Abstract
The efficiency of West Nile virus (WNV) transmission by competent mosquito vectors is driven by temperature and defined, in part, by the extrinsic incubation period, which is the time from a mosquito’s consumption of an infected bloodmeal until it becomes capable of transmitting the virus to the next vertebrate host. The extrinsic incubation period can be altered by a variety of factors involved in vector–pathogen interactions, and in North America, the WN02 strain of WNV emerged and displaced the founding NY99 strain reportedly because the duration of the extrinsic incubation period in Culex mosquitoes was shortened by a single positively selected mutation. However, recent work has suggested that this change is not universal and may depend on vector species or strain. In the current study, we estimated the extrinsic incubation periods at 22 and 30°C in Culex tarsalis Coquillett. We found that the time to transmission of the original North American WNV strain, NY99, was not different from two more recent California isolates of the WN02 genotype: one of the earliest California isolates from the southeastern deserts, and a more recent 2011 isolate from a hyperendemic region in the Central Valley. We conclude with a model-based assessment of the epidemiological effects of temperature on the duration of mosquitoes’ infectious life, which estimated that most mosquitoes have an infectious life of only a few days, but its duration expands markedly at warmer temperatures.
Keywords: Culex tarsalis, West Nile virus, transmission, temperature, incubation period
West Nile virus (WNV) is a flavivirus in the family Flaviviridae that was introduced to the United States in 1999 and has since emerged as an important cause of human disease (Hayes 2001, Petersen and Fischer 2012). The virus is now endemic in much of the United States and is maintained and amplified in nature through a cycle of female adult mosquitoes blood-feeding on susceptible bird hosts (Komar 2003). Since its introduction into North America, WNV has been the cause of ongoing outbreaks of febrile and neuroinvasive disease in humans (Hayes and Gubler 2006, Beasley et al. 2013). In both 2002 and 2003, there were ∼3,000 recorded cases of West Nile neuroinvasive disease in the United States, leading to ∼300 deaths each year (Hayes and Gubler 2006). From 2004 to 2011, there were lower levels of human cases every year than the 2002 and 2003 epidemic years; however, in 2012, there was a resurgence of human cases, with >5,000 total cases, 2,734 neuroinvasive cases, and 243 deaths, which indicated the potential for future resurgent and widespread outbreaks (Beasley et al. 2013).
There is evidence that WNV is changing. The original strain isolated in New York, NY99 (Lanciotti et al. 1999), persisted until 2002, when it was replaced broadly by a new genotype now known as WN02, with a single, positively selected mutation that resulted in an amino acid change, E-V159A (Ebel et al. 2004, Davis et al. 2005). This raised questions about the selective advantage that enabled such a rapid displacement, and subsequent work identified an association between WN02 and a shortened time required for transmission by Culex tarsalis Coquillett and Culex pipiens L. (Moudy et al. 2007). The time required from a mosquito’s consumption of an infectious bloodmeal to transmission is known as the extrinsic incubation period (EIP), and a shortened EIP can result in increased efficiency of viral transmission by allowing more mosquitoes to transmit virus earlier in their lives, raising the probability that they would become infectious within their lifespan. Warm temperatures shorten the EIP, and the temperature–EIP relationship has been characterized using both NY99 (Reisen et al. 2006, Goddard et al. 2003) and WN02 (Kilpatrick et al. 2008, Moudy et al. 2007, Anderson et al. 2012).
It is clear that vector competence – even within a single mosquito species – is highly variable among geographical locations and over time (Vaidyanathan and Scott 2007, Reisen et al. 2008, Kilpatrick et al. 2010), in part because of the interplay between vector and virus genetics. Recent findings have suggested that the shortened EIP for WN02 is not universal, particularly in the key vector species, Cx. tarsalis, in which a recent strain of WNV did not exhibit the early EIP advantages identified for the WN02 genotype (Anderson et al. 2012). Accordingly, we compared the EIP in the original North American strain, NY99, to two recent isolates from California that possess the WN02 envelope gene mutation. We tested the hypothesis that increased transmissibility of WN02 was more pronounced at warm temperatures (Kilpatrick et al. 2008) by comparing the viral strains at a warm temperature of 30°C and a cool temperature of 22°C, which extends the work at 26°C by Anderson at al. (2012). In addition, we used the resulting model to estimate epidemiologically relevant features of the transmission response curve as a function of time postinfection and temperature.
Materials and Methods
Mosquitoes
Experiments were conducted using the KNWR colony of Cx. tarsalis established from females collected in 2002 at the Kern National Wildlife Refuge (35.7458° N, 119.6179° W) in Kern County, CA, an area with a history of high activity of WNV (Reisen et al. 2009). Mosquitoes for experimentation were reared and held in an insectary at 24°C, with 40–60% humidity and a photoperiod of 14:10 (L:D) h. Larvae were fed ground fish food, and adults were offered 10% sucrose. Three to five days after emergence, adults were transferred to a BSL3 containment facility for experimentation.
