Abstract
Nesfatin-1 is a peptide secreted by peripheral tissues, central and peripheral nervous system. It is involved in the regulation of energy homeostasis related with food regulation and water intake. Nesfatin-1 can pass through the blood-brain barrier in both directions. It suppresses feeding independently from the leptin pathway and increases insulin secretion from pancreatic beta islet cells. That is why nesfatin-1 has drawn attention as a new therapeutic agent, especially for the treatment of obesity and diabetes mellitus. Its effects on nutrition have been studied in more detail in literature. On the other hand, its effects on other physiological parameters and mechanisms of action still need to be clarified. Synthesizing the research on nesfatin-1 can help us better understand this field. Hippokratia 2015, 19 (1): 4-10.
Keywords: Human nesfatin-1 protein, human nucleobinding 2 protein, nerve tissue proteins, anti-obesity agents, appetite depressants, L-type calcium channels
History of nesfatin-1
Nesfatin-1 was discovered by Oh-I and colleagues for the first time in 2006. They showed that nesfatin-1 is secreted from the hypothalamic nuclei, which are responsible for controlling appetite. In the same study, it was reported that nesfatin-1 suppresses food intake, even in obese mice with a knockdown leptin gene1. This finding indicates the efficacy of nesfatin-1 as an appetite suppressant, as it works independently of the leptin pathway. Thus, nesfatin-1 has raised interest in the area of treatment of obese individuals with the leptin gene mutation2.
What is nesfatin-1?
The hypothalamus contains areas responsible for the regulation of feeding behavior and the secretion of molecules from these areas. Nesfatin-1 is involved in the regulation of food intake. It is secreted from the hypothalamus and brain stem, and its secretion decreases during fasting1. This feature of nesfatin-1 indicates that nesfatin-1 is involved in the regulation of energy homeostasis.
Immunohistochemical studies have shown that the precursor of nesfatin-1, nonesterified fatty acid/nucleobinding 2 (NUCB2), is localized in many places such as the pituitary gland, hypothalamus, brain stem, the forebrain and midbrain nuclei, central amygdaloid nucleus, ventrolateral medulla, and cerebellum. Additionally, nesfatin-1 exists in the thoracolumbar sympathetic and sacral parasympathetic preganglionic neurons in the spinal cord in rats3,4. It has been shown that nesfatin-1 exists in peripheral tissues, in addition to the central nervous system (CNS). Specifically, it is secreted by peripheral adipose tissue, gastric mucosa, pancreatic endocrine beta cells, and testis tissue. Nesfatin-1 can pass through the blood brain barrier in both directions after secretion. Nesfatin-1 is stable in blood for 20 minutes after intravenous injection3,5-9.
NUCB1 shares 60% sequence homology with NUCB2 in the mouse genome. Very little is known about NUCB1 function. Recently, its distribution at the tissue, cellular and subcellular levels was determined to better understand its function. NUCB1 has widespread distribution in endocrine cells and its subcellular distribution is found coupled with Golgi-apparatus. These results can be accepted as a sign that this molecule may play a role in the regulation of hormone secretion10.
Gene of Nesfatin-1
Nesfatin-1 is an 82 amino acid (aa) length polypeptide derived from calcium and DNA binding protein NUCB2. It shares a homology of more than 85% between humans and other mammal species, and even demonstrates similarities with lower organisms7. NUCB2 is found in the plasma membrane and neuroplasma. NUCB2 has many parts for posttranslational modifications. Special prohormone convertase enzymes such as PC3/1 and PC2 converts NUCB2 to form nesfatin-1, nesfatin-2 and nesfatin-3; between 1-82 aa, 85-163 aa and 166-396 aa, respectively9,11. There is not yet any definitive information about the biological activity of nesfatin-2 and -312. Nesfatin-1 has three parts; N-terminal (N23), the middle part (M30) and C-terminal (C29). The middle part is described as an active part of nesfatin-1. It has the key role for the physiological effects of nesfatin-1, especially for an anorexic effect11,13,14.
The human NUCB2 gene is 55 kb in length and has 14 exon and 13 intron regions. The initiation region of transcription takes place before 246 bp than the initiation of translation region. The NUCB2 gene has a 5889 bp length promoter region, which is active in neuron-derived cell lines. The NUCB2 gene translation region is described in exon-3. Nesfatin-1 is coded in between exon-3 and 5 of the NUCB2 gene. The untranslated region (3’UTR) is described in exon-1411,13.
