRn binding is abrogated [PH-mIgG1-FcRn(−)]. Consistent with the study in hFcRn Tg mice, PH-mIgG1-FcγR(−) had higher Ag accumulation than did PH-mIgG1, which demonstrates the contribution of mFcγR to the intracellular uptake of a complex formed from an Ag and PH-mIgG1 Ab. In contrast, the plasma Ag concentration of PH-mIgG1-FcRn(−) was the same as that of PH-mIgG1, which means that a monomeric immune complex formed from an Ag with PH-mIgG1 is not internalized by mFcRn. Alternatively, the extent of Ag accumulation induced by NPH-mIgG1 and NPH-mIgG1-FcγR(−) was comparable, which is consistent with the fact that the Ab clearance of NPH-mIgG1 and NPH-mIgG1-FcγR(−) was comparable (data not shown). The different effect of FcγR binding on the levels of Ag accumulated by NPH-mIgG1 and PH-mIgG1 indicates that, although mFcγR contributes to the uptake of a monomeric mIgG1 immune complex, most of the internalized mIgG1 Ab is recycled back to plasma, regardless of whether it still binds hsIL-6R.
Enhancing mFcγRII and III binding but not mFcγRI and IV binding accelerates Ag clearance by a pH-dependent hIgG1 Ab in hFcRn Tg mice
Having determined FcγR as the receptor responsible for Ag clearance by a pH-dependent Ab, we were motivated to test whether enhancing FcγR binding could accelerate the Ag clearance. Because mice have four different FcγRs, namely mFcγRI, II, III, and IV, Fc engineering enabled us to prepare three Ab Fc variants with different profiles of enhanced mFcγR binding (Table I).
Table I. Mutations and FcγR binding affinity of hIgG1 Fc variants.
| Fc Variant |
KD (M) at pH 7.4 |
Mutations | ||||
|---|---|---|---|---|---|---|
| Mouse FcγRI | Mouse FcγRII | Mouse FcγRIII | Mouse FcγRIV | Human FcγRIIb | ||
| hIgG1 | 5.3 × 10−8 | 9.8 × 10−7 | 2.4 × 10−6 | 8.6 × 10−8 | 2.7 × 10−6 | — |
| hIgG1-FcγR(−) | ND | ND | ND | ND | NT | L235R/S239K |
| hIgG1-Fy | 7.6 × 10−9 | 1.0 × 10−8 | 5.5 × 10−9 | 1.4 × 10−7 | NT | K326D/L328Y |
| hIgG1-Fz | 2.4 × 10−9 | 1.1 × 10−7 | 4.8 × 10−7 | 5.3 × 10−10 | NT | S239D/I332E |
| v12 | ND | 3.2 × 10−7 | 1.3 × 10−6 | ND | 1.9 × 10−8 | E233D/G237D/P238D/H268D/P271G/A330R |
| hIgG1-FcRn(−) | 2.7 × 10−8 | 8.4 × 10−7 | 2.5 × 10−6 | 3.9 × 10−8 | NT | I253A |
| mIgG1-FcRn(−) | NT | NT | NT | NT | NT | H435A |
The KD of hIgG1 and Fc variants and the mutations introduced in the Fc region are shown. Mutation sites in the Fc region are described in EU numbering.
ND, not detected; NT, not tested.
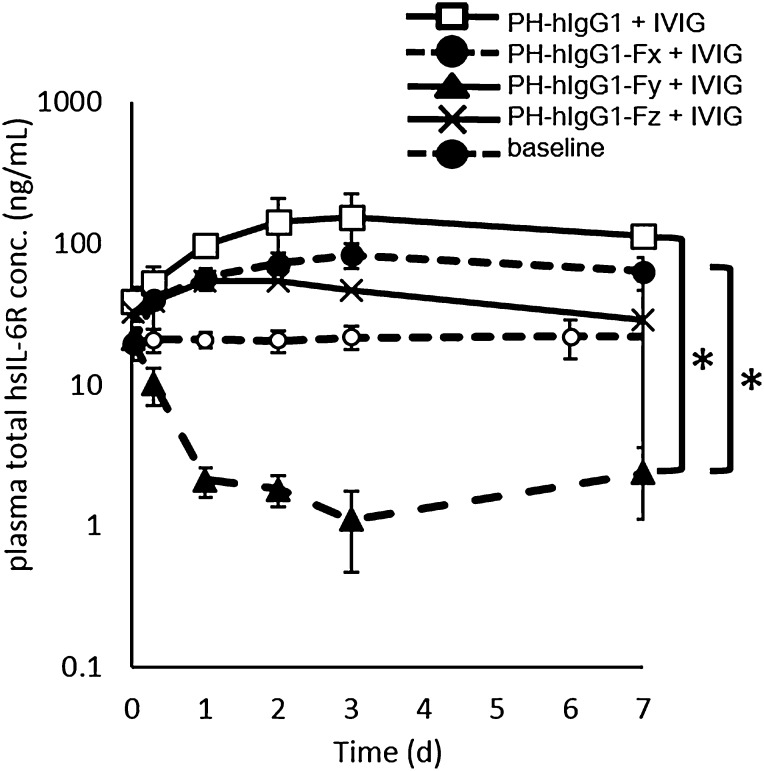
An afucosylated variant of PH-hIgG1 (PH-hIgG1-Fx), which was reported to have selectively higher affinity to mFcγRIV than to wild-type hIgG1 (23), showed Ag accumulation similar to PH-hIgG1. PH-hIgG1 with 100-fold higher affinity to both mFcγRII and III than to wild-type hIgG1 (PH-hIgG1-Fy) markedly reduced Ag plasma concentration to a level below the baseline. PH-hIgG1 with 20-, 5-, 5-, and 100-fold higher affinity to mFcγRI, II, III, and IV, respectively, than to wild-type hIgG1 (PH-hIgG1-Fz) showed only marginal reduction of Ag accumulation (Fig. 2). These results demonstrate that Ag clearance by a pH-dependent Ab could be accelerated by enhancing the binding affinity to mFcγRII and III, and thus suggest that mFcγRII and/or III are the main contributors to the intracellular uptake of monomeric immune complexes.