Viruses. We used three strains of WNV: 1) NY99 (strain 35211aaF9/23/99, GenBank accession AF196835), initially isolated from a flamingo that died in the Bronx Zoo; 2) COAV03 (strain COAV-997-2003, GenBank accession JF703162), which was the original strain of WNV isolated from Cx. tarsalis in Imperial County, CA, in 2003 and sequenced to show it has the E-V159A mutation characteristic of the WN02 strain; and 3) KERN11 (strain KERN-2000-2011, Gen Bank accession KR348980), which was isolated in 2011 from Cx. tarsalis collected in Kern County and sequenced to confirm the presence of the E-V159A mutation. Both of the California isolates we studied, COAV03 and KERN11, have been confirmed to be of the WN02 genotype (Andrade et al. 2011; A.C. Brault, personal communication) and not the SW03 genotype that is currently cocirculating with WN02 in California (Deardorff et al. 2006, McMullen et al. 2011). The NY99 and COAV03 strains had been passaged three times and the KERN11 strain twice in Vero cells prior to experimentation.
Infection and Transmission
Three- to five-day-old Cx. tarsalis were starved for 24 h and then allowed to feed for 1 h on a 1:5 mixture of viral stock and heparinized sheep blood (Hemostat Laboratories, Dixon, CA), warmed to 37°C in a Hemotek membrane-feeding apparatus (Discovery Workshops, Lancashire, UK). The NY99 stock had a viral titer of 9.03 log10 plaque-forming units (PFU) per ml, while COAV03 and KERN11 had titers of 9.00 and 9.02 log10 PFU/ml, respectively. After the blood mixture was removed, mosquitoes were anesthetized with CO2 and sorted into five groups of 25–30 blood-engorged females that were transferred into separate half-liter cages and held at a constant 22 or 30°C, which approximated average May and July temperatures in Kern County (NCDC 2015), in a programmable incubator with the same humidity and photoperiod, as described for rearing conditions above (Thermo Fisher Precision 818; Waltham, MA); all other mosquitoes were discarded. One milliliter of virus–blood mixture was stored at −80°C, and an aliquot was tested by plaque assay on Vero cells (Kramer et al. 2002) to confirm virus concentration at blood feeding. Procedures for mosquito maintenance and sample collection were as described for previous experiments (Reisen et al. 1993, 2006). Blood-fed females were provided with cotton pads soaked with 10% sucrose, which were replaced every other day.
Measuring Infection and Transmission
We were able to estimate the approximate range of EIPs at each temperature from prior research (Reisen et al. 2006), so we selected time points for each temperature that were expected to span the period from the earliest to maximal transmission. Therefore, mosquitoes incubated at 22°C were tested at five time points between 10 and 18 d postfeeding (DPF), whereas mosquitoes at 30°C were tested at four or five time points, depending on the number of infected mosquitoes, between 2 and 10 DPF. At each time point, mosquitoes were anesthetized with triethylamine and their expectorant was collected by the capillary tube method (Aitken 1977). Capillary tubes were filled with a 1:1 solution (by volume) of 10% sucrose and fetal bovine serum (Gibco, Grand Island, NY), and mosquitoes were allowed to feed for 20 min. Each mosquito and its expectorant was placed in a separate 2 ml centrifuge tube containing 300 µl viral transport media (Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 50 µg/ml gentamicin, 100 units/ml penicillin, 20 µg/ml streptomycin, and 5 µg/ml mycostatin) and stored at −80°C before testing.
Mosquito and expectorant samples were defrosted on wet ice, and mosquito bodies were homogenized with 3-mm glass beads at 30 vibrations/s for 2 min in a Retsch Tissuelyser (model 85210; Haan, Germany). Expectorant and body samples were clarified by centrifugation at 2,200 rpm for 20 min. Viral RNA was extracted from 50-µl aliquots using a 96-well MagMax system (ABI Life Technology, Waltham, MA) and then tested for WNV RNA by a singleplex semi-quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) using WNV primers and probes that target the envelope gene (Lanciotti et al. 2000) on a ViiA 7 platform (ABI Life Technology, Waltham, MA). The cutoff value for detection of viral RNA was 40 Ct. Some expectorant samples with a Ct value ≥35, which suggested low viral titer, were selected randomly for confirmation as positive for infectious virus by plaque assay on Vero cells.