Secretion fields of nesfatin-1
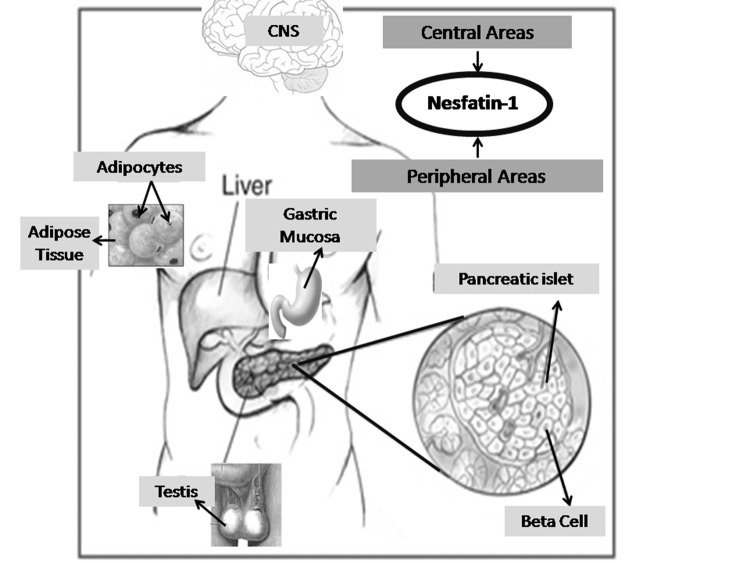
Nesfatin-1 and its precursor take place in many different parts of the CNS, such as the pituitary gland, arcuate (ARC) and paraventricular nuclei (PVN), which are hypothalamic, and the brain stem nuclei, supraoptic nucleus (SON), lateral hypothalamic region, and nucleus tractus solitarus (NTS). Additionally, it has been shown that nesfatin-1 takes place in the nuclei of the forebrain and midbrain, the central amygdaloid nucleus, ventrolateral medulla, the cerebellum and also in the thoracolumbar sympathetic and sacral parasympathetic spinal cord preganglionic nuclei of rats3,15 (Figure 1).
Figure 1. Schematic drawing demonstrating the secretion fields of nesfatin-1. CNS: central nervous system.
Several studies have shown that nesfatin-1 is also secreted by peripheral tissues besides CNS, such as adipose tissue, gastric mucosa, endocrine pancreatic beta cells, and the testis. Its expression level has been found 20-fold higher in the stomach oxyntic mucosa than in the brain1,3,6-9,15 (Figure 1).
Nesfatin-1 and food intake
Until now, the mechanism of food intake inhibition via nesfatin-1 has not been clarified. Possible mechanisms have been used to explain the injection of nesfatin-1 in different parts of the brain2,9. Nesfatin-1 can inhibit food intake in leptin-receptor-mutant rats via an injection in the brain1,3. That is why it is accepted that nesfatin-1 can inhibit food intake via melatonin system activation, independent of the leptin pathway. This presents the first explanation for the mechanism of inhibition of food intake by nesfatin-12,9,16.
Another possibility for the explanation of this mechanism is the direct inhibition of an orexigenic substance by nesfatin-1. In vitro studies have shown that nesfatin-1 causes hyperpolarization in ARC nuclei, which are responsible for neuropeptide-Y (NPY) secretion. This is accepted as the second explanation for the mechanism of food intake inhibition by nesfatin-12,3,9,16. Intracerebroventricular (icv) nesfatin-1 injection in rats inhibits food intake and dose-dependently in the dark. At the same time, peripheral nesfatin-1 injection can inhibit food intake2,16. Blockage of nesfatin-1 increases food intake2. Studies are in progress to explain the gaps in the mechanisms of nesfatin-1’s actions on different physiological parameters. Recently, it was shown that NPY and the α-melanocyte-stimulating hormone (α-MSH) have a controversial effect on PVN nesfatin-1/NUCB2 neurons that are activated by α-MSH and inhibited by NPY through induced and blocked cytosolic calcium ion, respectively17. Another recent study suggests that nesfatin-1/NUCB2 protein levels are increased by dehydration. This elevation is also found in positive correlation with plasma sodium concentration and plasma osmolality18.
Recently it has been shown that nesfatin-1 regulates the activity of gastric distention-sensitive neurons and gastric motility via the melanocortin pathway in the central nucleus of amygdala19. Furthermore, nesfatin-1 is described as an inhibitory neurotransmitter to regulate gastric motility through its action on the PVN and the lateral hypothalamic area (LHA)20. Further studies are in progress to explain the specific role of nesfatin-1 on feeding.
Nesfatin-1 and nervous system
Neurons that express NUCB2 have been found in many regions of the brain13. Some neurons in the parvocellular region (pPVH) express a NUCB2 melanocortin receptor (MC4) and receive signals from the fibers of α-MSH and agouti related protein (AgRP). α-MSH increases the expression and release of nesfatin-1. In a study, it was suggested that NUCB2 neurons in pPVH cause anorexia by secreting nesfatin-1; however, the efficacy of the mechanism of nesfatin-1 action still remains unclear2,9.
The central release of nesfatin-1 alters, depending on variations in metabolic activities. After a 24-hour fasting period, the transcription and translation of nesfatin-1 is decreased in PVH and SON. As a result of re-feeding after 24 hours fasting, nesfatin-1 production and secretion is activated in the SON. Peripheral injection of anorexic agents such as α-MSH and serotonin 5-HT receptor antagonist increases the amount of nesfatin-1, which is secreted from the hypothalamus in rodents. The peripheral application of an orexigenic dose of ghrelin causes an increase in the activity of nesfatin-1 secreting neurons in the ARC nucleus. Furthermore, peripheral injection of cholecystokinin (CCK), which is a satiety peptide, causes an increase in the activity of nesfatin-1 secreting neurons that are located in pPVN and NTS. These findings indicate that nesfatin-1 is affecting the feeding peptides which are involved in satiety signalling12. It is believed that hunger is influenced by leptin and ghrelin-mediated peripheric metabolic signals. Ghrelin reduces the activity of POMC neurons and increases the activity of AgRP neurons with its effects on ARC nucleus. Oh-I et al suggested that hunger decreases the NUCB2 activity of pPVH neurons through melanocortin pathways2,9,13.