FIGURE 2.
Ag clearance was enhanced by a pH-dependent binding Ab with increased binding affinity to mFcγRII and III but not mFcγRI and IV. The effect of binding affinity to mFcγRI, II, III, and IV on Ag clearance is shown in an hFcRn Tg mouse steady-state model with hsIL-6R concentration of ∼20 ng/ml in the presence of IVIG. PH-hIgG1 (□), PH-hIgG1-Fx (●), Fy (▴), and Fz (×) were i.v. administered as single doses of 1 mg/kg together with 1 g/kg IVIG. Plasma hsIL-6R concentration without Ab was set as baseline (○). The time profile of total hsIL-6R plasma concentration is shown. Each datum point represents the mean ± SD (n = 3 each). An asterisk indicates statistically different levels of hsIL-6R between PH-hIgG1-Fy and PH-hIgG1 or PH-hIgG1-Fx on day 7 under IVIG competition. Statistical significance was determined by a Dunnett test. *p < 0.05.
To accelerate Ag clearance by enhancing the FcγR binding, pH-dependent binding is indispensable
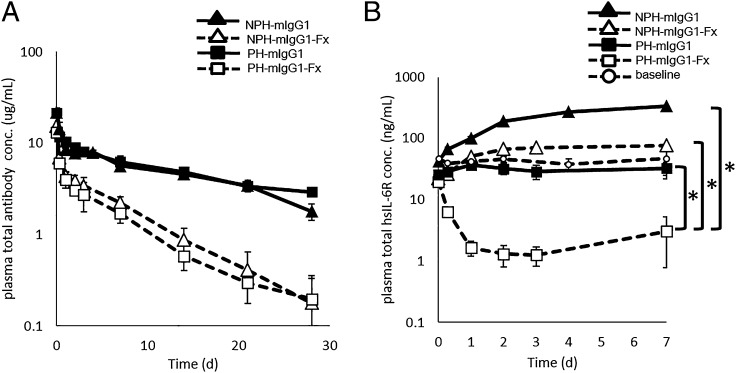
To examine whether Ag clearance could be accelerated simply by increasing mFcγRII/III binding without using a pH-dependent Ab, we compared the effect of enhancing the mFcγRII/III binding of a non–pH-dependent binding Ab (i.e., a conventional Ab) with that of a pH-dependent Ab in wild-type mice. In this study, we used wild-type mIgG1 as a control and an engineered mIgG1 with enhanced mFcγRII/III binding (mIgG1-Fx) (Table II). The Ab pharmacokinetics of pH-dependent and non–pH-dependent Abs was comparable when the same C region was used, and enhancing the mFcγRII/III binding accelerated the clearance of the Ab itself ∼5-fold (Fig. 3A). Because the Ag stays bound to a non–pH-dependent Ab and both the Ag and the Ab is cleared from circulation at the same rate, enhanced mFcγRII/III binding also reduced Ag accumulation of a non–pH-dependent Ab ∼4-fold, which is consistent with the 5-fold accelerated Ab clearance. Alternatively, Ag accumulation was reduced by ∼30-fold with the pH-dependent Ab with enhanced mFcγRII/III binding (Fig. 3A). These results demonstrate that just enhancing Fc binding to mFcγRII/III is not enough, and pH-dependent binding is indispensable to effectively accelerate the Ag clearance by enhancing the FcγR binding.
Table II. Mutations and FcγR binding affinity of mIgG1 Fc variants.
| Fc Variant |
KD (M) at pH 7.4 |
Mutations | ||||
|---|---|---|---|---|---|---|
| Mouse FcγRI | Mouse FcγRII | Mouse FcγRIII | Mouse FcγRIV | Human FcγRIIb | ||
| mIgG1 | ND | 1.1 × 10−7 | 2.1 × 10−7 | ND | NT | — |
| mIgG1-FcγR(−) | ND | ND | ND | ND | NT | P235K/S239K |
| mIgG1-Fx | ND | 4.6 × 10−10 | 6.5 × 10−10 | 8.7 × 10−7 | NT | S239D/K268D/A327D |
| mIgG1-Fy | ND | 1.2 × 10−9 | 3.6 × 10−9 | ND | NT | S239D/A327D |
The KD of mIgG1 and Fc variants and the mutations introduced in the Fc region are shown. Mutation sites in the Fc region are described in EU numbering.