Statistical Analysis
To characterize the probability of transmission as a function of time and temperature, we fitted a logistic regression model with the probability that mosquitoes transmitted the virus as the outcome, using R software (R Core Team 2013, Vienna, Austria). Viral strain, DPF, and temperature were explanatory variables, with NY99 as the reference strain, according to the following equation where is the probability of transmitting WNV:
Inclusion of coefficients for the strains COAV03 and KERN11 allowed us to determine whether their transmission curves differed from the reference NY99 strain. Secondarily, a separate model was used with the same structure to compare our results with those of a previous study that used only NY99 and the same Cx. tarsalis colony (Reisen et al. 2006). To compare the results of two experiments done several years apart with very different mosquito infection rates, we excluded uninfected mosquitoes from our analyses.
Epidemiological Modeling
The logistic regression function based on the combined data was used to define the cumulative probability of transmission over time, and we transformed the cumulative density function into a probability density function for the EIP at a given temperature. This allowed us to also estimate the probability density for the duration of mosquitoes’ infective life, which is equal to pEIP/-ln(p), based on the formula for vectorial capacity (Garrett-Jones 1964, 1970; Reisen 1989), where p is daily probability of mosquito survival. Statistical significance was defined as P < 0.05.
Results
Infection and Transmission
In total across both temperatures and all time points, 520 female Cx. tarsalis consumed blood containing ≥8.5 log10 PFU of WNV per ml from the Hemotek feeder and survived to be assessed for infection and transmission. Of those, 489, or 94%, had detectable viral RNA in their body by qRT-PCR at the time transmission was assessed and, therefore, were considered to be infected. Infection rates were similar at 22°C (94.7% or 233 of 246) and 30°C (93.4% or 256 of 274).
We tested the saliva from all infected females and found 164 WNV-positive samples (Table 1). At 22°C, saliva was positive at the first observed time point, 10 DPF, while at 30°C positive saliva was first detected at 4 DPF, which was the second time point. Ct values for positive expectorant samples ranged from 18.60 to 39.99. Of the 74 samples with Ct values ≥35, we randomly selected 48 to confirm the presence of infectious virus by Vero cell plaque assay. Of those samples, 36, or 75%, were positive by plaque assay, with no relationship between the Ct value and whether the sample was plaque assay positive (simple logistic regression, P = 0.55). This indicated that high Ct values were valid RNA-positive expectorant samples with very low viral titers that yielded variable results by plaque assay. As a result, we included all PCR-positive samples in our analysis. The percentage of mosquitoes that transmitted WNV increased as a function of time after bloodmeal ingestion (DPF; Fig. 1). There was little evidence for change in the amount of virus expectorated in saliva, as the only significant trend was a decline in Ct values over time for mosquitoes infected with the KERN11 strain at 30°C (P = 0.01), which indicates an increase in virus; however, all other strain and temperature combinations showed no significant relationship between Ct value and time (all P > 0.1). This suggested that, in general, once infectious, mosquitoes at later time points expectorated similar amounts of virus to those at earlier time points.
Table 1.
Extrinsic incubation of three strains of WNV at 22 and 30°C in Cx. tarsalis
| Temperature | Virus | Time point |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||||||||
| DPF | I | T | DPF | I | T | DPF | I | T | DPF | I | T | DPF | I | T | ||
| 22°C | NY99 | 10 | 100 (n = 16) | 13 | 12 | 95 (n = 19) | 11 | 14 | 100 (n = 5) | 0 | 16 | 100 (n = 15) | 60 | 18 | 100 (n = 14) | 43 |
| COAV03 | 10 | 100 (n = 14) | 7 | 12 | 89 (n = 19) | 12 | 14 | 100 (n = 19) | 32 | 16 | 55 (n = 20) | 45 | 18 | 100 (n = 20) | 60 | |
| KERN11 | 10 | 93 (n = 14) | 15 | 12 | 100 (n = 16) | 6 | 14 | 100 (n = 18) | 28 | 16 | 100 (n = 17) | 41 | 18 | 100 (n = 20) | 40 | |
| 30°C | NY99 | 2 | 90 (n = 21) | 0 | 5 | 100 (n = 19) | 5 | – | – | – | 8 | 100 (n = 20) | 65 | 10 | 100 (n = 16) | 100 |
| COAV03 | 2 | 76 (n = 25) | 0 | 4 | 77 (n = 22) | 38 | 6 | 100 (n = 23) | 22 | 8 | 95 (n = 21) | 57 | 10 | 100 (n = 20) | 65 | |
| KERN11 | 2 | 100 (n = 21) | 0 | 5 | 84 (n = 25) | 18 | – | – | – | 8 | 100 (n = 21) | 48 | 10 | 100 (n = 20) | 60 | |
DPF, days postfeeding; I, percent infected; n, number tested; T, percent of blood-fed mosquitoes transmitting.