Nesfatin-1 and sympathetic nervous system
In a study it has been shown that an icv injection of nesfatin-1 in rats, both increased the sympathetic activity of kidneys and induced an increase in blood pressure. Additionally, it has been found that nesfatin-1 performed this effect in a dose-dependent manner up to a certain point. In the same study, the relationship between nesfatin-1 and the melanocortin pathway and activation of sympathetic system were examined. For this purpose, the melanocortin 3/4 receptor antagonist SHU9119 was used. As a result of this application, the blood pressure-raising and sympathetic nervous system-activating effects of nesfatin-1 were eliminated. For this reason, it is considered that nesfatin-1 has made the sympathetic nervous system activation on kidney through the central melanocortin system21.
Nesfatin-1 and stress
It is supposed that nesfatin-1 plays a role in the regulation of stress responses under stress conditions. Stress causes anorexia. Probably, nesfatin-1 shows this effect by activating NUCB2 secreting neurons in pPVH. It is known that there are posterior pituitary extensions of magnocellular neurons and neurons in SON, which are responsible for ensuring the water balance. Likely, the regulative functions of NUCB2 neurons in this area are affected by water balance, indirectly. Surprisingly, the NUCB2 neurons in mPVH, SON, ARH, lateral hypothalamus/zona interca (LHA/ZI) and NTS are not affected by hunger or melanocortins. For this reason, their function still remains unclear3,9,13.
The central level of nesfatin-1 can be increased by acute stress; on the other hand, the plasma level of nesfatin-1 is not influenced by acute stress22-24. Recently, it has been shown that chronic stress may increase the plasma level of nesfatin-124.
Nesfatin-1 and anxiety
Several studies show that nesfatin-1 exerts a regulatory effect on anxiety as well as on feeding behavior. The central application of nesfatin-1 has been shown to increase anxiety behaviors25. In another study, the differences between serum nesfatin-1 levels, because of the altered feeding behavior in depressed situations, was investigated. Serum nesfatin-1 levels have been found to be higher in patients with depression than in controls26. These studies show that nesfatin-1 may have an intermediary role in anxiety behaviors in addition to its effects on feeding behaviors.
Nesfatin-1 and endocrine system
Many substances affecting feeding behavior, neuroendocrine regulation, autonomous control, visceral functions, sleep, mood and pain, are also secreted in the areas associated with nesfatin-1 in the brain.Therefore, it is plausible that nesfatin-1 is related to other functions besides nutrition13.The abundance of nesfatin-1 in endocrine tissues indicates that it may have a role in hormone secretion. Its presence in the stomach indicates that it may play an unexplained role in food intake. Moreover, its presence in the pancreas indicates that nesfatin-1 may be involved in insulin and glucagon-mediated glucose metabolism. The presence of nesfatin-1 in the peripheral tissues strengthens the idea that it has also an integral regulative role in energy homeostasis and neuroendocrine-related functions as well as editing the anorexic signal in the hypothalamus9,13. Additionally, the presence of nesfatin-1 in plasma is regarded as evidence that it can act as a hormone27.
The expression of NUCB2 is increased in female rats at puberty and an increase in luteinizing hormone (LH) is observed parallel with this rise. Injection of nesfatin-1 in adult rats does not form a difference in LH level. These results reveal that it has a role in transition to puberty28.
It is thought that decreased or increased serum levels of nesfatin-1 can act as a disease marker, according to the possible role of nesfatin-1 in the endocrine system. For this purpose, serum levels of nesfatin-1 were measured in different patient populations. Two of these studies reached controversial results. One study concluded that lower nesfatin-1 concentration may play role in the development of polycystic ovary syndrome (PCOS)29, and the other one concluded higher nesfatin-1 level may play a role in PCOS30. This difference may be related to study design, patients or experimental condition30. We believe that these differences can be attributed to genetic variations and should be confirmed by genetic analysis. That is why determining polymorphic focus for the nesfatin-1 gene can provide a better understanding of nesfatin-1’s role in the development of various diseases.
