Abstract
Giant cell arteritis (GCA) is an immune-mediated disease of unknown etiology. Varicella zoster virus (VZV) antigen was found in all of 4 GCA-positive temporal arteries (TAs) but was not present in any of 13 normal TAs. All 4 GCA-positive TAs contained viral antigen in skip areas, mostly in the adventitia and media and least in the intima. Despite formalin fixation, VZV DNA was detected in 2 of 4 GCA-positive, VZV antigen–positive TAs. Skeletal muscle was attached to 3 of 4 TAs, and VZV antigen was found in 2 and VZV DNA in 1. VZV may cause GCA.
Keywords: varicella zoster virus, temporal artery, giant cell arteritis
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection is followed by varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines, leading to virus reactivation, manifest as herpes zoster (shingles), pain and rash usually restricted to 1–2 dermatomes. The most common complication of zoster is postherpetic neuralgia (chronic radicular pain); other serious disorders after zoster include meningoencephalitis, myelitis, and VZV vasculopathy [1]. VZV vasculopathy results from productive virus infection in cerebral arteries and is increasingly recognized as a treatable cause of transient ischemic attacks and stroke [2].
Although all cases of VZV vasculopathy had been in intracerebral arteries [3], multiple clinical-virological case studies from 2011 to 2014 revealed that VZV also infects the extracranial temporal artery (TA), as well as the ophthalmic and retinal arteries, producing symptoms, signs, and laboratory abnormalities identical to those seen in giant cell arteritis (GCA), including the presence of anti-VZV immunoglobulin G (IgG) and immunoglobulin M antibodies in the cerebrospinal fluid of some of these patients [4–6]. All of these TAs had negative results of pathological tests for GCA, including the 5 of 24 (21%) archived GCA-negative TAs in which VZV infection was found [7]. Further indication of the strong association of VZV with GCA was demonstrated by GCA pathology (inflammation, necrosis, and abundant multinucleated giant cells in the arterial media) in multiple regions (skip areas) adjacent to those containing VZV antigen and VZV DNA, as well as in skeletal muscle adjacent to the infected TA [5]. Finally, VZV antigen and VZV DNA were found in skip areas in the TA of a patient who developed clinical features of GCA and ipsilateral ophthalmic-distribution herpes zoster, followed 2 weeks later by VZV encephalitis and 2 months later by ischemic optic neuropathy, but for whom results of pathological analysis of the TA were negative for GCA [8].
The repeated detection of VZV in multiple GCA-negative TAs, as well as in a GCA-positive TAs, prompted virological analysis of TA biopsy specimens from patients with pathologically confirmed GCA and of control TAs removed at routine autopsy from adults ages >50 years. Here, we analyzed 4 GCA-positive TAs and 13 normal TAs for the presence of VZV to investigate a causal link between VZV and GCA.
METHODS
Immunohistochemical Analysis
One hundred 5-µm sections of 4 formalin-fixed, paraffin-embedded GCA-positive TAs and 13 normal TAs were cut and baked for 1 hour at 60°C. Every other section (50 slide sections/TA) was then deparaffinized and immunostained with a 1:500 dilution of mouse monoclonal anti-VZV gE IgG1 antibody (Santa Cruz Biotechnology, Dallas, Texas), followed by a 1:1000 dilution of secondary biotinylated goat anti-mouse antibody (Dako, Carpinteria, California) and prediluted streptavidin-alkaline phosphatase (BD Biosciences, San Diego, California). The color reaction was developed using the fresh fuchsin substrate system (Dako) with levamisole (Dako) at a final concentration of 24 µg/mL as described elsewhere [7]. When a section was found to contain VZV antigen, at least 2 adjacent sections were stained as described above except that mouse anti-VZV gE IgG antibody was replaced with a 1:500 dilution of control mouse IgG1 antibody (Dako). Positive controls consisted of a VZV-infected cadaveric TA maintained for 14 days in vitro and then stained with mouse anti-VZV gE IgG antibody as described above. To further control for VZV specificity in TAs, one GCA-positive TA was immunostained with rabbit anti-VZV IgG and rabbit anti-HSV antibody [7].