Fig. 1.
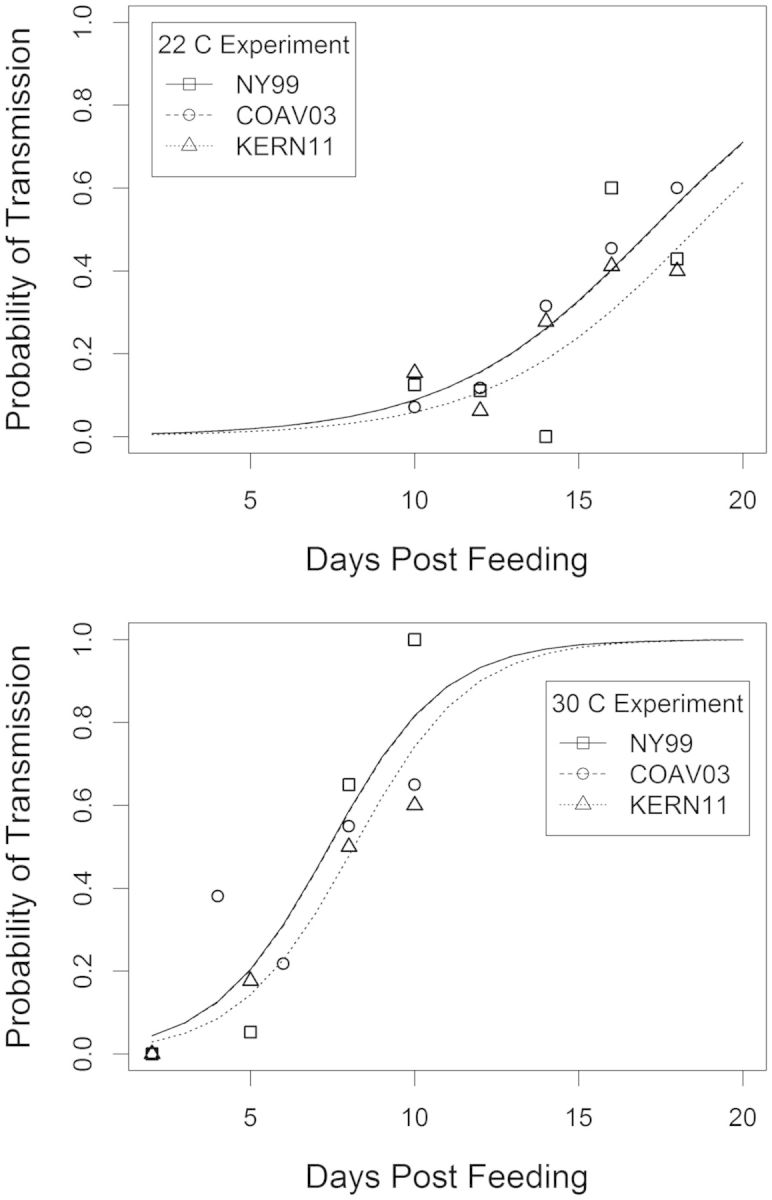
Probability of female Cx. tarsalis transmitting by day for each virus strain, with observed data (points) shown with fitted logistic regression curves (lines), at both temperatures tested.
Strain Comparisons
There was a difference in the infection rate between some viral strains when analyzed using a pairwise test for proportions with an adjustment for multiple comparisons. The infection rate, across all temperatures and time points tested, for COAV03 (89.3% or 184 of 206) was significantly lower than both NY99 (97.9% or 142 of 145; P = 0.010) and KERN11 (96.4% or 163 of 169; P = 0.030); there was no significant difference between the NY99 and KERN11 strains (P = 0.66).
The overall probability of transmission by infected Cx. tarsalis did not differ significantly between the COAV03 and NY99 strains (β = 0.00; P = 0.96) nor between KERN11 and NY99 (β = −0.05; P = 0.35) or COAV03 and KERN11 (β = −0.05; P = 0.34). Based on the median EIPs from the logistic regression model, there was no difference in the time to transmission between the NY99 and COAV03 strains, while transmission of KERN11 occurred very slightly later, but the time difference was not significant (Table 2). When transmission of NY99 strains was compared between our current experiment and an earlier experiment that used the same viral strain and mosquito colony (Reisen et al. 2006), there was no significant difference between these experiments (P = 0.87). Because we did not find any significant difference between the NY99 strain and more recent WN02 strains nor between the earlier 2006 experiment and our results, all current virus strains were grouped with results from the earlier experiment for all temperatures to examine the epidemiological effects of temperature on transmission potential.
Table 2.
