Abstract
Introduction:
Anaplastic thyroid carcinoma (ATC) has the most aggressive progression among thyroid malignancies. Most of the patients have metastasis, especially to the lungs, liver and regional lymph nodes, at the time of diagnosis. Gastrointestinal tract metastasis of ATC has been rarely reported. We report a case who presented with gastrointestinal bleeding and was diagnosed with ATC accompanied with gastric, skin, lung and adrenal gland metastases.
Case report:
A 72-year-old male patient presented with one month history of neck mass, weight loss and weakness and three-day-history of melena. On examination his thyroid gland was tender on palpation and hyperplasic, multiple, painful, solid, and fixed nodules were palpated.Ultrasonographic neck examination demonstrated an enlarged thyroid gland and multiple hypoechoic nodules including cystic degenerative areas; the largest 28 x 23 mm in size. Thyroid fine needle aspiration biopsy was performed and biopsy results indicated ATC. Gastroscopy, performed due to the gastrointestinal bleeding, detected a 4 x 6 mm polypoid lesion on sternal pili of the gastric cardia and histopathological examination of its biopsy demonstrated metastasis of ATC.
Conclusion:
We reported a case of ATC with gastric, skin, lung and adrenal gland metastases, initially presenting with gastrointestinal bleeding due to the gastric metastasis. Hippokratia 2015, 19 (1): 85-87.
Keywords: Thyroid, anaplastic carcinoma, gastric metastasis, skin metastasis
Introduction
Thyroid carcinomas account for approximately 3% of all malignancies, whereas anaplastic thyroid carcinoma (ATC) accounts for 1.3-9.8% of total thyroid carcinomas1,2. ATC, which has the most aggressive progression among thyroid malignancies, is likely to give metastasis to the lungs, liver and regional lymph nodes. Gastrointestinal metastasis of ATC has been rarely reported in the literature3. We report a case who presented withgastrointestinal bleedingand was diagnosed with ATC accompanied with gastric, skin, lung and adrenal gland metastases.
Case report
A 72-year-old male patient, who was a retired worker, presented to the emergency department complaining of melena for the preceding three days. His anamnesis revealed a neck mass, weight loss and weakness within the previous one month. He reported that the mass expanded rapidly over the last 15 days and he had lost approximately 5 kg within that period. His medical history also included irregular drug use for Diabetes Mellitus for 10 years.
On physical examination, his general status was moderate-to-poor, he was conscious and cooperated. His blood pressure was 110/70 mmHg, pulse rate was 88/min and rhythmic. On head and neck examination, conjunctivas were pale and thyroid gland was tender on palpation; hyperplastic, multiple, painful, solid, and fixed nodules were palpated. A solid, fixed skin nodule measuring 1.5 cm was palpated in the right inguinal region. Rectal examination demonstrated melena.
In laboratory analyses, hemoglobin was 6.91 g/dL, hematocrit 21%, white blood cells 15,400/mm3, erythrocyte sedimentation rate 121 mm/h, fasting plasma glucose 130 mg/dL, urea 88 mg/dL, and creatinine 1.72 mg/dL. Other biochemical parameters were within the normal ranges. Thyroid function tests and anti-thyroid antibodies were also within the normal ranges (fT3: 2.1 pg/mL, fT4: 1 ng/dL, TSH: 0.42 uIU/mL, antiTG: 0.77I U/mL, anti-TPO: 0.16I U/mL).