Nesfatin-1 and diabetes
A research study has shown an anti-hyperglycemic effect of nesfatin-1. In hyperglycemic db/db mice (mimics of Type 2 diabetes model), the intravenous administration of nesfatin-1 decreased the blood glucose level significantly. The results obtained indicate that the anti-hyperglycemic effect of nesfatin-1 is peripheral and dependent on time, dose, and insulin. It is reported that this effect of nesfatin-1 is insulin-dependent because it did not reduce the blood glucose level in streptozotocin-mediated diabetes model. It is complementary to insulin for the reduction of the blood glucose level. However, the intracellular mechanism of nesfatin-1’s effect on reducing blood glucose level is still unknown. In this research, as a result of icv administration of nesfatin-1 to db/db mice, food intake was inhibited, but high blood glucose level was not affected, which is why this study proves the anti-hyperglycemic effect of nesfatin-1 to be independent of its anorexic effect. The anorexic and anti-hyperglycemic effects of nesfatin-1 have impacts respectively on nutrition and glucose metabolism. These results indicate that nesfatin-1 plays a significant role in the metabolic control mechanisms of the body. It is considered that in metabolic disorders, particularly in the treatment of type-2-diabetes and obesity, nesfatin-1 may be used as a potential therapeutic agent; thus, nesfatin-1 is the target of studies conducted in this field31. The result of this study may suggest that because of its effect on increasing insulin and oscillation, nesfatin-1 may be used as an anti-diabetic agent. One of the other studies suggested that nesfatin-1 plays a role type 2 diabetes mellitus (T2DM) via stimulating free acid utilization32. In another study, nesfatin-1 has been described as a novel stimulatory agent for glucagon secretion in beta cells. Additionally, they showed decreased expression level of nesfatin-1 in type-2-diabetes islets which may provide a compensatory mechanism to overcome hyperglucagonemia33. However, the precise physical and pathophysiological effects of nesfatin-1 on glucose and energy mechanism still remain unknown.
Nesfatin-1 and the immune system
A study has shown that in the subarachnoid hemorrhage model, nesfatin-1 is of an anti-apoptotic and anti-inflammatory nature in rats. In rats that developed a subarachnoid hemorrhage for experimental purposes, brain TNF- α, interleukin-1β (IL-1β) and interleukin-6 (IL-6) levels increased while antioxidant enzyme levels decreased. Compared to the group that received placebo, there was a greater level recovery in neurological disorders and oxidative brain damage in the group that was administered nesfatin-1. Furthermore, with respect to morphological changes of the basillar artery, there was a recovery in the group that was administered nesfatin-1. The increase in plasma pro-inflammatory cytokine because of subarachnoid hemorrhage was suppressed in the group that was administered nesfatin-1. These results indicate that nesfatin-1 has positive effects on the prevention of brain damage arising from oxidative mechanisms34.
Recently, a decreased serum level of nesfatin-1 was found to be associated with the obstructive sleep apnea syndrome (OSAS), concluded with its anti-inflammatory action35. On the other hand, a study that examined emphysema-type chronic obstructive pulmonary disease (COPD) patients, suggested that NUCB2/nesfatin-1 can be a novel factor associated with systemic inflammation in COPD, and introduced it as a novel inflammatory factor in stable emphysema type COPD36. We suggest that nesfatin-1 level plays a key role for inflammatory or anti-inflammatory function of nesfatin-1. Thus, more information is needed for a clear understanding of nesfatin-1 for inflammation processes.
Nesfatin-1 and the cardiovascular system
Brain nesfatin-1 signaling also plays a role in the regulation of cardiovascular response under stress conditions; for instance, the intra-cerebro-spinal injection of nesfatin-1 elevates arterial blood pressure37. In studies conducted with melanocortin and oxytocin receptor antagonists, the nesfatin-1 effects on nutrition and increased blood pressure were removed. In PVN, nesfatin-1, co-localized with oxytocin, stimulates the secretion of oxytocin by means of depolarization. It is also known that nesfatin-1 activates the melanocortin pathway by means of oxytocin. That is why it is thought that its hypertensive effect is associated with either central oxytocin or melanocortin pathways. However, there has yet been no detailed information on whether nesfatin-1 affects these two systems simultaneously or in a certain order, and on other components of this relationship9,12,37.
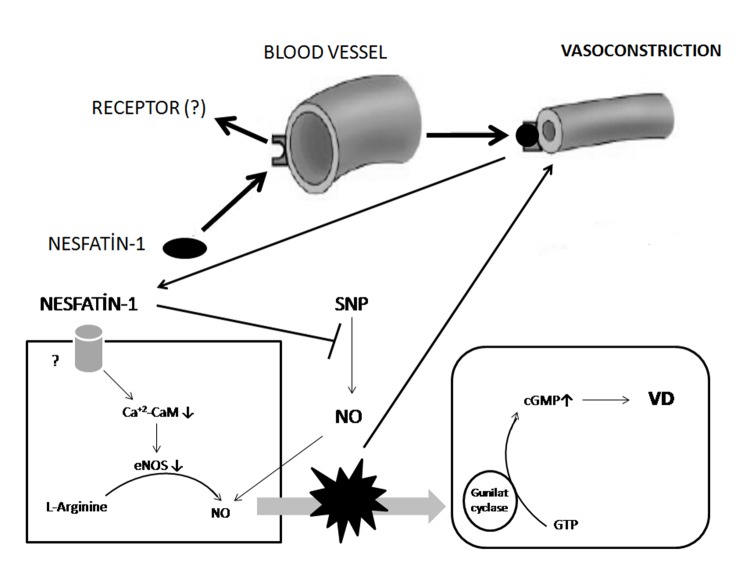
It has been shown that intravenous nesfatin-1 application induces vasoconstriction via inhibition of nitric oxide (NO) production and causes high blood pressure (BP)38. In one of our recent studies, we have shown that chronic peripheral nesfatin-1 decreases plasma level of endothelial nitric oxide synthase (eNOS) specifically in chronic restraint stressed rats39. According to these results, we can suppose that chronic peripheral nesfatin-1 effect on BP is related with (eNOS) and NO. It can elevate BP via vasoconstriction (Figure 2).