ND, not detected; NT, not tested.
FIGURE 3.
pH-dependent Ag binding is required for FcγR-mediated Ag clearance. The effect of pH-dependent Ag binding on FcγRII- or III-mediated Ag clearance and Ab pharmacokinetics in a normal mouse steady-state model with hsIL-6R concentration of ∼20 ng/ml is shown. NPH-mIgG1 (▴), NPH-mIgG1-Fx (△), PH-mIgG1 (▪), and PH-mIgG1-Fx (□) were i.v. administered as single doses of 1 mg/kg. Time profiles of (A) Ab plasma concentration and (B) total hsIL-6R plasma concentration are shown. Each datum point represents the mean ± SD (n = 3 each). An asterisk indicates statistically different levels of hsIL-6R between PH-mIgG1-Fx and NPH-mIgG1-Fx or PH-mIgG1 on day 7. Statistical significance was determined by a Dunnett test. *p < 0.05.
Inhibitory receptor mFcγRII is the main contributor to Ag clearance by a pH-dependent Ab in mice
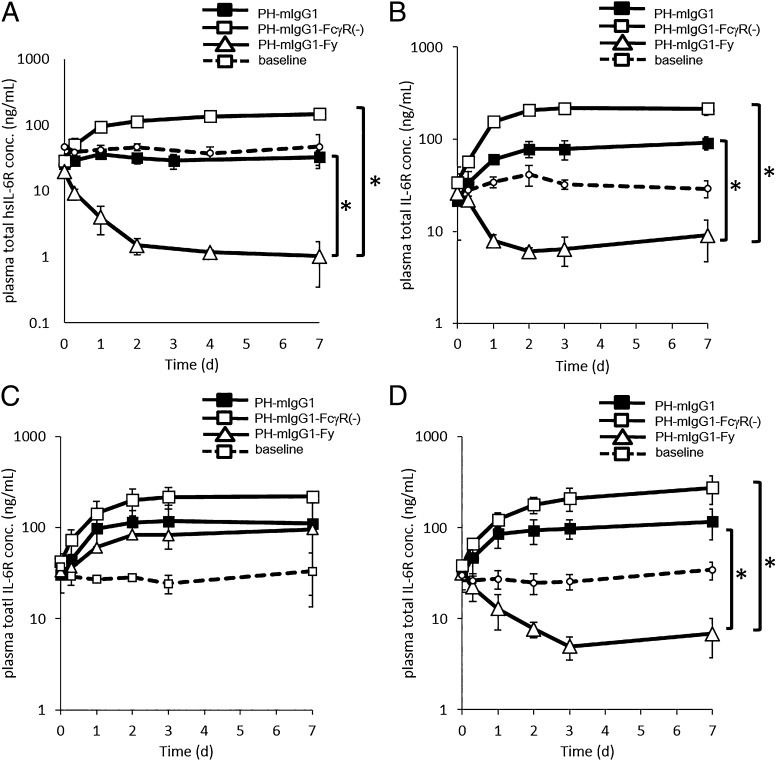
The studies using PH-hIgG1-Fy and PH-hIgG1-Fx (Figs. 2, 3A) suggested that mFcγRII and/or III contribute mainly to the Ag clearance achieved by a pH-dependent Ab. However, in hFcRn Tg mice or wild-type mice it was difficult to examine the effect of mFcγRII and III separately. Because mFcγRII and III have high sequence homology, it was not feasible to selectively enhance the binding affinity to one or the other. To distinguish between the contribution of mFcγRII and III, we used wild-type mice and three types of knockout mice that lacked either a common γ-chain (FcR γ-chain knockout mice), FcγRII (FcγRII knockout mice), or FcγRIII (FcγRIII knockout mice). We engineered mIgG1 to prepare two variants: one with diminished binding to all mFcγRs [mIgG1-FcγR(−)] and one with 100-fold enhanced binding to both mFcγRII and III (mIgG1-Fy) (Table II). The pH-dependent Abs with mIgG1, mIgG1-FcγR(−), and mIgG1-Fy [PH-mIgG1, PH-mIgG1-FcγR(−), and PH-mIgG1-Fy] were injected to wild-type mice, FcR γ-chain knockout mice, FcγRII knockout mice, and FcγRIII knockout mice, respectively (Fig. 4).
FIGURE 4.
mFcγRII is the main contributor to the Ag clearance by a pH-dependent binding Ab. The effect of mFcγRII and mFcγRIII on Ag sweeping is shown in a mouse steady-state model with hsIL-6R concentration of ∼20 ng/ml. PH-mIgG1 (▪), PH-mIgG1-FcγR(−) (□), and PH-mIgG1-Fy (△) were i.v. administered as single doses of 1 mg/kg in (A) wild-type mice, (B) common γ-chain knockout mice, (C) FcγRII knockout mice, and (D) FcγRIII knockout mice. Time profiles of total hsIL-6R plasma concentration in each type of mouse are shown. Each datum point represents the mean ± SD (n = 3 each). In (A), (B), and (D), an asterisk indicates statistically different levels of hsIL-6R between PH-mIgG1-Fy and PH-mIgG1 or PH-mIgG1-FcγR(−) on day 7. Statistical significance was determined by a Dunnett test. *p < 0.05.
