Abstract
MicroRNAs (miRNAs) are short noncoding RNAs with a length of approximately 22 nucleotides that are involved in posttranscriptional regulation of gene expression. miRNAs cover an important role in all biological processes, and aberrant miRNA expression is commonly associated with cancer. Recent discoveries have associated the involvement of miRNA in an increasingly large number of biological processes, including cachexia. The cachexia syndrome is a debilitating state of cancer that is, at least in part, associated with apoptosis. The mechanism through which tumors promote the characteristic distal loss of muscle and fat mass during the cachectic process is still not deeply investigated. In this review, we briefly describe the role of miRNAs in cancer development and cachexia.
Keywords: microRNA, cancer, cachexia
MicroRNAs (miRNAs) are short noncoding RNAs of approximately 22 nucleotides that are found in all eukaryotic cells and play important roles in almost all biological pathways [1]. In the 1990s, 2 independent studies identified the lin-4 gene in Caenorhabditis elegans as a small noncoding RNA. After that, for a few years, no major discoveries were made concerning miRNA functions [2, 3]. Only in 2002 was the first association between miRNA deregulation and cancer made, by studying a frequently deleted chromosomal region in chronic lymphocytic leukemia (CLL; 13q14). This region contains 2 miRNA genes expressed in the same polycistronic RNA, miR-15a and miR-16-1, whose deletion causes the development of the indolent form of CLL [4]. The association between miRNA deregulation and cancer induced many laboratories to focus their research on the study of these small noncoding RNAs in a wide range of cancers, generating large amounts of data supporting the idea that aberrant miRNA expression is the rule rather than the exception in cancer [5]. miRNAs are involved in the control of several cancer-relevant processes, such as proliferation [6], apoptosis [7], and migration and invasion [8]. Apart from cancer, miRNAs are involved in many other diseases, such as metabolic disorders [9].
Cachexia is a syndrome characterized by a reduction of muscle and fat body masses, and it is often associated with many types of chronic diseases, including cancer [10]. The weight loss during cachexia is not related to starvation but, rather, to tumor presence and the abnormal production of cytokines. In general, patients with lung or pancreas cancers at an advanced stage have asthenia, physical weakness, and difficulty of concentration, symptoms that are often associated with cancer-induced cachexia. The chemical treatment of tumors in patients with cachexia is often characterized by a low dose-limiting toxicity and, unfortunately, by a grade of weight loss that turns out to be positively correlated with patient mortality [11, 12]. Recently, miRNAs have been discovered to play a very important role during the cachectic muscle-wasting process. Many recent studies reported the detection of miRNAs in body fluids such as serum, plasma, milk, urine, and spinal fluid [13–15]. Experimental evidence also describes the presence of miRNAs associated with microvesicles in body fluids. These circulating miRNAs were found in microvesicles with diameters usually ranging from 30 nm to 1 µm [16]. Although the microvesicle secretion mechanism is still not well understood, there is clear evidence that microvesicles are able to interact with target cells by fusing with the plasma membrane and consequently delivering their load [17]. Recent studies demonstrate that cancer-derived microvesicles containing miRNA were able to induce apoptosis of skeletal muscle cells [18].
Role of miRNAs in Cancer
The first evidence of a link between miRNA deregulation and cancer has been described by Calin et al in 2002 [4]. They were the first to associate the frequent deletion of miR-15a/miR-16-1 with CLL, concluding that these 2 miRNAs exert a tumor-suppressor function [4]. This study is considered a forerunner of miRNA research in cancer, motivating, in recent years, many research groups to focus their efforts on studying the causes of aberrant miRNA expression in tumors. Generally, miRNA deregulation in cancer tissues as opposed to normal ones could be related to aberrant epigenetic changes in the methylation status of miRNA promoters [19]. The altered function and/or expression of enzymes implicated in miRNA biogenesis is another mechanism that can cause aberrant expression of miRNAs in cancer. For instance, the expression of Drosha and Dicer, 2 proteins actively involved in miRNA biogenesis, was decreased in 39% of patients with ovarian cancer [20]. Transcription factor activity also plays an important and multifaceted role in the regulative mechanism of miRNA expression [21]. A very important and representative example of cancer-related transcriptional regulation of miRNA expression is the induction of miR-17/miR-92 cluster transcription by the MYC oncogene [22]. miRNAs can also decisively intervene in the modulation of cellular methylation by regulating the expression of the enzymes responsible for epigenetic control. In the past few years, our group identified the miR-29 family as a key regulator for the ex novo expression of DNA methyltransferases DNMT3a and DNMT3b in lung cancer [23]. Recently, the transcriptional effect of hepatocyte growth factor receptor c-MET on miRNAs has been investigated. In particular, our laboratory found that c-MET is able, through specific transcriptional factors such as AP1 and ELK-1 under its control, to promote the expression of the miR-221/miR-222 onco-miRNA cluster and to induce a negative feedback with miR-27a. Our data suggest that the effect of c-MET deregulation in cancer is at least in part linked to a deregulated miRNA expression pathway [24, 25]. Finally, because miRNA genes are transcribed by RNA polymerase II, their expression can be also regulated by nuclear receptors [21]. For example an aberrant expression of nuclear receptors such as estrogen receptor α and androgen receptor in cancer is involved in the deregulation of numerous miRNAs [26].