Median EIPs, in DPF, estimated for three WNV strains
| Temperature | NY99 | COAV03 | KERN11 |
|---|---|---|---|
| 14°C | 88 | 88 | 94 |
| 18°C | 31 | 31 | 33 |
| 22°C | 17 | 17 | 18 |
| 26°C | 11 | 11 | 12 |
| 30°C | 8 | 8 | 8 |
Transmission as a Function of Time and Temperature
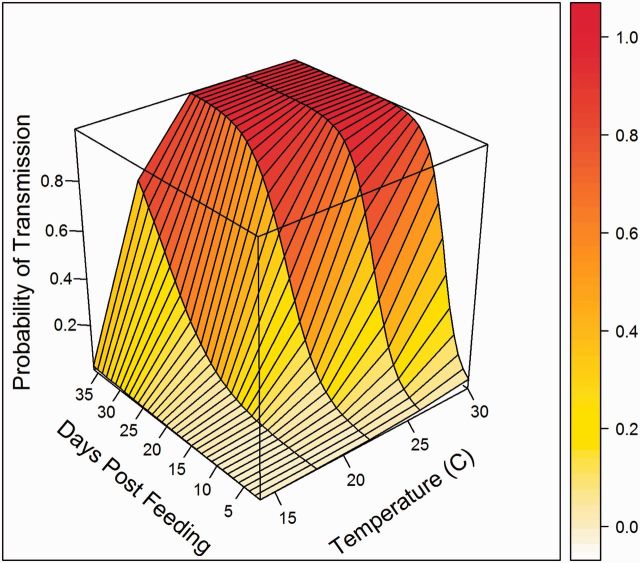
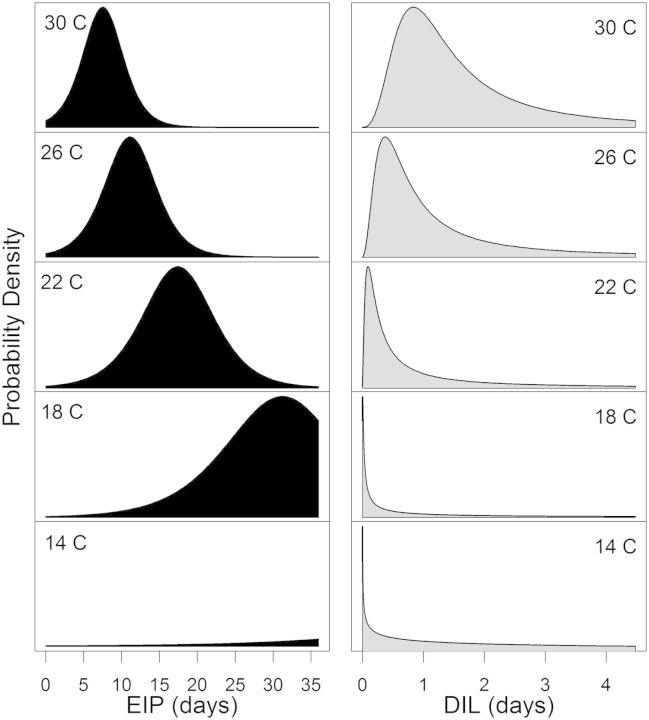
Using the pooled data, the joint effect of time and temperature, represented by the interaction term, was positively associated with the probability of transmission (β3 = 0.03; P = 0.01). The coefficients from the final logistic regression model (Table 3; Fig. 2) characterized the cumulative probability of transmission over time for any given temperature, and from this function the probability density functions for the EIP were derived at each temperature tested. At the warmest temperature tested, 30°C, our model-based estimates suggested that all mosquitoes would be capable of transmission by 18 DPF, while at our coolest temperatures, 14 to 18°C, transmission had barely begun at the same time point (Fig. 3). From the distributions of EIPs, we also calculated the probability distribution for the duration of infectious life at each of the temperatures used in this study, assuming daily survival of 80% (Reisen et al. 1992, 1995). Very few mosquitoes would be expected to live long enough to become infectious at the lowest temperatures tested (Fig. 3). Infectious life remained short for most mosquitoes (<4 d) even at the warmest temperatures, although it markedly expanded between 22 and 30°C.
Table 3.
Coefficients from the final logistic model for the probability of mosquito transmitting as a function of time and temperature
| Coefficient | Estimate | 95% CI | P-value |
|---|---|---|---|
| Intercept | −9.44 | (−11.83, −7.05) | <0.0001 |
| DPF | −0.34 | (−0.48, −0.20) | <0.0001 |
| Temperature | 0.17 | (0.09, 0.25) | <0.0001 |
| DPF × temperature | 0.03 | (0.02, 0.04) | <0.0001 |
Fig. 2.