PH-mIgG1-Fy markedly accelerated the Ag clearance and reduced Ag plasma concentration to a level below the baseline in wild-type mice. The increased Ag clearance shown by PH-mIgG1-Fy in wild-type mice was mostly diminished in FcγRII knockout mice, but it was largely maintained in FcR γ-chain knockout mice and FcγRIII knockout mice. The difference in Ag clearance among the different mice was not significant when using PH-mIgG1-FcγR(−), which lacked mFcγR binding. These results demonstrate that mFcγRII, which is an inhibitory FcγR, contributes strongly to the Ag clearance by a pH-dependent Ab in mice, which indicates that mFcγRII contributes to the intracellular uptake of monomeric immune complexes.
Fc engineering to selectively enhance the hFcγRIIb binding accelerates the Ag clearance by a pH-dependent hIgG1 Ab in hFcγRIIb Tg mice
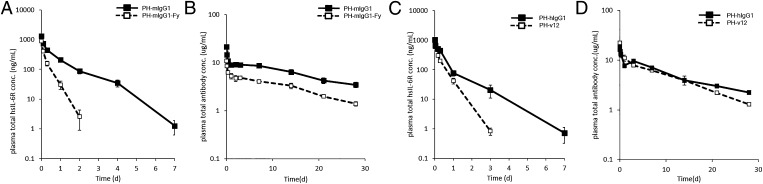
To evaluate in vivo efficacy, we generated eight lines of hFcγRIIb Tg mice and confirmed their expression of hFcγRIIb mRNA and protein by RT-PCR and flow cytometry (data not shown), respectively. Two of these lines, nos. 90 and 23-1, were used for further study. To translate the enhanced Ag clearance of a pH-dependent Ab when binding affinity to mFcγRII is increased into the clinical situation, we generated a pH-dependent Ab with an hIgG1 variant that had selectively increased binding affinity to hFcγRIIb, and not to any other hFcγRs or mFcγRs (PH-v12) (24). PH-v12 shows ∼100-fold enhanced binding to hFcγRIIb compared with PH-hIgG1 (Table I). The effect of the increased binding to hFcγRIIb on Ag clearance by this pH-dependent hIgG1 Ab was evaluated in hFcγRIIb Tg mice.
Because a technical issue of hFcγRIIb Tg mice made it impossible to establish an hsIL-6R steady-state model using an infusion pump, we assessed the effect of the PH-v12 Ab on Ag clearance with an Ag/Ab coinjection study, as previously described (4, 18). To compare mFcγRII and hFcγRIIb, we first evaluated PH-mIgG1 and PH-mIgG1-Fy in the Ag/Ab coinjection study using FcγRIII knockout mice, because PH-mIgG1-Fy in FcγRIII knockout mice results in selective increased binding to mFcγRII. As observed in the hsIL-6R steady-state model, PH-mIgG1-Fy enhanced the Ag clearance over that of PH-mIgG1 (Fig. 5A) without significantly compromising the Ab half-life (Fig. 5B). Next, we evaluated PH-hIgG1 and PH-v12 in the Ag/Ab coinjection study using hFcγRIIb Tg mice. Similar to PH-mIgG1-Fy in wild-type mice, PH-v12 also accelerated the Ag clearance over that of PH-hIgG1 in both hFcγRIIb Tg mice line nos. 90 and 23-1 (Fig. 5C, data for no. 23-1 not shown), but not in wild-type mice (Supplemental Fig. 1). Furthermore, increasing the binding affinity to either mFcγRII or hFcγRIIb did not alter the Ab pharmacokinetics (Fig. 5D).
FIGURE 5.
A pH-dependent binding Ab with Fc engineering to selectively increase the hFcγRIIb binding enhanced Ag clearance while maintaining Ab pharmacokinetics in hFcγRIIb Tg mice. The effect of Abs on the total hsIL-6R plasma concentration was evaluated in a coinjection model. hsIL-6R and Ab were i.v. administered as single doses of 50 μg/kg for hsIL-6R and 1 mg/kg for Ab. PH-mIgG1 (▪) and PH-mIgG1-Fy (□) were each coinjected with hsIL-6R in FcγRIII knockout mice, and time profiles of (A) total hsIL-6R plasma concentration and (B) Ab plasma concentration are shown. PH-hIgG1 (▪) and PH-v12 (□) were each coinjected with hsIL-6R in hFcγRIIb Tg mice, and time profiles of (C) total hsIL-6R plasma concentration and (D) Ab plasma concentration are shown. Each datum point represents the mean ± SD (n = 3 each).
Discussion
In this study, we report the involvement of inhibitory FcγRII in the intracellular uptake of monomeric immune complex in vivo and show that this function can be exploited in a therapeutic Ab that targets soluble Ag by engineering enhanced FcγRII binding into the Fc region. These findings were only revealed by using a pH-dependent Ab that dissociates the Ag in acidic endosome, a property that enabled us to separately evaluate 1) the intracellular uptake rate by measuring Ag clearance, and 2) the recycling property of the Ab after internalization by comparing Ag clearance and Ab clearance.