Some miRNAs also play an important role in drug resistance. miRNAs such as miR-19, miR-21, and the miR-221/miR-222 cluster were upregulated several fold in association with this phenomenon [24, 27, 28], while others, such as miR-130a and miR-298, were downregulated, suggesting an important biological role of miRNAs in drug resistance [29, 30]. It is very important to highlight that the role of miRNAs in drug resistance is tissue specific. For instance, miR-27a is indirectly related to drug susceptibility in ovarian cancer, but it can be directly involved in drug resistance in leukemia [31].
miRNAs have been shown to also have a role as cancer biomarkers in patients. Recently, our group analyzed the miRNA expression profile of 82 patients with colon cancer. Our data demonstrated that a miRNA profile was able to discriminate between colorectal recurrences in lymph nodes and liver, colorectal liver metastasis, and primary hepatic tumor [32].
miRNA and Cachexia
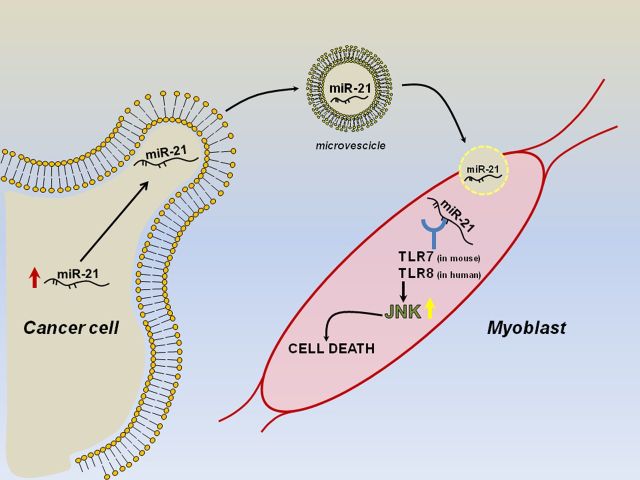
Cachexia is a syndrome consisting in the loss of muscle proteins and fat mass, which has very important clinical consequences in aging, as well as in diseases such as diabetes, chronic heart failure, and cancer [33]. Such syndrome is incurable and occurs in malignancies such as pancreatic and lung cancer. Patients with cachexia cannot be treated with standard therapies because they could easily lead to their death [18]. The loss of skeletal muscle during cachexia is a result of protein degradation subsequent to an altered metabolism in response to tumor progression [34]. Recently, many efforts have been made to better investigate the molecular mechanisms underlying cachexia. In silico and in vivo approaches found that miR-206 and miR-21 were sufficient and required for atrophy. Their ability to target the transcription factor YY1 and the translational initiator factor eIF4E3 downstream places them as fine regulators of muscle loss and weakness in catabolic conditions [33]. Overexpression of the Pax7 transcription factor has been recently reported in both tumor-bearing mice and patients with pancreatic cancer and weight loss. This abnormal expression of Pax7 induces stem cell differentiation impairment, resulting in muscle atrophy. These data suggest that the muscle microenvironment is able to affect tumor-induced muscle wasting in a consistent way [35]. It has been shown that secreted miRNAs within exosomes can regulate gene expression in recipient cells by targeting their messenger RNAs [17]. More recently, it has been demonstrated that miRNAs can also directly bind proteins, acting as ligands [36]. As early as 2012, our group showed that tumor-secreted miR-21 and miR-29a were able to bind as ligands to human Toll-like receptor 8 (TLR8), corresponding to murine TRL7, in immune cells, inducing the TLR-mediated prometastatic inflammatory response [37]. More recently, our group found that muscle cachexia is caused by a high level of shedding from microvesicles containing miR-21 in lung and pancreatic cancer cells. Such microvesicles fuse with myoblasts and cause them to die by apoptosis. This process depends on the expression of TLR7/8 in the myoblasts. The identification of this process may bring us to develop novel approaches to the treatment of cachexia (Figure 1) [18].
Figure 1.
Schematic representation of miR-21 function in cachexia. Abbreviations: TLR7, Toll-like receptor 7; TLR8, Toll-like receptor 8.