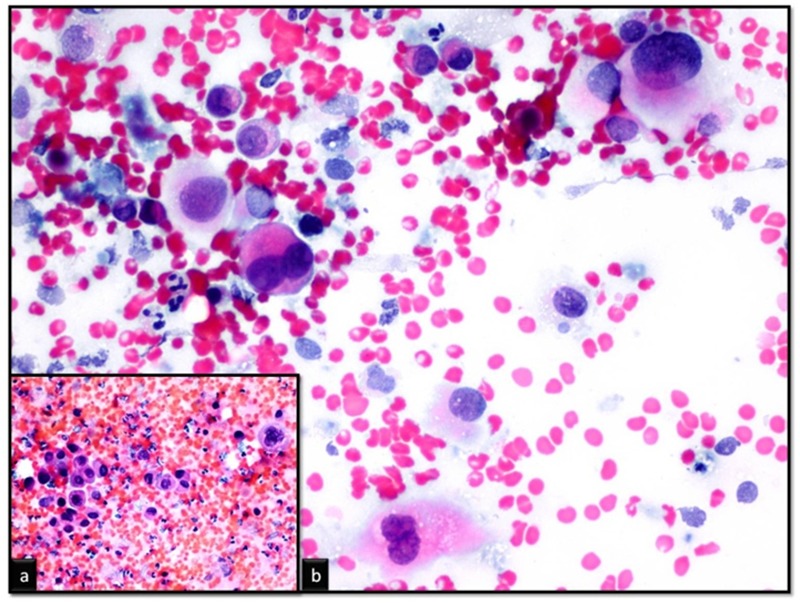
Ultrasonographic (US) examination of the neck demonstrated an enlarged thyroid gland; the right lobe was 55 x 49 x 64 mm and the left 33 x 30 x 56 mm.The left thyroid lobe had multiple hypoechoic nodules including cystic degenerative areas; the largest one was 28 x 23 mm in dimensions. A lesion was observed, almost completely filling the right thyroid lobe and isthmus, causing lobulation in thyroid contour, including irregular and heterogeneous configurations, and containing microcalcifications and secondary nodules. On the right cervical chain several oval-shaped lymph nodeswere detected, of which the largest section was 26 x 19 mm and echogenic hilum was not visible on the superior, which also showed irregular echo. Thyroid fine needle aspiration biopsy was performed to the nodules and biopsy results indicated ATC (Figure 1).
Figure 1. Thyroid fine needle aspiration biopsy showing giant, with hyperchromatic and eccentric located nuclei, large cytoplasmic, pleomorphic and dishesive cells (Pap stain, a: x200 b: x400).
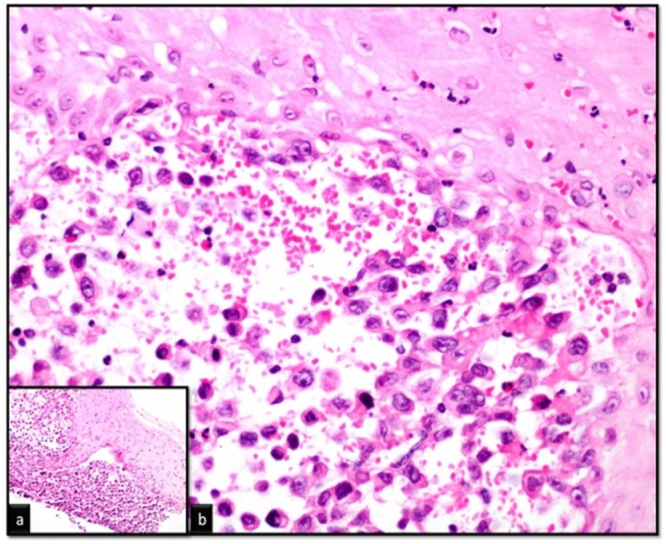
Gastroscopy, performed due to the gastrointestinal bleeding, showed normal gastric fundus, corpus, antrum and pylori. However, a 4 x 6 mm polypoid lesion was detected on sternal pili of the gastric cardia and biopsy was taken from that lesion (Figure 2). Histopathological examination demonstrated metastasis of ATC (Figure 3). Also excisional biopsy of the nodular skin lesion from the right inguinal region was performed and histopathology showed that the lesion was consistent with metastasis of ATC.
Figure 2. Endoscopic (gastroscopy) view of a 4 x 6 mm polypoid lesion detected on sternal pili of the gastric cardia repre- senting metastatic nodule.
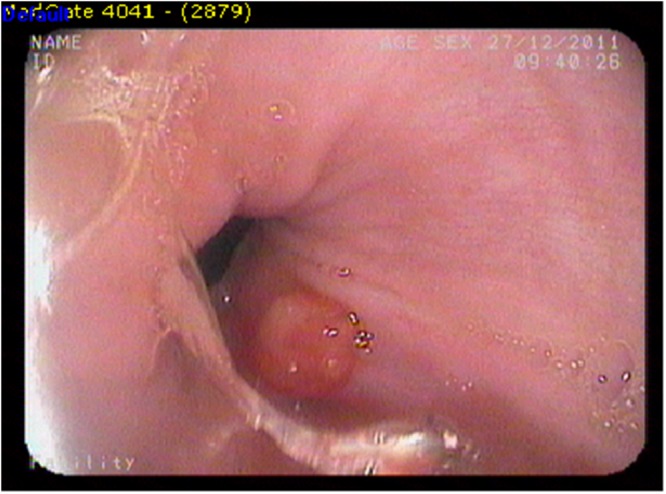
Figure 3. Histopathological examination of the polypoid lesion showing under the squamous epithelium of epithelial cells infiltrating groups of pleomorphic appearance (Hematoxylin & Eosin stain, a: x200, b: x400).