Figure 2. Schematic drawing demonstrating the peripheral effect of nesfatin-1 on vessels. SNP: sodium nitroprusside, NO: nitric oxide, CaM: calmodulin, eNOS: endothelial nitric oxide synthase, cGMP: cyclic guanosine monophosphate, GTP: guanozin trifosfat, VD: vasodilatation.
Nesfatin-1 and L-type Calcium (Ca+2) channels
A study conducted with a hypothalamic neural cell culture of rats has shown that the pharmacological characteristics of nesfatin-1 resemble the G protein-coupled receptors. In this study indicating that nesfatin-1 increases intracellular Ca+2concentration, the use of specific inhibitors in calcium channel subunits shows that the channels activated by nesfatin-1 are L, P and Q-type calcium channels. This study suggests that nesfatin-1 is expressed in hypothalamus and brainstem neurons and this peptide stimulates the cellular intake of calcium as a result of interaction with the G protein-coupled receptors in hypothalamic neural cell culture studies40.
Another study has revealed that nesfatin-1 increases insulin secretion by activating L-type Ca+2 channels in pancreatic islet beta cells (Figure 1). The intracellular increase in calcium occurs independently of protein kinase A and phospholipase A2. However, the relationship between this effect and voltage-dependent Ca+2channels is still unknown. Its effect of increasing intracellular Ca+2level is glucose-dependent. With the increase in postprandial plasma glucose level, nesfatin-1 elevates the level of intracellular Ca+2stimulated by glucose in pancreatic islet beta cells, as a result of which insulin oscillation increases41.
In another study, we have observed that chronic peripheral nesfatin-1 administration can increase the expression level of heart L-type Ca+2 channels α1c subunit protein, especially under chronic stress conditions (Data not shown)42. We suppose this effect of nesfatin-1 on heart can lead to serious damage of cardiomyocytes.
Major clinical applications of nesfatin-1
Positive clinical applications
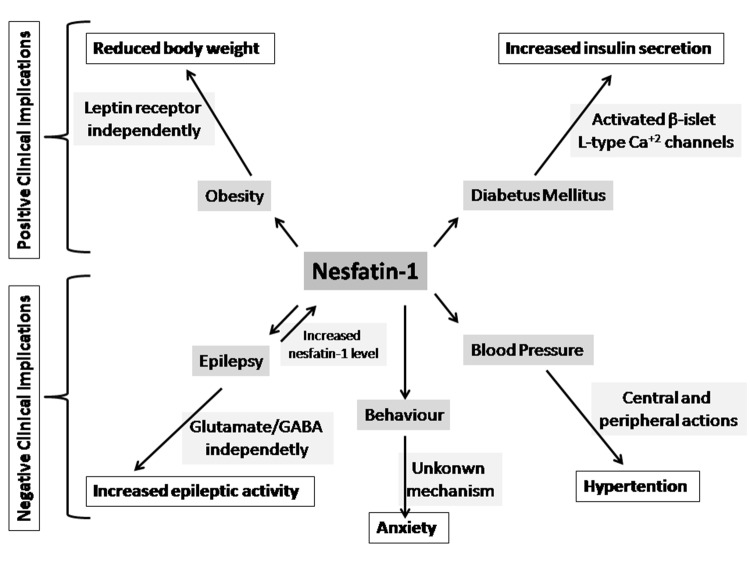
It is known that most obese people are marked with leptin-resistance43. It has been shown that intraperitoneal nesfatin-1 administration can significantly inhibit food intake in leptin resistant animal models. Additionally, a significant decrease in body weight by chronic peripheral nesfatin-1 administration has been shown in non-obese mice too44. Because leptin-independent signaling system that contributes to feeding suppression is induced by central and peripheral nesfatin-1 administration1,45(Figure 3). It seems that nesfatin-1 and its analogues are possible candidates of anti-obesity drugs in leptin-resistant obese people. So that nesfatin-1 may be a potential target for the treatment of obesity-related disorders. Also, subcutaneous and intranasal routes are the recommended way of use for the administration of nesfatin-1 based on the evidence of experimental mice and rat models46. However, this preliminary pre-clinical data needs further confirmation.
Figure 3. Major clinical applications of nesfatin-1. GABA: gamma aminobutyric acid.
The increased insulin secretion by activation of L-type Ca+2 channels in pancreatic islet beta cells via nesfatin-1 administration brings a hope to use nesfatin-1 for the treatment of diabetes mellitus47 (Figure 3).
It has been reported that NUCB2/nesfatin-1 has a central role in driving the puberty onset. In a study by Garca-Galiano et al, the increase in the hypothalamic NUCB2 expression has been found along the pubertal transition, with detectable elevations of its mRNA levels at LHA, PVN, SON and, threefold increase has been observed in the total protein content of NUCB2 between late-infantile and peripubertal periods of female rats28. These results support that NUCB2/nesfatin-1 is involved in the control of female puberty.