First, by using various pH-dependent Abs, we revealed that inhibitory FcγRII is capable of intracellular uptake of monomeric immune complexes without cross-linking the receptor (which is reflected by the accelerated Ag elimination mediated by FcγRII), and that after internalization of immune complexes followed by dissociation of the Ag, it does not transfer the Ab into the lysosome but rather the Ab is efficiently recycled back to the cell surface (which is reflected by the rather long half-life of Ab compared with Ag) (Supplemental Fig. 3C). This recycling property of the monomeric immune complex after FcγRII-mediated internalization makes it difficult to examine FcγRII-mediated internalization in vivo using a non pH-dependent Ab (i.e., a conventional Ab), and we assume that this is the reason why FcγRII was not thought to be involved in the process of internalizing a monomeric immune complex.
Previous studies using conventional Abs have shown that FcγR binding does not affect Ab pharmacokinetics (16, 17), and they suggested that FcγR does not contribute to the uptake of a monomeric immune complex. Thus, it seemed plausible that FcγR binding is not involved in the Ag clearance of a conventional Ab because Ag bound to the Ab exhibits almost the same clearance as the Ab itself. Indeed, our study also showed that FcγR binding does not affect the Ag clearance of a conventional mIgG1 Ab in vivo (Fig. 1C). However, because these findings are based on the use of a conventional Ab that recycles most of the complexes back into circulation after FcγR-mediated internalization (Supplemental Fig. 3D), the actual ability of FcγR to take up monomeric immune complexes into the cell in vivo could not be evaluated. Alternatively, when we used a pH-dependent Ab, which dissociates the Ag within acidic endosome from where it is transferred to lysosome and degraded, the Ag clearance directly reflected the uptake of immune complexes into the cell (because all the internalized Ags are transferred to lysosome and degraded) and, consequently, our study could evaluate the intracellular uptake of immune complexes by FcγR.
This study showed that both abrogating Fc binding to FcγR and administering a large amount of endogenous hIgG i.v. reduced the amount of hsIL-6R eliminated by a pH-dependent Ab, whereas abrogating Fc binding of non–pH-dependent Ab to FcγR did not affect the level of hsIL-6R (Fig. 1). This indicates that, in the case of wild-type IgG1, FcγR contributes to the uptake of monomeric immune complexes of wild-type IgG1, and after internalization, Abs are recycled with high efficiency in vivo (Supplemental Fig. 3C, 3D). Moreover, a pH-dependent hIgG1 Ab with enhanced FcγRII and FcγRIII binding improved the FcγR-mediated uptake of monomeric immune complexes and increased the hIL-6R clearance in mice by 20-fold, even when competing with high levels of endogenous hIgG (Fig. 2).
Next, various FcγR knockout mice were used to identify which type of FcγR contributes to the uptake of a monomeric immune complex by measuring the Ag clearance. Whereas Ag clearance was largely maintained in FcR γ-chain knockout mice and FcγRIII knockout mice, it was clearly diminished in FcγRII knockout mice, demonstrating that FcγRII, which is an inhibitory FcγR, is the main contributor to intracellular uptake of monomeric immune complexes in vivo. Although it has long been said that FcγRII could take multivalent immune complexes up into the cell in a noninflammatory way (13), to our knowledge this is the first report revealing that inhibitory FcγRIIb can efficiently internalize monomeric immune complexes without cross-linking the receptor in vivo. Furthermore, studies using PH-mIgG1-Fy in FcγRIII knockout mice and PH-v12 in hFcγRIIb Tg mice (Abs with enhanced binding to FcγRII/III in mice or FcγRIIb in humans, respectively) showed that Ag clearance was accelerated without shortening Ab half-life (Fig. 5). This indicates that a pH-dependent Ab internalized by mFcγRII or hFcγRIIb is efficiently recycled back to the cell surface while the dissociated Ag is transferred to lysosome in vivo. Furthermore, we assume that whereas Ab with enhanced FcγR binding could associate with cell surface FcγRs efficiently, the rather fast dissociation rate constant of mFcγRII or human FcγRIIb from the Ab (Tables I, II, Supplemental Fig. 2) also enables a recycled Ab to be released from the receptor at the cell surface back to the circulation.
Whereas this study focused on monomeric Ag/Ab immune complexes and revealed that the efficiency of FcγRII to recycle them after FcγRII-dependent internalization is rather high, most previous studies designed to assess the fate of immune complexes after FcγRII-dependent internalization used multivalent immune complexes containing three or more Abs. However, the in vivo behavior of multivalent immune complexes still remains to be elucidated. It has been said that multivalent immune complexes were eliminated by FcγR in vivo and the fact that multivalent immune complexes are constitutively eliminated through FcγRII expressed on liver sinusoidal endothelial cells in mice (11–15) indicates that multivalent immune complexes bound to mFcγRII would be internalized and transferred to lysosome in vivo. Alternatively, it was also shown by an in vitro study that hFcγRIIb has a recycling capability and that an immune complex internalized by hFcγRIIb is constitutively recycled to the cell surface after internalization (25, 26). Considering these conflicting observations, the in vivo behavior of FcγRII needs further evaluation. From the results shown in the present study, we assume that the fate of multivalent immune complexes after FcγRII-dependent cellular uptake could also be fruitfully examined using a pH-dependent Ab against a multimeric Ag that forms immune complexes containing more than two Fc. Further studies could elucidate the differential intracellular trafficking of monomeric and multivalent immune complexes after FcγRII-mediated internalization (27). Considering that the Ag/Ab ratio, which changes during an immunological reaction, would affect the type of immune complex formed, further understanding of intracellular regulation of monomeric and multivalent immune complexes may provide some insight into the function of the immune complex (28).