Quantitative Polymerase Chain Reaction (qPCR) Amplification of VZV DNA in TA Sections Containing VZV Antigen
Every section of each VZV antigen-positive TA was scraped with a scalpel, pooled, placed into 200 µL lysis buffer with proteinase K, and incubated overnight at 56°C (DNeasy Blood and Tissue Kit; Qiagen; Germantown, Maryland). DNA was extracted as per the manufacturer's protocol, followed by qPCR analysis with primers for VZV and for glyceraldehyde-3-phosphate-dehydrogenase (GAPdH) as described previously [8]. Similarly, any VZV antigen–positive section of skeletal muscle adjacent to a TA was scraped and treated as described above to detect amplifiable VZV DNA by real-time qPCR. VZV DNA copy number was determined using known concentrations of VZV DNA as PCR standards [9].
RESULTS
Using antibodies directed against VZV from 2 different species, immunohistochemical staining revealed VZV antigen in all 4 GCA-positive TAs that was not seen with cognate control antibodies (Figure 1). No VZV antigen was detected with either anti-VZV IgG antibody in 13 normal TAs. Among the 4 GCA-positive TAs, VZV antigen was found in 3, 5, 12, and 13 skip areas, mostly in the adventitia and media. PCR amplified VZV DNA in 2 of the 4 GCA-positive TAs that contained VZV antigen. Skeletal muscle was attached to 3 of 4 GCA-positive TAs, and analysis of these 3 skeletal muscles revealed VZV antigen in 2 and VZV DNA in 1.
Figure 1.
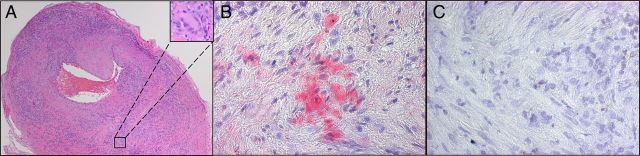
A, Hematoxylin–eosin staining of a temporal artery cross-section reveals extensive inflammation in the adventitia, media, and intima; disruption of the media and internal elastic lamina; a thickened intima; and the presence of giant cells (inset), diagnostic of giant cell arteritis. B and C, Varicella zoster virus (VZV) antigen (B; red) was detected in the adventitia of the same temporal artery by using rabbit anti–VZV 63 antibody but by using rabbit anti–herpes simplex virus type 1 antibody (C) or normal rabbit serum (data not shown).
DISCUSSION
Immunohistochemical analysis detected VZV antigen in all of 4 GCA-positive TAs but not in normal TAs from individuals aged >50 years, the same age group in which GCA develops. The distribution of VZV antigen predominately in the arterial adventitia and media and least in the intima in GCA-positive TAs is identical to that of VZV in intracerebral arteries of patients who died of VZV vasculopathy [10], indicating that after reactivation from cranial nerve ganglia, VZV spreads transaxonally to intracerebral and extracranial arteries, initially infecting the adventitia and then extending transmurally. Despite formalin fixation, PCR amplified VZV DNA in 2 of 4 VZV antigen-positive TAs. The pathological relevance of VZV in GCA-positive TAs was further indicated by the presence of virus in skip areas, paralleling a characteristic feature of GCA pathology, as well as by the detection of VZV antigen and VZV DNA in skeletal muscle adjacent to VZV antigen-positive TAs.
Clearly, analysis of additional GCA-positive TAs, as well as GCA-negative TAs from patients with symptoms, signs, and laboratory abnormalities characteristic of GCA, are needed. If VZV is proven to be a cause of GCA, treatment would consist of antiviral agents, as well as corticosteroids, for what is likely a VZV-induced immunopathological disorder.
Notes
Acknowledgments. We thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
Financial support. This work was supported by the National Institutes of Health (grant AG032958 to D. G. and M. A. N.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection of the nervous system. In: Johnson MV, Adams HP, Fatemi SA, eds. Neurobiology of disease. 2nd ed New York: Oxford University Press, 2015. In press. [Google Scholar]
- 2.Nagel MA, Gilden D. Update on varicella zoster virus vasculopathy. Curr Infect Dis Rep 2014; 407:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis and treatment. Lancet Neurol 2009; 8:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salazar R, Russman AN, Nagel MA, et al. Varicella zoster virus ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol 2011; 68:517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel MA, Khmeleva N, Boyer PJ, Choe A, Bert R, Gilden D. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci 2013; 335:229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci 2013; 325:180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology 2013; 80:2017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teodoro T, Nagel MA, Geraldes R, et al. Biopsy-negative, varicella zoster virus (VZV)-positive giant cell arteritis, zoster, VZV encephalitis and ischemic optic neuropathy, all in one. J Neurol Sci 2014; 343:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird NL, Bowlin JL, Yu X, et al. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 2014; 458–459:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: Analysis of virus-infected arteries. Neurology 2011; 77:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