Wireframe plot of the probability of female Cx. tarsalis transmitting over time and temperature.
Fig. 3.
Probability density functions for EIPs (left panel) and durations of infectious life (DIL: right panel) at each temperature tested. The height of each curve is proportional to the percentage of mosquitoes that had the specified EIP or duration of infectious life.
Discussion
The EIP of WNV in mosquitoes is a key determinant of transmission, and a shorter EIP enhances transmission by allowing more mosquitoes to become infectious within their lifespan. The EIP for WNV, as with other arboviruses, is affected by a number of variables. In particular, vector competence for the virus can differ within and among mosquito species. A better understanding of the causes and consequences of variation in the EIP for currently circulating strains of WNV would allow public health agencies to estimate transmission risk more accurately. For example, the California Department of Public Health has used temperature-based estimates of the EIP to define thresholds for environmental temperatures at which there is a higher risk of arboviral transmission in the California State Mosquito-borne Virus Surveillance and Response Plan (CDPH et al. 2014).
We did not find evidence for shortened EIPs in Cx. tarsalis for the California WN02 strains tested at cool (22°C) or warm (30°C) temperatures, agreeing with an earlier report for Cx. tarsalis at moderate temperatures (26°C; Anderson et al. 2012) and suggesting that the reported acceleration of the extrinsic incubation for the WN02 genotype (Ebel et al. 2004, Moudy et al. 2007, Kilpatrick et al. 2008) is not universal. An earlier comparison of four Culex species from California using NY99 found no difference in the EIP at a single temperature (Goddard et al. 2003), but similar species comparisons have not been done for WN02. Both Cx. tarsalis and the Cx. pipiens complex are competent vectors following infection with the NY99 strain, but there is variation in competence between and within these species (Goddard et al. 2002; Turell et al. 2002, 2005; Reisen et al. 2005, 2008; Kilpatrick et al. 2010).
Differences among laboratory colonies cannot be excluded as a cause for differences in vector competence within the same species. The Cx. tarsalis in this study came from a colony started in 2002 from mosquitoes collected in Kern County, at the southern end of California’s Central Valley, which is very close to the source of the colony that showed faster transmission of WN02 isolates (Moudy et al. 2007). Another colony of Cx. tarsalis from California’s Coachella Valley, where populations are genetically distinct from Cx. tarsalis found in the rest of the California (Venkatesan et al. 2007), transmitted WN02 strains at the same rate as NY99 (Anderson et al. 2012). Colonized mosquitoes go through genetic bottlenecks, which can lead to low levels of diversity within the same colony compared with natural populations, and high levels of genetic variability among colonies owing to genetic drift. Genetic drift increases with time, and it is possible that at least some of the discrepancies in findings may result from the differences in colony establishment dates from the 1950s (Moudy et al. 2007) to the 2000s (Anderson et al. 2012 and this study).
Our estimates for time to transmission are slightly longer than those of an earlier study of NY99 in Cx. tarsalis (Reisen et al. 2006), but when compared with a nonlinear degree-day model for transmission of WN02 in Cx. pipiens (Kilpatrick et al. 2008), transmission in our study occurred earlier at each temperature. For example, we estimated that 50% of Cx. tarsalis were capable of transmitting WNV 11 d after infection at 26°C; using the Kilpatrick et al. (2008) model, we interpolated that at 26°C Cx. pipiens would have required 21 d to complete the median EIP of the WN02 strain and 33 d for the same percent to complete the EIP of NY99 (Kilpatrick et al. 2008).
Our faster rates of transmission were, in part, because of the exclusion of uninfected mosquitoes from our analyses. While some prior studies also excluded the uninfected mosquitoes (Reisen et al. 2006), most based estimates for time to transmission on all mosquitoes that consumed an infected bloodmeal (Moudy et al. 2007, Kilpatrick et al. 2008, Anderson et al. 2012). Despite the fact that both our study and the Reisen study from 2006 used Cx. tarsalis from the same colony, our mosquitoes had a much higher infection rate. This was attributed to Reisen et al. (2006) using an in vivo infection system, where donor passerines exhibited highly variable viremias that rarely exceeded 8 log10 PFU/ml and resulted in variable vector infection rates. In addition, the Kern National Wildlife Refuge colony went through dozens of generations between the two experiments. Random assortment of mosquitoes into groups for testing also may lead to an imbalanced distribution of infected and uninfected mosquitoes, which introduces “noise” into the data. As there was little difference in the infection rate among the viruses tested herein, this decision did not significantly alter our results.