We applied the findings on FcγRII gained by our study, that is, that a pH-dependent Ab could accelerate Ag clearance in an FcγRII-dependent manner, to enhance the therapeutic potential of an mAb. We have recently shown that when Fc is engineered to confer FcRn binding at neutral pH, monomeric immune complexes can be taken up into the cell in an FcRn-dependent manner, and this will accelerate the Ag clearance of a pH-dependent Ab (18). However, this study showed that FcRn does not contribute to the uptake of monomeric immune complexes formed by wild-type hIgG1 (Fig. 1B), which is not surprising given that wild-type hIgG1 has negligible binding affinity to hFcRn at neutral pH (18). Alternatively, wild-type IgG1 does bind to FcγR at neutral pH (29–31), which is consistent with our finding that monomeric immune complexes can be taken up into the cell in an FcγR-mediated manner. Therefore, enhancing this natural IgG1 uptake pathway by increasing the Fc binding affinity to FcγR also enables us to increase the Ag clearance of a pH-dependent Ab (Fig. 2). As our study using FcγR knockout mice revealed (Fig. 4), immune complexes were mainly taken up by mFcγRII, so the Ag clearance of a pH-dependent Ab could be successfully accelerated by increasing the binding affinity to mFcγRII at neutral pH. Importantly, the ability of an increased binding affinity to mFcγRII to enhance Ag clearance was not observed when a non–pH-dependent, or conventional, Ab was used (Fig. 3B)—because Ag stays bound to the Ab within acidic endosome and is efficiently recycled back to the cell surface as an immune complex after mFcγRII-mediated internalization—and note that this novel application of Fc engineering to increase the binding affinity to mFcγRII and thus enhance the clearance of soluble Ag could only be revealed using a pH-dependent Ab.
As mentioned in the Introduction, the use of Fc engineering to modulate Fc–FcγR interaction has been limited to membrane-bound Ags and, to the best of our knowledge, this is the first report regarding soluble Ags. With the clinical application of this approach in mind, we confirmed that Fc engineering to enhance hFcγRIIb binding could also accelerate the Ag clearance of a pH-dependent Ab in hFcγRIIb Tg mice (Fig. 5). Importantly, the half-life of the Fc-engineered Ab was comparable to that of wild-type hIgG1, which indicated that the Ab was efficiently recycled back to plasma after hFcγRIIb-mediated internalization. As previously reported, our Fc engineering could selectively enhance the binding to inhibitory hFcγRIIb over that to other activating FcγRs, including the highly homologous Arg131 allotype of hFcγRIIa (24, 32). Because activating hFcγRIIa is highly expressed on platelets and contributes to platelet activation (33, 34), we think that selective enhancement of FcγRIIb binding would be important for clinical application from the point of safety and pharmacokinetics.
In conclusion, the present study revealed a hitherto unknown function of inhibitory FcγRIIb to take up monomeric immune complexes into the cell and subsequently recycle them back to the cell surface with high efficiency. We demonstrated that this newly discovered function of inhibitory FcγRIIb could be exploited to accelerate the clearance of soluble Ag when the Fc of a pH-dependent Ab, not a conventional Ab, is engineered to increase the binding affinity to inhibitory FcγRIIb.
Supplementary Material
Acknowledgments
We thank colleagues in Chugai Research Institute for Medical Science, Inc., and Chugai Pharmaceutical Co. Ltd.; Otoya Ueda and Hiroshi Hino for critical discussion and technical support; Yosuke Kawase, Hiromi Tateishi, Masahiro Morita, Mami Kakefuda, Kiyoharu Sato, Chisato Goto, and Kaoru Matsumoto for manipulation of mouse embryos and breeding the transgenic mice; Satomi Uchida for excellent technical assistance; Miho Saito and Wakako Hatakeyama for carrying out SPR analysis; Sachiyo Masujima and Akie Takara for Ab generation; and Toshiko Nishimura for carrying out in vivo studies.
The online version of this article contains supplemental material.