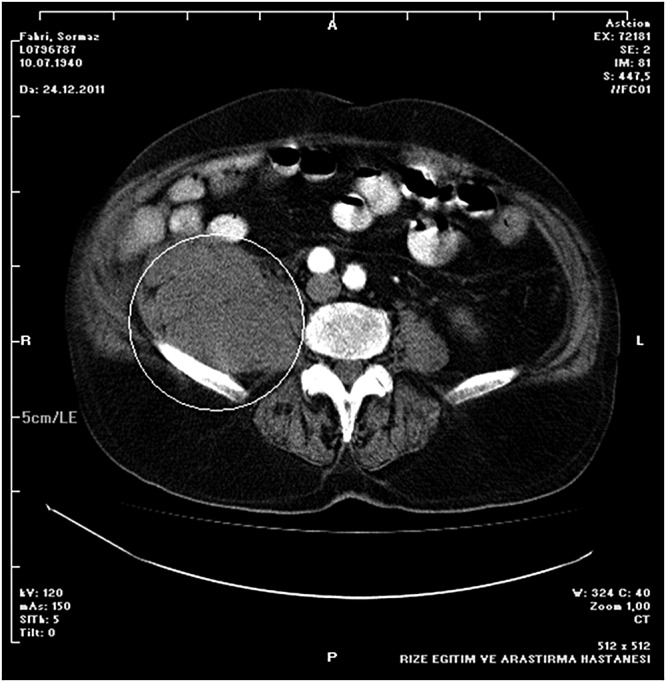
Abdominal US and computed tomography (CT) demonstrated approximately 10 x 7 cm hypoechoic and lobular mass lesions in both adrenal glands, the largest being in the left; based on the radiological appearance, they were considered metastatic (Figure 4).
Figure 4. Abdominal computed tomography axial image demonstrated a 10 x 7 cm lobular mass lesions in the right adrenal gland (area in the circle).
On thoracic CT, nodular lesions were detected on the 2nd, 4th, 6th, 8th, and 9th segments of the right lung and on the 3rd, 4th, and 6th segments of the left lung, the largest being 1 x 1.5 cm in size. Radiological appearance was interpreted in favor of metastasis.
Cranial magnetic resonance imaging showed no remarkable pathological finding except for ischemic changes. Based on clinical, laboratory and radiological findings, the patient was diagnosed with ATC accompanied with lung, gastric, adrenal and skin metastases. The patient, whose general status and performance score were poor, received supportive treatment. His general status worsened rapidly and he died due to respiratory and cardiac arrest.
Discussion
Anaplastic thyroid carcinoma is an undifferentiated carcinoma of the thyroid gland and is primarily a disease of the elderly. In an American and German prospective study which included 5,583 cases of thyroid carcinoma, 67% of patients with ATC were over 70 years of age. In that study, females constituted 70% and males 30% of ATC patients4. In many series, patients were elder women with female to male ratio was 3:15.
There are studies emphasizing that this type of tumor is more prevalent in regions where endemic goiter is common2and thus with improvement in iodine supplementation, the incidence of this tumor would be expected to decline. In studies, 25% of the ATC patients had a prior history of goiter and 10% had family history of goiter4,6. The reported case, as well, was living in a region where goiter is endemic and he also had a history of goiter.
ATC commonly presents with local symptoms. Metastases are found in 50% of patients at the time of diagnosis, with 25% developing metastasis during the course of disease. It has been accepted that early distant metastasis of the tumor occurs via hematogenous spread. Lungs (80%), bone (6-16%), and brain (5-13%) are the most common sites of metastasis4.
