Abstract
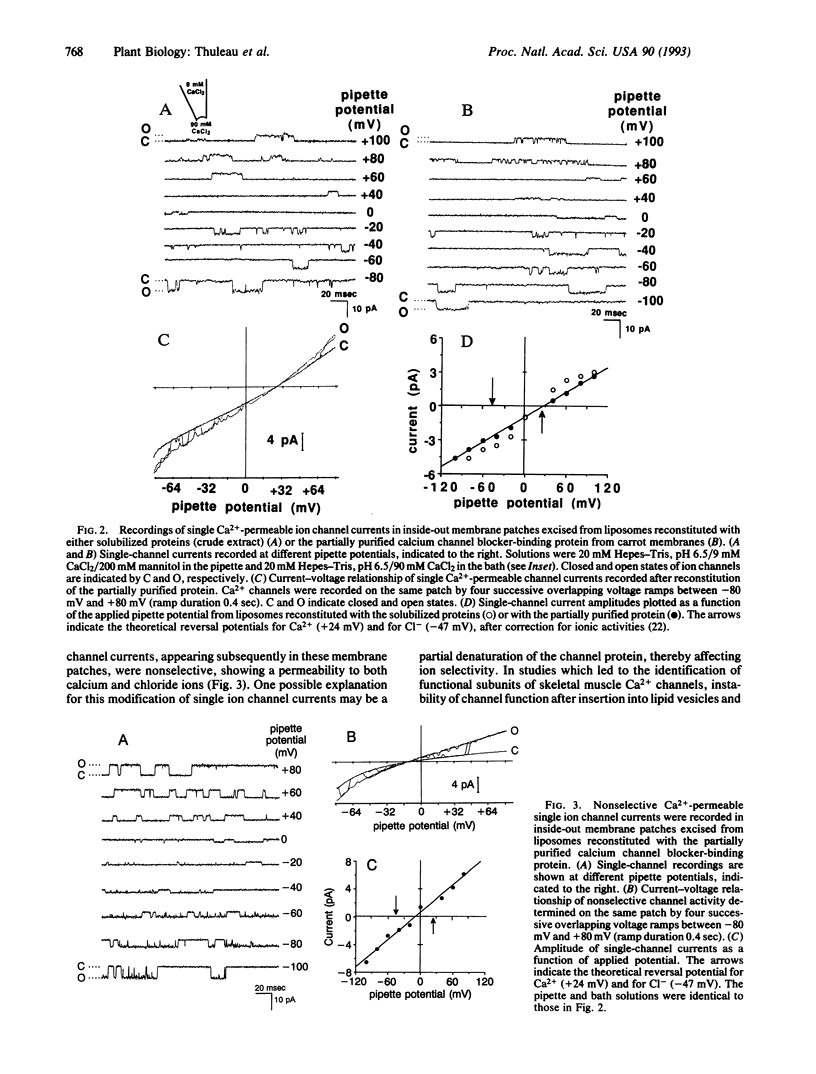
Calcium channels have been suggested to play a major role in the initiation of a large number of signal transduction processes in higher plant cells. However, molecular components of higher plant Ca2+ channels remain unidentified to date. Calcium channel blockers of the phenylalkylamine family and bepridil specifically inhibit Ca2+ influx into carrot (Daucus carota L.) cells. By using a phenylalkylamine azido derivative, a 75-kDa carrot membrane protein has been previously identified. Here we have partially purified this Ca2+ channel blocker-binding protein by lectin-affinity and ion-exchange chromatographies. The protein fraction containing the 75-kDa binding protein was incorporated into giant liposomes. Single-channel patch-clamp studies on these proteoliposomes showed the presence of Ca2+-permeable channel currents. These Ca2+-permeable channels were not stable. Recordings after durations of 2-10 min showed the appearance of nonselective ion channels with a permeability to calcium and chloride ions. These nonselective Ca2+-permeable ion channels, in contrast, were stable and were recorded for extended durations. The addition of the Ca2+ channel-blocker bepridil (10 M) led to the inhibition of these nonselective Ca2+-permeable channels by reducing the probability of channel opening. These results suggest that the 75-kDa Ca2+ channel blocker-binding protein from carrot cells plays a role in channel sensitivity to Ca2+ channel inhibitors and may constitute one of the components of Ca2+ channels in higher plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrejauskas E., Hertel R., Marmé D. Specific binding of the calcium antagonist [3H]verapamil to membrane fractions from plants. J Biol Chem. 1985 May 10;260(9):5411–5414. [PubMed] [Google Scholar]
- Bush D. S., Jones R. L. Measuring intracellular ca levels in plant cells using the fluorescent probes, indo-1 and fura-2 : progress and prospects. Plant Physiol. 1990 Jul;93(3):841–845. doi: 10.1104/pp.93.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Keller B. U. A membrane fusion strategy for single-channel recordings of membranes usually non-accessible to patch-clamp pipette electrodes. FEBS Lett. 1987 Nov 16;224(1):172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Reconstitution of the voltage-sensitive calcium channel purified from skeletal muscle transverse tubules. Biochemistry. 1986 Jun 3;25(11):3077–3083. doi: 10.1021/bi00359a002. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V., Oeken H. J., Hofmann F., Pelzer D., Cavalié A., Trautwein W. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986 Sep 4;323(6083):66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Fricker M. D., Read N. D., Trewavas A. J. Role of Calcium in Signal Transduction of Commelina Guard Cells. Plant Cell. 1991 Apr;3(4):333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey H. J., Venis M. A., Trewavas A. J. Partial purification of a protein from maize (Zea mays) coleoptile membranes binding the Ca2+-channel antagonist verapamil. Biochem J. 1989 Jan 1;257(1):95–100. doi: 10.1042/bj2570095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Horne W. A., Abdel-Ghany M., Racker E., Weiland G. A., Oswald R. E., Cerione R. A. Functional reconstitution of skeletal muscle Ca2+ channels: separation of regulatory and channel components. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3718–3722. doi: 10.1073/pnas.85.11.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., French R. J., Krueger B. K. Voltage-dependent calcium channels from brain incorporated into planar lipid bilayers. Nature. 1984 Mar 1;308(5954):77–80. doi: 10.1038/308077a0. [DOI] [PubMed] [Google Scholar]
- Riquelme G., Lopez E., Garcia-Segura L. M., Ferragut J. A., Gonzalez-Ros J. M. Giant liposomes: a model system in which to obtain patch-clamp recordings of ionic channels. Biochemistry. 1990 Dec 25;29(51):11215–11222. doi: 10.1021/bi00503a009. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Reeves J. P., Smilowitz H., Tsien R. W. Calcium channels in planar lipid bilayers: insights into mechanisms of ion permeation and gating. Science. 1986 Mar 28;231(4745):1564–1566. doi: 10.1126/science.2420007. [DOI] [PubMed] [Google Scholar]
- Sanders C. L., Dagle G. E., Mahaffey J. A. Incidence of brain tumours in rats exposed to an aerosol of 239PuO2. Int J Radiat Biol. 1992 Jul;62(1):97–102. doi: 10.1080/09553009214551871. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Thuleau P. Ca2+ Channels in Higher Plant Cells. Plant Cell. 1991 Jun;3(6):555–559. doi: 10.1105/tpc.3.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Thuleau P., Graziana A., Canut H., Ranjeva R. A 75-kDa polypeptide, located primarily at the plasma membrane of carrot cell-suspension cultures, is photoaffinity labeled by the calcium channel blocker LU 49888. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):10000–10004. doi: 10.1073/pnas.87.24.10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuleau P., Graziana A., Rossignol M., Kauss H., Auriol P., Ranjeva R. Binding of the phytotoxin zinniol stimulates the entry of calcium into plant protoplasts. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5932–5935. doi: 10.1073/pnas.85.16.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]