In a study by Aydın et al, both saliva and serum levels of nesfatin-1 were found 160-fold higher in patients who had been recently diagnosed with epilepsy than in controls and, after anti-epileptic treatment, a 10-fold increase has been observed in saliva and serum levels of nesfatin-1 in epileptic patients (Figure 3). Therefore, it was suggested that elevated nesfatin-1 levels might contribute to the pathophyisology of epilepsy and remarkably increased nesfatin-1 may be a candidate biomarker both for the diagnosis of epilepsy and for monitoring the response to anti-epileptic treatment47. Additionally, a recent study reported that increased serum nesfatin-1 concentration could be used as a marker to identify patients who have suffered from a recent epileptic seizure48(Figure 3). Consequently, nesfatin-1 seems promising as a biomarker in epilepsy. Besides this, it has been recently shown that nesfatin-1 causes epileptic activity and increases penicillin induced epileptic activity via an unknown mechanism independently glutamate and/or gamma aminobutyric acid (GABA)49(Figure 3).
Negative clinical applications
Besides its positive clinical effects, studies have reported some negative clinical implications of nesfatin-1. For instance, a novel action of nesfatin-1 to stimulate autonomic nervous system activity has been identified37. Tanida et al have shown that icv injection of nesfatin-1 stimulated renal sympathetic nerve activity and significantly increased blood pressure through the central melanocortin system21(Figure 3). In a study by Yosten et al, significant increases in mean arterial pressure were observed following icv administration of nesfatin-1 and it has been suggested that nesfatin-1 may interact with the central melanocortin system to stimulate sympathetic nerve activity and increase mean arterial pressure37. As we see from the literature, intracerebral administration of nesfatin-1 induces hypertension, thus indicating its role in central cardiovascular control50(Figure 3). Today, we know that hypertension is one of the major risk factors for cardiovascular diseases51. In this respect, the blood pressure increasing effect of nesfatin-1 is a negative factor, especially for patients with cardiovascular diseases.
In addition to its role as a satiety peptide, nesfatin-1 may also be involved in the mediation of anxiety-related responses. In a recent study, an increase in anxiety behavior has been shown, following the central application of nesfatin-1. However, the relation between nesfatin-1 and stress-related behaviors like anxiety has not yet been fully understood25 (Figure 3).
Recently nesfatin-1 was provided as a regulatory factor for thyroid function in T2DM patients52and in aging53because of its crucial role in energy balance and glucose metabolism.
Limitations of current knowledge and what needs to be further studied
Although the anorexigenic effect of the hypothalamic peptide nesfatin-1 has been identified, the recently established crosstalk between nesfatin-1, corticotropin-releasing factor, oxytocin and melanocortin pathways involved in hypothalamic nesfatin-1’s anorexigenic action needs to be further clarified. Additionally, the participation of nesfatin-1 in the regulation of biological functions and its interaction with other neuropeptides remain to be investigated3.
Despite, the increasing number of studies focusing on the biological actions of nesfatin-1 and expression of endogenous NUCB2/nesfatin-1 in tissues, the knowledge about the receptors mediating those effects is limited. Therefore, it is necessary to clarify key components for understanding of NUCB2/nesfatin-1 signaling system and action in the brain as well as the periphery, along with their regulation3.
It is known that circulating nesfatin-1 may cross the blood-brain barrier and be involved in the central regulation of feeding behavior in a leptin-independent manner. However, the origin of circulating nesfatin-1 in the general circulation is still unknown5,6. In a study by Stengel et al, it has been shown that a single icv injection of nesfatin-1, although not modulating the 24h cumulative food intake, reduced body weight in rats. Although this evidence indicates a central action of nesfatin-1 to increase energy expenditure, the mechanism of its action also needs further examination54.
In a recent study, it is reported that intracerebral administration of nesfatin-1 induces hypertension, which suggests a role of nesfatin-1 in central cardiovascular control. However, it is not known whether it is able to directly control heart performance and this subject needs still to be further clarified50.
Conclusion
If we consider that nesfatin-1 has been gaining attention as an effective therapeutic agent for various diseases, we suppose that it is important to know more about nesfatin-1 from different viewpoints. For this purpose, in this review, we tried to summarize the literature about nesfatin-1 research and to point out the gaps within this field in hope of conducting further experiments with nesfatin-1.
Conflict of interest
The authors report no conflict of interest.
Acknowledgement
Ceylan Ayada and mran Toru are equal contributors in the present manuscript. We would like to present our special thanks to Prof. Dr. Gnfer Turgut, Prof. Dr. Osman Gen, Prof. Dr. Sebahat Turgut for their scientific supervision and to Tarny Renee Nash, Jack Eastham for their linguistic review.