DISCUSSION
miRNAs are a class of small noncoding RNAs that play a critical role in the regulation of a large number of biological processes and diseases, including cancer [38]. In 2002, our laboratory was the first to associate miRNA deregulation with cancer. This led to a constantly growing number of studies and publications about miRNA expression and cancer. The ubiquitous expression of miRNAs and the pervasive role of certain deregulated miRNAs in cancer processes such as proliferation, apoptosis, and invasion led investigators worldwide to increase their efforts in the understanding of the biology of miRNAs in cancer. In the last 10 years, great progress has been made, but many additional years of study are needed for a complete understanding of the connection between miRNAs and cancer. Recently, new mechanisms of miRNA action have been discovered. For instance, the ability of miRNAs to act as protein ligands or their ability to spread through the body when included within microvesicles yielded new horizons of miRNA research in cancer. It has been recently reported that miRNAs have a role in cachexia, and miR-21 in particular appears to be a key regulator of this process [33]. Our group has recently characterized a new biological mechanism that involves microvesicles and miRNAs: their ability to act as TRL7/8 ligands in humans and mice. This process is very important in tumor-induced cachexia. We discovered that microvesicles were able to shuttle overexpressed miR-21 from cancer cells in tumors to myoblasts, inducing apoptosis. This is a revolutionary mechanism that could enormously contribute to a better understanding of tumor-induced cachexia. We believe that this finding is another important step toward the comprehension of miRNA function, proving once again the key role that they play in tumors.
Notes
Acknowledgments. We thank Giulia Romano, Dario Veneziano, and Federica Calore for revisions to the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant CA152758 to C. M. C.).
Potential conflict of interest. Both authors: No reported conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol 2012; 6:590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern-formation in C-elegans. Cell 1993; 75:855–62. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843–54. [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99:15524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009; 10:704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Brit J Cancer 2006; 94:776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene 2006; 25:6176–87. [DOI] [PubMed] [Google Scholar]
- 8.Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer 2010; 126:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004; 432:226–30. [DOI] [PubMed] [Google Scholar]
- 10.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012; 16:153–66. [DOI] [PubMed] [Google Scholar]
- 11.Tisdale MJ. Cancer cachexia. Br J Cancer 1991; 63:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002; 2:862–71. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Li J, Huang B, et al. Exosomes: novel biomarkers for clinical diagnosis. Sci World J 2015; 2015:657086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke M, Hoefig K, Merz H, et al. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol 2010; 28:655–61. [DOI] [PubMed] [Google Scholar]
- 15.Allegra A, Alonci A, Campo S, et al. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int J Oncol 2012; 41:1897–912. [DOI] [PubMed] [Google Scholar]
- 16.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res 2011; 1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 17.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654–9. [DOI] [PubMed] [Google Scholar]
- 18.He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci U S A 2014; 111:4525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabbri M, Calore F, Paone A, Galli R, Calin GA. Epigenetic regulation of miRNAs in cancer. Adv Exp Med Biol 2013; 754:137–48. [DOI] [PubMed] [Google Scholar]
- 20.Merritt WM, Lin YG, Han LY, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 2008; 359:2641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y, Kim M, Han J, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 2004; 23:4051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435:839–43. [DOI] [PubMed] [Google Scholar]
- 23.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 2007; 104:15805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garofalo M, Di Leva G, Romano G, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009; 16:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Acunzo M, Romano G, Palmieri D, et al. Cross-talk between MET and EGFR in non-small cell lung cancer involves miR-27a and Sprouty2. Proc Natl Acad Sci U S A 2013; 110:8573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci 2011; 1:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Z, Li Y, Huang K, Wagar N, Shim H. Regulation of miR-19 to breast cancer chemoresistance through targeting PTEN. Pharm Res 2011; 28:3091–100. [DOI] [PubMed] [Google Scholar]
- 28.Shi GH, Ye DW, Yao XD, et al. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin 2010; 31:867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acunzo M, Visone R, Romano G, et al. miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene 2012; 31:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol 2012; 180:2490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Wu H, Liu X, et al. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 2008; 76:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drusco A, Nuovo GJ, Zanesi N, et al. MicroRNA profiles discriminate among colon cancer metastasis. PLoS One 2014; 9:e96670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soares RJ, Cagnin S, Chemello F, et al. Involvement of microRNAs in the regulation of muscle wasting during catabolic conditions. J Biol Chem 2014; 289:21909–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol 1999; 31:1373–90. [DOI] [PubMed] [Google Scholar]
- 35.He WA, Berardi E, Cardillo VM, et al. NF-kappaB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest 2013; 123:4821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eiring AM, Harb JG, Neviani P, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 2010; 140:652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbri M, Paone A, Calore F, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012; 109:E2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis 2012; 33:1126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]