Abstract
Chemical insecticides are effective for controlling Lutzomyia and Phlebotomus sand fly (Diptera: Psychodidae) vectors of Leishmania parasites. However, repeated use of certain insecticides has led to tolerance and resistance. The objective of this study was to determine lethal concentrations (LCs) and lethal exposure times (LTs) to assess levels of susceptibility of laboratory Lutzomyia longipalpis (Lutz and Nieva) and Phlebotomus papatasi (Scopoli) to 10 insecticides using a modified version of the World Health Organization (WHO) exposure kit assay and Centers for Disease Control and Prevention (CDC) bottle bioassay. Sand flies were exposed to insecticides coated on the interior of 0.5-gallon and 1,000-ml glass bottles. Following exposure, the flies were allowed to recover for 24 h, after which mortality was recorded. From dose–response survival curves for L. longipalpis and P. papatasi generated with the QCal software, LCs causing 50, 90, and 95% mortality were determined for each insecticide. The LCs and LTs from this study will be useful as baseline reference points for future studies using the CDC bottle bioassays to assess insecticide susceptibility of sand fly populations in the field. There is a need for a larger repository of sand fly insecticide susceptibility data from the CDC bottle bioassays, including a range of LCs and LTs for more sand fly species with more insecticides. Such a repository would be a valuable tool for vector management.
Keywords: Phlebotomus papatasi, Lutzomyia longipalpis, insecticide resistance, CDC bottle bioassay, WHO
Since their introduction in the 1940s, synthetic chemical insecticides remain an effective tool for controlling insects that are vectors of disease agents (Hemingway and Ranson 2000, World Health Organization [WHO] 2006). Unfortunately, insecticides have been used indiscriminately, exerting tremendous selective pressure for insecticide resistance (Feyereisen 1995, WHO 2006). The insecticide resistance phenotype is defined as a heritable, genetic change in response to insecticide exposure (Feyereisen 1995, Scott 1999, Hemingway et al. 2002). Increasing the insecticide dosage in response to resistance only exacerbates the problems of resistance by increasing the frequency of the genetic trait(s) in a vector population (Feyereisen 1995). Two resistance phenotypes observed in the field are target site insensitivity and metabolic detoxification resistance (Mallet 1989, Brogdon and McAllister 1998a, Rivero et al. 2010). Today, there is evidence of target site insensitivity and metabolic detoxification resistance to all classes of synthetic insecticides in all major vector species (Nauen 2007, Rivero et al. 2010). Acquiring data on vector species’ susceptibility to insecticides will support the strategies directed at effectively managing these vector populations (Surendran et al. 2005). The following two techniques are commonly used to measure a vector species’ susceptibility to insecticides: 1) the WHO exposure kit bioassay and 2) the Centers for Disease Control and Prevention (CDC) bottle bioassay (CDC 2010, WHO 2013).
The WHO exposure kit bioassay is widely accepted because it can measure insecticide susceptibility in many species of insect vectors worldwide (Braverman et al. 2004, Ocampo et al. 2011, Faraj et al. 2012, Aïzoun et al. 2013, Chen et al. 2013). The assays can be run with live insects collected in the field or with their progeny reared in the laboratory. The WHO bioassay is a standardized protocol that consists of an exposure kit containing tubes lined with filter papers that are impregnated with a specific concentration of an insecticide (WHO 1998, 2013). Despite its accepted use, the WHO bioassay is expensive, filter papers are not available for some insecticides, and there is a limited range of concentrations that can be purchased for some insecticides (Perea et al. 2009, Aïzoun et al. 2013).
The CDC bottle bioassay is an inexpensive and portable alternative to the WHO bioassay, especially in regions where there is little money to implement the WHO bioassay (Perea et al. 2009, Aïzoun et al. 2013). The CDC bottle bioassay requires fewer test insects than the WHO bioassay (Aïzoun et al. 2013). The protocol consists of coating the interior of a glass bottle with an insecticide that has been diluted in a solvent. The solvent is then allowed to evaporate, leaving the insecticide coated to the glass surface. Once the bottles are treated, insects are introduced into the bottles and exposed to the insecticide for a specified amount of time (Brogdon and McAllister 1998b, CDC 2010, Aïzoun et al. 2013). Insect mortality can be scored at distinct time intervals during the exposure test (e.g., every 15 min for 1-h), and percent mortality at each time interval is plotted (Brogdon and McAllister 1998b). The CDC bottle bioassay can also be used as an end-point assay where mortality is only measured at the end of the exposure test. Susceptibility is measured by simply comparing mortality rates between insect populations (Perea et al. 2009).