References
- 1.Oh-I S, Shimizu H. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 2.Cowley MA, Grove KL. To be or NUCB2, is nesfatin the answer? Cell Metab. 2006;4:421–422. doi: 10.1016/j.cmet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Stengel A, Tach Y. Nesfatin-1 role as possible new potent regulator of food intake. Regul Pept. 2010;163:18–23. doi: 10.1016/j.regpep.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 5.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–2228. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–2381. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643–648. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 8.Stengel A. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–238. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garca-Galiano D, Navarro VM, Gaytan F, Tena-Sempere M. Expanding roles of NUCB2/nesfatin-1 in neuroendocrine regulation. J Mol Endocrinol. 2010;45:281–290. doi: 10.1677/JME-10-0059. [DOI] [PubMed] [Google Scholar]
- 10.Williams P, Tulke S, Ilegems E, Berggren PO, Broberger C. Expression of nucleobindin 1 (NUCB1) in pancreatic islets and other endocrine tissues. Cell Tissue Res. 2014;358:331–342. doi: 10.1007/s00441-014-1948-z. [DOI] [PubMed] [Google Scholar]
- 11.Palasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, Wiaderkiewicz R, et al. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides. 2012;46:105–112. doi: 10.1016/j.npep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Ramanjaneya M, Chen J. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–3180. doi: 10.1210/en.2009-1358. [DOI] [PubMed] [Google Scholar]
- 13.Yamada M, Horiguchi K, Umezawa R, Hashimoto K, Satoh T, Ozawa A, et al. Troglitazone, a Ligand of Peroxisome Proliferator-Activated Receptor, Stabilizes NUCB2 (Nesfatin) mRNA by Activating the ERK1/2 Pathway: Isolation and Characterization of the Human NUCB2 Gene. Endocrinology. 2010;151:2494–2503. doi: 10.1210/en.2009-1169. [DOI] [PubMed] [Google Scholar]
- 14.Aydin S. Role of NUCB2/nesfatin-1 as a possible biomarker. Curr Pharm Des. 2013;19:6986–6992. doi: 10.2174/138161281939131127143422. [DOI] [PubMed] [Google Scholar]
- 15.Goebel-Stengel M, Wang L. Central and peripheral expression and distribution of NUCB2/nesfatin-1. Curr Pharm Des. 2013;19:6935–6940. doi: 10.2174/138161281939131127124814. [DOI] [PubMed] [Google Scholar]
- 16.Stengel A, Tach Y. Role of brain NUCB2/nesfatin-1 in the regulation of food intake. Curr Pharm Des. 2013;19:6955–6959. doi: 10.2174/138161281939131127125735. [DOI] [PubMed] [Google Scholar]
- 17.Sedbazar U, Ayush EA, Maejima Y, Yada T. Neuropeptide Y and α-melanocyte-stimulating hormone reciprocally regulate nesfatin-1 neurons in the paraventricular nucleus of the hypothalamus. Neuroreport. 2014;25:1453–1458. doi: 10.1097/WNR.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura M, Matsuura T, Ohkubo J, Maruyama T, Ishikura T, Hashimoto H, et al. A role of nesfatin-1/NucB2 in dehydration-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2014;307:R225–R236. doi: 10.1152/ajpregu.00488.2013. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Guo F, Sun X, Gao S, Li Z, Gong Y, et al. Effects of exogenous nesfatin-1 on gastric distention-sensitive neurons in the central nucleus of the amygdala and gastric motility in rats. Neurosci Lett. 2014;582:65–70. doi: 10.1016/j.neulet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Guo FF, Xu L, Gao SL, Sun XR, Li ZL, Gong YL. The effects of nesfatin-1 in the paraventricular nucleus on gastric motility and its potential regulation by the lateral hypothalamic area in rats. J Neurochem. 2015;132:266–275. doi: 10.1111/jnc.12973. [DOI] [PubMed] [Google Scholar]
- 21.Tanida M, Mori M. Nesfatin-1 stimulates renal sympathetic nerve activity in rats. Neuroreport. 2011;22:309–312. doi: 10.1097/WNR.0b013e328346107f. [DOI] [PubMed] [Google Scholar]
- 22.Goebel M, Stengel A, Wang L, Tach Y. Restraint stres activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Ma Q, Duan HF, Qian LJ. Effects of stress on L type calcium channels of rat ventricular myocytes. Chin J Applied Physiol. 2003;19:216–219. [PubMed] [Google Scholar]
- 24.Yoshida N, Maejima Y. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2010;2:775–784. doi: 10.18632/aging.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merali Z, Cayer C. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology (Berl) 2008;201:115–123. doi: 10.1007/s00213-008-1252-2. [DOI] [PubMed] [Google Scholar]
- 26.Ari M, Ozturk OH. High plasma nesfatin-1 level in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:497–500. doi: 10.1016/j.pnpbp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet MS, Pecchi E. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garca-Galiano D, Navarro VM, Roa J, Ruiz-Pino F, Snchez-Garrido MA, Pineda R, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30:7783–7792. doi: 10.1523/JNEUROSCI.5828-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deniz R, Gurates B, Aydin S, Celik H, Sahin I, Baykus Y, et al. Nesfatin-1 and other hormone alterations in polycystic ovary syndrome. Endocrine. 2012;42:694–699. doi: 10.1007/s12020-012-9638-7. [DOI] [PubMed] [Google Scholar]