- FcRn
- neonatal FcR
- h
- human
- hs
- human soluble
- m
- mouse
- mIgG1-Fx
- a variant of mouse IgG1 with enhanced mouse FcγRII/III binding
- mIgG1-Fy
- a variant of mouse IgG1 with 100-fold enhanced binding to both mouse FcγRII and FcγRIII
- NPH-IgG1
- a non–pH-dependent Ab against human soluble IL-6R
- NPH-mIgG1
- non–pH-dependent anti–human soluble IL-6R Ab with mouse IgG1
- NPH-mIgG1-FcγR(−)
- a non–pH-dependent anti–human soluble IL-6R Ab with mouse IgG1 variant in which mouse FcγR binding is abrogated
- PH-hIgG1-FcγR(−)
- variant of pH-dependent Ab against human soluble IL-6R in which mouse FcγR binding is abrogated
- PH-hIgG1-FcRn(−)
- variant of pH-dependent Ab against human soluble IL-6R in which human neonatal FcR binding is abrogated
- PH-hIgG1-Fx
- afucosylated variant of pH-dependent Ab against human soluble IL-6R with higher affinity to mouse FcγRIV than to wild-type human IgG1
- PH-hIgG1-Fy
- variant of human IgG1 that has 100-fold higher affinity to both mouse FcγRII and FcγRIII than to wild-type human IgG1
- PH-hIgG1-Fz
- variant of human IgG1 that has higher affinity to all mouse FcγRs than to wild-type human IgG1
- PH-IgG1
- pH-dependent Ab against human soluble IL-6R
- PH-mIgG1-FcγR(−)
- pH-dependent anti–human soluble IL-6R Ab with mouse IgG1 variant in which mouse FcγR binding is abrogated
- PH-mIgG1-FcRn(−)
- pH-dependent anti−human soluble IL-6R Ab with mouse IgG1 variant in which mouse neonatal FcR binding at acidic pH is abrogated
- PH-v12
- a pH-dependent human IgG1 variant with selectively increased binding affinity to human FcγRIIb and not to any mFcγRs
- Tg
- transgenic.
Disclosures
All authors are full-time employees, part-time employees, or contractors of Chugai Pharmaceutical Co. Ltd.
References
- 1.Clynes R., Takechi Y., Moroi Y., Houghton A., Ravetch J. V. 1998. Fc receptors are required in passive and active immunity to melanoma. Proc. Natl. Acad. Sci. USA 95: 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clynes R. A., Towers T. L., Presta L. G., Ravetch J. V. 2000. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 6: 443–446. [DOI] [PubMed] [Google Scholar]
- 3.Li F., Ravetch J. V. 2012. Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement. Proc. Natl. Acad. Sci. USA 109: 10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igawa T., Ishii S., Tachibana T., Maeda A., Higuchi Y., Shimaoka S., Moriyama C., Watanabe T., Takubo R., Doi Y., et al. 2010. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 28: 1203–1207. [DOI] [PubMed] [Google Scholar]
- 5.Byrd J. C., O’Brien S., Flinn I. W., Kipps T. J., Weiss M., Rai K., Lin T. S., Woodworth J., Wynne D., Reid J., Molina A., et al. 2007. Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin. Cancer Res. 13: 4448–4455. [DOI] [PubMed] [Google Scholar]
- 6.Davda J. P., Hansen R. J. 2010. Properties of a general PK/PD model of antibody-ligand interactions for therapeutic antibodies that bind to soluble endogenous targets. MAbs 2: 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haringman J. J., Gerlag D. M., Smeets T. J., Baeten D., van den Bosch F., Bresnihan B., Breedveld F. C., Dinant H. J., Legay F., Gram H., et al. 2006. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 54: 2387–2392. [DOI] [PubMed] [Google Scholar]
- 8.Jayson G. C., Mulatero C., Ranson M., Zweit J., Jackson A., Broughton L., Wagstaff J., Hakansson L., Groenewegen G., Lawrance J., et al. European Organisation for Research and Treatment of Cancer (EORTC) 2005. Phase I investigation of recombinant anti-human vascular endothelial growth factor antibody in patients with advanced cancer. Eur. J. Cancer 41: 555–563. [DOI] [PubMed] [Google Scholar]
- 9.Martin P. L., Cornacoff J., Prabhakar U., Lohr T., Treacy G., Sutherland J. E., Hersey S., Martin E. 2005. Reviews preclinical safety and immune-modulating effects of therapeutic monoclonal antibodies to interleukin-6 and tumor necrosis factor-α in cynomolgus macaques. J. Immunotoxicol. 1: 131–139. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J. J., Krzyzanski W., Wang Y. M., Li H., Rose M. J., Ma M., Wu Y., Hinkle B., Perez-Ruixo J. J. 2010. Pharmacokinetics of anti-hepcidin monoclonal antibody Ab 12B9m and hepcidin in cynomolgus monkeys. AAPS J. 12: 646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arend W. P., Sturge J. C. 1979. Composition and biologic properties of soluble IgG-anti-IgG immune complexes: effects of variations in the specificity of rabbit antibodies to different structural components of human IgG. J. Immunol. 123: 447–454. [PubMed] [Google Scholar]
- 12.Benacerraf B., Sebestyen M., Cooper N. S. 1959. The clearance of antigen antibody complexes from the blood by the reticuloendothelial system. J. Immunol. 82: 131–137. [PubMed] [Google Scholar]