Sand flies (Diptera: Psychodidae: Phlebotominae) are among the insect vectors that require resistance monitoring because they have been actively targeted with insecticides. Many sand fly species in the genera Lutzomyia and Phlebotomus are capable of vectoring Leishmania parasites, infection with which causes leishmaniasis, a disease currently infecting millions of people worldwide (Guerin et al. 2002, Rutledge and Gupta 2009). To control sand flies, populations around the world have been exposed to the four main classes of insecticides— 1) organochlorines, 2) organophosphates, 3) carbamates, and 4) pyrethroids—via residual spraying, ultra-low volume spraying, insecticide-treated clothing, and insecticide-treated nets. These exposures are either intentional in directed vector control efforts or are inadvertent as part of vector control efforts targeted against other insect vectors (Alexander and Maroli 2003, Surendran et al. 2005, Alexander et al. 2009, Henriquez et al. 2009, Rutledge and Gupta 2009, Dinesh et al. 2010, Faraj et al. 2012, Hassan et al. 2012, Saeidi et al. 2012).
Some sand fly populations have been found to be tolerant or resistant to the insecticides used in the Middle East, southern Asia, and South America. In Montes Claros, Brazil, 29 of 80 (36.3%) Lutzomyia longipalpis (Lutz and Nieva) survived a 0.05% deltamethrin exposure (Alexander et al. 2009). In a Delft Island population from Sri Lanka, 11 of 80 Phlebotomus argentipes Annandale & Brunetti (14%) had insensitive acetylcholinesterase, and 20 (25%) had elevated esterases, of which both of these findings are associated with resistance to malathion (Surendran et al. 2005). P. argentipes was found to be DDT-resistant throughout the Muzaffarpur, Vaishali, and Patna districts of the Bihar state, India, and in the Amahibelha village of the Sunsari district, Nepal, as only 43 and 62% of populations died from DDT exposure, respectively (Dinesh et al. 2010). In the Surogia village of Khartoum State, Sudan, 51 Phlebotomus papatasi (Scopoli) (79.7%) had insensitive acetylcholinesterase, which is associated with malathion and propoxur resistance. Both of these insecticides have been extensively used in this region as part of the antimalaria mosquito control program (Hassan et al. 2012).
Many of the examples demonstrating reduced insecticide susceptibility in sand flies have been determined using the WHO bioassay. However, a few studies have used the CDC bottle bioassay to measure the susceptibility status of sand fly populations to insecticides (Santamaría et al. 2003, Alexander et al. 2009, Henriquez et al. 2009). These studies have been completed entirely in the New World. The CDC bottle bioassay is preferred over the WHO bioassay because the susceptibility results can be generated quickly, the bottles can be prepared with any insecticide, the results are reproducible with fewer insects and fewer replicates, and the results allow one to infer the detoxification mechanism conferring resistance (Santamaría et al. 2003).
It is imperative to develop expansive baseline susceptibility data to different insecticides in different sand fly species and in flies from different geographic regions (CDC 2010). In addition, these bioassays require baseline data from known susceptible sand fly populations to assess insecticide-susceptibility in field populations and for the calculation of relative risk ratios (e.g., lethal concentration causing 50% mortality [LC50] in a field population / LC50 control population). These data will provide vector management programs the information necessary to ensure appropriate and effective insecticide application (Maharaj 2011). Potentially, the CDC bottle bioassay is one tool that could be incorporated into sand fly surveillance programs to a greater extent worldwide, especially in regions where Leishmania transmission is a concern.