- 30.Ademoglu EN, Gorar S, Carlıoglu A, Yazıcı H, Dellal FD, Berberoglu Z, et al. Plasma nesfatin-1 levels are increased in patients with polycystic ovary syndrome. J Endocrinol Invest. 2014;37:715–719. doi: 10.1007/s40618-014-0089-2. [DOI] [PubMed] [Google Scholar]
- 31.Su Y, Zhang J. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Xu H, Xu H, Wang PF, Cai GJ, Song HF, et al. Nesfatin-1 stimulates fatty-acid oxidation by activating AMP-activated protein kinase in STZ-induced type 2 diabetic mice. PLoS One. 2013;8:e83397. doi: 10.1371/journal.pone.0083397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riva M, Nitert MD, Voss U, Sathanoori R, Lindqvist A, Ling C, et al. Nesfatin-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res. 2011;346:393–405. doi: 10.1007/s00441-011-1268-5. [DOI] [PubMed] [Google Scholar]
- 34.Ozsavci D, Erşahin M. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery. 2011;68:1699–1708. doi: 10.1227/NEU.0b013e318210f258. [DOI] [PubMed] [Google Scholar]
- 35.Shen P, Han Y, Cai B, Wang Y. Decreased levels of serum nesfatin-1 in patients with obstructive sleep apnea syndrome. Sleep Breath. 2015;19:515–522. doi: 10.1007/s11325-014-1039-0. [DOI] [PubMed] [Google Scholar]
- 36.Leivo-Korpela S, Lehtimki L, Hmlainen M, Vuolteenaho K, Kbi L, Jrvenp R, Kankaanranta H, Saarelainen S, Moilanen E. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediators Inflamm. 2014;2014:232167. doi: 10.1155/2014/232167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:330–336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamawaki H, Takahashi M, Mukohda M, Morita T, Okada M, Hara Y. A novel adipocytokine, nesfatin-1 modulates peripheral arterial contractility and blood pressure in rats. BiochemBiophys Res Commun. 2012;418:676–681. doi: 10.1016/j.bbrc.2012.01.076. [DOI] [PubMed] [Google Scholar]
- 39.Ayada C, Turgut G, Turgut S, Gl Z. The effect of chronic peripheral nesfatin-1 application on blood pressure in normal and chronic restraint stressed rats: related with circulating level of blood pressure regulators. Gen Physiol Biophys. 2015;34:81–88. doi: 10.4149/gpb_2014032. [DOI] [PubMed] [Google Scholar]
- 40.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, et al. Nesfatin-1: Distribution and Interaction with a G Protein-Coupled Receptor in the Rat Brain. Endocrinology. 2007;148:5088–5094. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 41.Nakata M, Manaka K. Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca (2+) influx through L-type channels in mouse islet β-cells. Endocr J. 2011;58:305–313. doi: 10.1507/endocrj.k11e-056. [DOI] [PubMed] [Google Scholar]
- 42.Ayada C, Turgut G, Turgut S. The effect of nesfatin -1 on heart L-type Ca2+ channel a1c subunit in rats subjected to chronic restrained stress. Bratisl Lek Listy. 2015;116:236–329. doi: 10.4149/bll_2015_061. [DOI] [PubMed] [Google Scholar]
- 43.Myers MG, Cowley MA, Mnzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu H, Oh-I S. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu H, Inoue K, Mori M. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu H, Oh-I S, Okada S, Mori M. Nesfatin-1: an overview and future clinical application. Endocr J. 2009;56:537–543. doi: 10.1507/endocrj.k09e-117. [DOI] [PubMed] [Google Scholar]
- 47.Aydin S, Dag E, Ozkan Y, Erman F, Dagli AF, Kilic N, Sahin I, Karatas F, Yoldas T, Barim AO, Kendir Y. Nesfatin-1 and ghrelin levels in serum and saliva of epileptic patients: hormonal changes can have a major effect on seizure disorders. Mol Cell Biochem. 2009;328:49–56. doi: 10.1007/s11010-009-0073-x. [DOI] [PubMed] [Google Scholar]
- 48.Aydin S, Dag E, Ozkan Y, Arslan O, Koc G, Bek S, Kirbas S, Kasikci T, Abasli D, Gokcil Z, Odabasi Z, Catak Z. Time-dependent changes in the serum levels of prolactin, nesfatin-1 and ghrelin as a marker of epileptic attacks young male patients. Peptides. 2011;32:1276–80. doi: 10.1016/j.peptides.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Erken HA, Ko ER, Gen O, Erken G, elik HT, Gke EC, et al. Proconvulsant effect of NUCB2/nesfatin-1. Int J of Pept Res and Ther. 2015;21:21–38. [Google Scholar]
- 50.Angelone T, Filice E, Pasqua T, Amodio N, Galluccio M, Montesanti G, et al. Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury. Cell Mol Life Sci. 2013;70:495–509. doi: 10.1007/s00018-012-1138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaněčkov I, Maletnsk L, Behuliak M, Nagelov V, Zicha J, Kuneš J. Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol. 2014;223:R63–R78. doi: 10.1530/JOE-14-0368. [DOI] [PubMed] [Google Scholar]
- 52.Liu F, Yang Q, Gao N, Liu F, Chen S. Decreased plasma nesfatin-1 level is related to the thyroid dysfunction in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:128014. doi: 10.1155/2014/128014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li JB, Nishida M, Kaimoto K, Asakawa A, Chaolu H, Cheng KC, et al. Effects of aging on the plasma levels of nesfatin-1 and adiponectin. Biomed Rep. 2014;2:152–156. doi: 10.3892/br.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mnnikes H, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]