Even at the shortest EIPs, most mosquitoes have a relatively brief duration of infectious life, although it rapidly increases at temperatures >22°C. This increases transmission efficiency at warmer temperatures, as many more vectors are capable of transmitting within their expected lifetime. But even at the warmest temperatures tested, infectious life for most mosquitoes would not be expected to last >2 d. As part of the vectorial capacity equation (Garrett-Jones 1964, 1970; Reisen 1989), these estimates suggest that a large number of Cx. tarsalis females biting relative to the vertebrate host population size are required for transmission to proceed at high rates, or that parameter estimates for extrinsic incubation rates at constant temperatures in the laboratory or survival from mark–release–recapture studies differ from those of natural mosquito populations. To isolate the effects of temperature on the duration of infectious life, we based our estimates on the assumption that mosquito survival is constant across all temperatures. Field mark–release–recapture studies on Cx. tarsalis have found that daily survival decreased at warmer temperatures, which would offset some of the increases in infectious life owing to faster extrinsic incubation.
In summary, our study did not support earlier findings that the E-V159A mutation led to earlier transmission by Cx. tarsalis, suggesting that the EIP may be nuanced by more than a single amino acid change in the viral envelope. Epidemiological models applied to our results expressed the range of EIPs and the implications for mosquitoes’ infective life spans. These findings further elucidated why transmission of WNV by Cx. tarsalis can intensify as temperatures warm. Such models could be used by public health and mosquito control districts to more accurately estimate when WNV transmission to humans might occur, which allows for more efficient allocation of resources and efforts for intervention.
Acknowledgments
We thank Ying Fang, Sandra Garcia, Sarah Wheeler, and Veronica Armijos (Center for Vectorborne Diseases, Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, University of California, Davis) for technical support and Dr. Philip Kass (Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis) for statistical advice and editing. This research was funded by Grant 2013-17 from the Mosquito Research Foundation. CMB acknowledges additional support from the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security and Fogarty International Center, National Institutes of Health.
References Cited
- Aitken T. H. G. 1977. An in vitro feeding technique for artificially demonstrating virus transmission by mosquitoes. Mosq. News. 37: 130–133. [Google Scholar]
- Anderson J. F., Main A. J., Cheng G., Ferrandino F. J., Fikrig E. 2012. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am. J. Trop. Med. Hyg. 86: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C. C., Maharaj P. D., Reisen W. K., Brault A. C. 2011. North American West Nile virus genotype isolates demonstrate differential replicative capacities in response to temperature. J. Gen. Virol. 92: 2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley D. W., Barrett A. D., Tesh R. B. 2013. Resurgence of West Nile neurologic disease in the United States in 2012: What happened? What needs to be done? Antiviral Res. 99: 1–5. [DOI] [PubMed] [Google Scholar]
- Bellamy R. E., Kardos E. H. 1958. A strain of Culex tarsalis Coq. reproducing without blood meals. Mosq. News 18: 132–134. [Google Scholar]
- [CDPH] California Department of Public Health, Mosquito and Vector Control Association of California, and University of California. 2014. California Mosquito-borne Virus Surveillance and Response Plan. <http://westnile.ca.gov/resources.php> (accessed 1 December 2014). [Google Scholar]
- Davis C. T., Ebel G. D., Lanciotti R. S., Brault A. C., Guzman H., Siirin M., Lambert A., Parsons R. E., Beasley D. W., Novak R. J., et al. 2005. Phylogenetic analysis of North American West Nile virus isolates, 2001-2004: Evidence for the emergence of a dominant genotype. Virology 342: 252–265. [DOI] [PubMed] [Google Scholar]
- Deardorff E., Estrada-Franco J., Brault A. C., Navarro-Lopez R., Campomanes-Cortes A., Paz-Ramirez P., Solis-Hernandez M., Ramey W. N., Davis C. T., Beasley D. W., et al. 2006. Introductions of West Nile virus strains to Mexico. Emerg. Infect. Dis. 12: 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel G. D., Carricaburu J., Young D., Bernard K. A., Kramer L. D. 2004. Genetic and phenotypic variation of West Nile virus in New York, 2000-2003. Am. J. Trop. Med. Hyg. 71: 493–500. [PubMed] [Google Scholar]
- Garrett-Jones C. 1964. Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature 204: 1173–1175. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. 1970. Problems in epidemiological entomology as applied to malariology. Misc. Publ. Entomol. Soc. Am. 7: 168–180. [Google Scholar]