Hermann et al7investigated 892 cases with thyroid carcinoma and found distant metastasis in 151 cases. For ATC distant metastasis were most frequently found (86%) in lungs (4 solitary mass, others with diffuse involvement) and only 17.3% in bones; whereas, for metastatic well-differentiated carcinoma, the rate of lung and bone metastases were 55.4% (diffuse spread) and 64.8%, respectively. Vinkatesh et al8 investigated 121 ATC cases and found metastasis in 53% of the cases, of which 88% were lung and 15% were bone metastases. In the study by Besic and Gazic3, autopsy was performed in 45 of 205 patients with ATC and it was found that 41 of them had metastasis. The most common sites of metastases found were the lungs (78%), intrathoracic lymph nodes (58%), neck lymph nodes (51%), pleura (29%), adrenal glands (24%), liver (20%), brain (18%), heart (18%), and retroperitoneal lymph nodes (18%). Less common sites of distant metastases were reported to be the pericardium (13%), bones (13%), kidneys (13%), mesentery or peritoneum (13%), skin (9%), pancreas (4%), stomach (4%), diaphragm (4%), pituitary gland (2%), ovary (2%), jejunum (2%), axillary lymph nodes (2%), and gingival mucosa (2%)3.
The number of studies reporting skin metastasis of ATC is limited in the literature7,9,10.Thyroid cancer metastases to skin are rare. Dahl et al reported in 1997 six patients and reviewed the literature to find totally 43 patients with skin metastases from thyroid cancer (all types) and ATC accounted for 15%10. Given its rarity compared to the other types, it would appear that ATC has a greater tendency to metastasize to cutaneous sites than the other types of thyroid cancer. The scalp was reported as the most common site of cutaneous metastases10. Tronnier et al9reported skin metastasis in two cases with follicular carcinoma of thyroid while Çelik et al11 reported an ATC case with skin metastasis. Excisional biopsy of the skin nodular lesion from the right inguinal area of the present case was found to be consistent with metastasis of ATC.
As the survival period of the reported patient was very short after diagnosis, radiological demonstration of lung, liver and adrenal gland metastases could not be verified histopathologically. However, when these radiological images were correlated with the clinical picture, they were considered to be metastasis. Moreover, because of his short survival period, bone scintigraphy could not be obtained; thus, bone metastasis of the patient who had severe bone pain could not be evaluated.
ATC still remains a fatal solid tumor and has the most aggressive progression among thyroid malignancies. Prognosis has been reported to be better in young cases and in those without distant metastasis2. Herein we reported a case of ATC with gastric, skin, lung and adrenal gland metastases, initially presenting with gastrointestinal bleeding due to the gastric metastasis.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R CollRadiol) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 3.Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013;23:709–713. doi: 10.1089/thy.2012.0252. [DOI] [PubMed] [Google Scholar]
- 4.Hundahl SA1, Cady B, Cunningham MP, Mazzaferri E, McKee RF, Rosai J, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer. 2000;89:202–217. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: A 50-yearexperience at single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 6.Bakiri F1, Djemli FK, Mokrane LA, Djidel FK. The relative roles of endemic goiter and socioeconomic development status in the prognosis of thyroid carcinoma. Cancer. 1998;82:1146–1153. doi: 10.1002/(sici)1097-0142(19980315)82:6<1146::aid-cncr20>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Hermann M, Kober F, Keminger K. [Distant metastases of malignant thyroid cancer. A retrospective study of 892 cases] Oncologie. 1987;10:350–355. doi: 10.1159/000216442. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990;66:321–330. doi: 10.1002/1097-0142(19900715)66:2<321::aid-cncr2820660221>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Tronnier M, Winzer M, Wolff HH. Cutaneous metastases from follicular thyroid carcinoma: histology, immunohistology, and electron microscopy. A report of two cases. Dermatologica. 1991;183:286–289. doi: 10.1159/000247702. [DOI] [PubMed] [Google Scholar]
- 10.Dahl PR, Brodland DG, Goellner JR, Hay ID. Thyroidcarcinoma metastatic to the skin: a cutaneous manifestationof a widely disseminated malignancy. J Am Acad Dermatol. 1997;36:531–537. doi: 10.1016/s0190-9622(97)70239-1. [DOI] [PubMed] [Google Scholar]
- 11.Çelik GE, Akkoca Ö, Karabiyikoglu G, et al. Metastases of a Thyroid Anaplastic Carcinoma. Ankara Üniversitesi Tip Fakültesi Mecmuasi. 1996;49:113–116. [Google Scholar]