The objective of this study was to quantify, using a modified version of the WHO exposure kit assay and the CDC bottle bioassay, the susceptibility of laboratory L. longipalpis and P. papatasi to 10 insecticides that are incorporated globally in vector control efforts. Specifically, for each insecticide, a dose–response survival curve was produced. From each curve, LC50, LC90, and LC95 values were determined. These doses can now be used for comparison in future studies to assess sand fly susceptibility to insecticides.
Materials and Methods
Sand Flies
Insecticide-susceptible L. longipalpis and P. papatasi sand fly colonies at Utah State University (USU) were derived from long-established colonies maintained at the Walter Reed Army Institute of Research (Silver Spring, MD). The original colonies are >30 years old and have never been exposed to insecticides. All life stages were reared at USU at 25°C, 85% relative humidity, and a photoperiod of 16:8 (L:D) h according to methods described by Lawyer et al. (1991) and Modi and Rowton (1999). Larvae were fed a composted 1:1 mixture of rabbit feces and rabbit food (Young et al. 1981; Volf and Volfova 2011). Adults were provided 30% sucrose–water solution daily on saturated cotton balls, and adult female L. longipalpis and P. papatasi were blood-fed on anesthetized mice placed inside holding cages twice weekly.
Insecticides
Ten technical-grade insecticides were used in this study: four pyrethroids [cypermethrin (Sigma-Aldrich, St. Louis, MO), deltamethrin (Sigma-Aldrich, St. Louis, MO), lambda(λ)-cyhalothrin (Sigma-Aldrich, St. Louis, MO), and permethrin (Chem Service, Inc., West Chester, PA)]; three organophosphates [chlorpyrifos (Sigma-Aldrich, St. Louis, MO), fenitrothion (Sigma-Aldrich, St. Louis, MO), and malathion (Chem Service, Inc., West Chester, PA)]; two carbamates [bendiocarb (Sigma-Aldrich, St. Louis, MO), and propoxur (Sigma-Aldrich, St. Louis, MO)]; and the organochlorine DDT (Sigma-Aldrich, St. Louis, MO). The concentrations of each insecticide to which L. longipalpis and P. papatasi were exposed are provided in Table 1. The diagnostic doses for Anopheles and Aedes mosquitoes were used as starting reference points for initial insecticide exposure (CDC 2010). Concentrations higher and lower than these diagnostic doses were determined to derive the dose–response survival curves for the two sand fly species. All insecticide dilutions were prepared in acetone, stored in glass bottles, wrapped in aluminum foil, and refrigerated while not being used (CDC 2010).
Table 1.
Concentrations of 10 insecticides used in the CDC bottle bioassays to expose L. longipalpis and P. papatasi sand flies
| Insecticide class | Insecticide | Species | Concentration (µg insecticide per bottle) |
|---|---|---|---|
| Pyrethroid | Cypermethrin | L. longipalpis | 1, 5, 10, 25, 50, 100, 125, 150 |
| P. papatasi | 0.5, 1, 5, 10, 25, 50, 75, 100, 125, 150, 175, 200, 225, 250 | ||
| Deltamethrin | L. longipalpis | 0.01, 0.1, 1, 5, 10, 25, 50, 75 | |
| P. papatasi | 5, 10, 25, 50, 100, 125, 150, 200 | ||
| λ-Cyhalothrin | L. longipalpis | 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 25, 50, 75 | |
| P. papatasi | 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 25, 50 | ||
| Permethrin | L. longipalpis | 1, 5, 10, 25, 50, 75, 100 | |
| P. papatasi | 5, 10, 25, 50, 75, 100, 125, 150, 175, 200, 225, 250 | ||
| Organophosphate | Chlorpyrifos | L. longipalpis | 0.01, 0.05, 0.1, 0.5, 1, 5, 10 |
| P. papatasi | 0.001, 0.01, 0.05, 0.1, 0.5, 1, 5, 10 | ||
| Fenitrothion | L. longipalpis | 0.1, 0.5, 1, 3, 5, 10, 25, 50 | |
| P. papatasi | 0.1, 0.5, 1, 3, 5, 10, 25, 50 | ||
| Malathion | L. longipalpis | 5, 8, 10, 12, 15, 25, 50, 100 | |
| P. papatasi | 10, 25, 50, 100, 125, 150 | ||