- Goddard L. B., Roth A. E., Reisen W. K., Scott T. W. 2002. Vector competence of California mosquitoes for West Nile virus. Emerg. Infect. Dis. 8: 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard L. B., Roth A. E., Reisen W. K., Scott T. W. 2003. Extrinsic incubation period of West Nile virus in four California Culex (Diptera: Culicidae) species. Proc. Mosq. Vector Control Assoc. Calif. 71: 70–75. [Google Scholar]
- Hayes C. G. 2001. West Nile virus: Uganda, 1937, to New York City, 1999. Ann. N. Y. Acad. Sci. 951: 25–37. [DOI] [PubMed] [Google Scholar]
- Hayes E. B., Gubler D. J. 2006. West Nile virus: Epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 57: 181–194. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A. M., Kramer L. D., Campbell S. R., Alleyne E. O., Dobson A. P., Daszak P. 2005. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 11: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M., Meola M. A., Moudy R. M., Kramer L. D. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 4: e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A. M., Fonseca D. M., Ebel G. D., Reddy M. R., Kramer L. D. 2010. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am. J. Trop. Med. Hyg. 83: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. 2003. West Nile virus: Epidemiology and ecology in North America. Adv. Virus Res. 61: 185–234. [DOI] [PubMed] [Google Scholar]
- Kramer L. D., Wolfe T. M., Green E. N., Chiles R. E., Fallah H., Fang Y., Reisen W. K. 2002. Detection of encephalitis viruses in mosquitoes (Diptera: Culicidae) and avian tissues. J. Med. Entomol. 39: 312–323. [DOI] [PubMed] [Google Scholar]
- Lanciotti R. S., Roehrig J. T., Deubel V., Smith J., Parker M., Steele K., Crise B., Volpe K. E., Crabtree M. B., Scherret J. H., et al. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286: 2333–2337. [DOI] [PubMed] [Google Scholar]
- Lanciotti R. S., Kerst A. J., Nasci R. S., Godsey M. S., Mitchell C. J., Savage H. M., Komar N., Panella N. A., Allen B. C., Volpe K. E., et al. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen A. R., May F. J., Li L., Guzman H., Bueno R., Jr, Dennett J. A., Tesh R. B., Barrett A. D. 2011. Evolution of new genotype of West Nile virus in North America. Emerg. Infect. Dis. 17: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy R. M., Meola M. A., Morin L.-L. L., Ebel G. D., Kramer L. D. 2007. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am. J. Trop. Med. Hyg. 77: 365–370. [PubMed] [Google Scholar]
- [NCDC] National Climatic Data Center. 2015. Data tools: 1981-2001 normals, Bakersfield Meadows Field Airport, CA US monthly normals. <http://www.ncdc.noaa.gov/cdo-web/datatools/normals> (accessed 18 April 2015). [Google Scholar]
- Petersen L.R., Fischer M. 2012. Unpredictable and difficult to control–the adolescence of West Nile virus. N. Engl. J. Med. 367: 1281–1284. [DOI] [PubMed] [Google Scholar]
- R Core Team 2013. R: A language and environment for statistical computing computer program, version 3.1. Vienna, Austria. [Google Scholar]
- Reisen W. K. 1989. Estimation of vectorial capacity: Introduction. Bull. Soc. Vector Ecol. 14: 39–40. [Google Scholar]
- Reisen W. K., Milby M. M., Presser S. B., Hardy J. L. 1992. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987-1990. J Med Entomol. 29: 582–598. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Meyer R. P., Presser S. B., Hardy J. L. 1993. Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 30: 151–160. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Lothrop H. D., Hardy J. L. 1995. Bionomics of Culex tarsalis (Diptera: Culicidae) in relation to arbovirus transmission in southeastern California. J. Med. Entomol. 32: 316–327. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Fang Y., Martinez V. M. 2005. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 42: 367–375. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Fang Y., Martinez V. M. 2006. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43: 309–317. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Barker C. M., Fang Y., Martinez V. M. 2008. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J. Med. Entomol. 45: 1126–1138. [DOI] [PubMed] [Google Scholar]
- Reisen W. K., Carroll B. D., Takahashi R., Fang Y., Garcia S., Martinez V. M., Quiring R. 2009. Repeated West Nile virus epidemic transmission in Kern County, California, 2004-2007. J. Med. Entomol. 46: 139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell M. J., Sardelis M. R., O'Guinn M. L., Dohm D. J. 2002. Potential vectors of West Nile virus in North America. Curr. Top. Microbiol. Immunol. 267: 241–252. [DOI] [PubMed] [Google Scholar]
- Turell M. J., Dohm D. J., Sardelis M. R., O’Guinn M.L., Andreadis T. G., Blow J.A. 2005. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 42: 57–62. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R., Scott T. W. 2007. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis. 7: 193–198. [DOI] [PubMed] [Google Scholar]
- Venkatesan M., Westbrook C. J., Hauer M. C., Rasgon J. L. 2007. Evidence for a population expansion in the West Nile virus vector Culex tarsalis. Mol. Biol. Evol. 24: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]