- 13.Ganesan L. P., Kim J., Wu Y., Mohanty S., Phillips G. S., Birmingham D. J., Robinson J. M., Anderson C. L. 2012. FcγRIIb on liver sinusoidal endothelium clears small immune complexes. J. Immunol. 189: 4981–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurlander R. J., Ellison D. M., Hall J. 1984. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J. Immunol. 133: 855–862. [PubMed] [Google Scholar]
- 15.Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. 1985. Characterization of monoclonal antibodies to Treponema pallidum. J. Immunol. 134: 585–592. [PubMed] [Google Scholar]
- 16.Leabman M. K., Meng Y. G., Kelley R. F., DeForge L. E., Cowan K. J., Iyer S. 2013. Effects of altered FcγR binding on antibody pharmacokinetics in cynomolgus monkeys. MAbs 5: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hand P. H., Calvo B., Milenic D., Yokota T., Finch M., Snoy P., Garmestani K., Gansow O., Schlom J., Kashmiri S. V. 1992. Comparative biological properties of a recombinant chimeric anti-carcinoma mAb and a recombinant aglycosylated variant. Cancer Immunol. Immunother. 35: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igawa T., Maeda A., Haraya K., Tachibana T., Iwayanagi Y., Mimoto F., Higuchi Y., Ishii S., Tamba S., Hironiwa N., et al. 2013. Engineered monoclonal antibody with novel antigen-sweeping activity in vivo. PLoS One 8: e63236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dall’Acqua W. F., Woods R. M., Ward E. S., Palaszynski S. R., Patel N. K., Brewah Y. A., Wu H., Kiener P. A., Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169: 5171–5180. [DOI] [PubMed] [Google Scholar]
- 20.Richards J. O., Karki S., Lazar G. A., Chen H., Dang W., Desjarlais J. R. 2008. Optimization of antibody binding to FcγRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 7: 2517–2527. [DOI] [PubMed] [Google Scholar]
- 21.Roopenian D. C., Christianson G. J., Sproule T. J. 2010. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol. Biol. 602: 93–104. [DOI] [PubMed] [Google Scholar]
- 22.Rashid A., Auchincloss H., Jr., Sharon J. 1992. Comparison of GK1.5 and chimeric rat/mouse GK1.5 anti-CD4 antibodies for prolongation of skin allograft survival and suppression of alloantibody production in mice. J. Immunol. 148: 1382–1388. [PubMed] [Google Scholar]
- 23.Nimmerjahn F., Ravetch J. V. 2005. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310: 1510–1512. [DOI] [PubMed] [Google Scholar]
- 24.Mimoto F., Katada H., Kadono S., Igawa T., Kuramochi T., Muraoka M., Wada Y., Haraya K., Miyazaki T., Hattori K. 2013. Engineered antibody Fc variant with selectively enhanced FcγRIIb binding over both FcγRIIa(R131) and FcγRIIa(H131). Protein Eng. Des. Sel. 26: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergtold A., Desai D. D., Gavhane A., Clynes R. 2005. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 23: 503–514. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C. Y., Booth J. W. 2010. Divergent intracellular sorting of FcγRIIA and FcγRIIB2. J. Biol. Chem. 285: 34250–34258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weflen A. W., Baier N., Tang Q. J., Van den Hof M., Blumberg R. S., Lencer W. I., Massol R. H. 2013. Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol. Biol. Cell 24: 2398–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker K., Qiao S. W., Kuo T. T., Aveson V. G., Platzer B., Andersen J. T., Sandlie I., Chen Z., de Haar C., Lencer W. I., et al. 2011. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8−CD11b+ dendritic cells. Proc. Natl. Acad. Sci. USA 108: 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferis R., Lund J. 2002. Interaction sites on human IgG-Fc for FcγR: current models. Immunol. Lett. 82: 57–65. [DOI] [PubMed] [Google Scholar]
- 30.Lux A., Nimmerjahn F. 2013. Of mice and men: the need for humanized mouse models to study human IgG activity in vivo. J. Clin. Immunol. 33(Suppl. 1): S4–S8. [DOI] [PubMed] [Google Scholar]
- 31.Overdijk M. B., Verploegen S., Ortiz Buijsse A., Vink T., Leusen J. H., Bleeker W. K., Parren P. W. 2012. Crosstalk between human IgG isotypes and murine effector cells. J. Immunol. 189: 3430–3438. [DOI] [PubMed] [Google Scholar]
- 32.Mimoto F., Kadono S., Katada H., Igawa T., Kamikawa T., Hattori K. 2014. Crystal structure of a novel asymmetrically engineered Fc variant with improved affinity for FcγRs. Mol. Immunol. 58: 132–138. [DOI] [PubMed] [Google Scholar]
- 33.Pollreisz A., Assinger A., Hacker S., Hoetzenecker K., Schmid W., Lang G., Wolfsberger M., Steinlechner B., Bielek E., Lalla E., et al. 2008. Intravenous immunoglobulins induce CD32-mediated platelet aggregation in vitro. Br. J. Dermatol. 159: 578–584. [DOI] [PubMed] [Google Scholar]
- 34.Tomiyama Y., Kunicki T. J., Zipf T. F., Ford S. B., Aster R. H. 1992. Response of human platelets to activating monoclonal antibodies: importance of Fc gamma RII (CD32) phenotype and level of expression. Blood 80: 2261–2268. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.