| Carbamate | Bendiocarb | L. longipalpis | 0.01, 0.1, 1, 5, 10, 25, 50, 75, 100, 125, 150, 175, 200 |
| P. papatasi | 0.01, 0.1, 0.5, 1, 5, 10, 25 | ||
| Propoxur | L. longipalpis | 0.01, 0.1, 0.5, 1, 5, 10, 25, 50, 75, 100 | |
| P. papatasi | 0.01, 0.1, 0.5, 1, 5, 10, 25, 50, 75 | ||
| Organochlorine | DDT | L. longipalpis | 10, 25, 50, 75, 100, 150, 200, 300, 350, 450 |
| P. papatasi | 5, 10, 25, 50, 75, 100, 150, 200, 300, 350 |
Preparation of Exposure Bottles
On the day prior to exposing the sand flies, 0.5-gallon glass bottles (1,892.5 ml) (unknown maker) or 1,000-ml glass bottles (Fisher Scientific, Pittsburgh, PA) were prepared by coating them with insecticide. For both bottle sizes, the concentration of insecticide in each bottle was determined to be X µg per bottle (CDC 2010). For a 250-ml bottle, 1 ml of insecticide at 10 µg insecticide/ml acetone gives a concentration of 10 µg/250 ml bottle. To maintain an equivalence of 10 µg insecticide/250 ml bottle to compensate for the larger bottle sizes, 4.0 ml of 10 µg insecticide/ml acetone is needed to coat the interior of the 1,000-ml bottle, and 7.57 ml of 10 µg insecticide/ml acetone is needed to coat the interior of the 0.5-gallon bottle. The bottles were coated with insecticide by swirling the acetone:insecticide solution on the bottom, on the sides, and on the lid. The bottle was then placed on a mechanical bottle roller under a chemical hood for 30 min to dry. During this time, the lids were slowly loosened to allow the acetone to evaporate. After 30 min, the caps were removed, and the bottles were rolled until all of the acetone had evaporated. The bottles were then left open to dry overnight. For each test replicate, one bottle serving as a control was coated with either 7.57 or 4.0 ml of acetone, depending on its volume. All bottles were reused throughout the duration of the experiment. To clean a bottle with residual insecticide, the bottle and lid was first triple-rinsed with acetone; filled with warm, soapy water; drained; rinsed and filled with cold water; drained; and autoclaved for at least 20 min. After being autoclaved, the bottles were left to dry for at least one day before being used again (CDC 2010). Each cleaned bottle also underwent testing to determine the presence of residual insecticide. Ten sand flies were aspirated into each bottle and were left in the bottle for at least 3 h. If no mortality was observed at the end of the 3 h, the bottles were cleared and allowed to be reused. If mortality was observed, the bottles were cleaned again and retested until no mortality was observed.
Insecticide Exposure Tests
Approximately 12 h after the bottles were prepared with insecticide, adult sand flies at least 2 d posteclosion were aspirated from the main colony and gently blown into each bottle: 40–50 flies into each 0.5-gallon bottle and 20–30 flies into each 1,000-ml bottle. Approximately equal numbers of un-fed female and male flies were used for each replicate. At least three replicates were completed for each concentration of every insecticide.
Both species were exposed for the same length of time to each insecticide. In preliminary tests, exposure time for all 10 insecticides was 60 min, but it was soon discovered that for some insecticides, 60 min of exposure was either too short or too long because sand fly survival was nearly 0 or 100% for most of the insecticide concentrations (Brogdon and McAllister 1998b). Therefore, the range of exposure times was adjusted to 30 or to 120 min, depending on unexpected and actual sand fly survival rates (Table 2) (CDC 2010).
Table 2.
Length of exposure of L. longipalpis and P. papatasi to 10 insecticides with the CDC bottle bioassay
| Insecticide | Exposure time (Min) |
|---|---|
| Cypermethrin | 60 |
| Deltamethrin | 60 |
| λ-cyhalothrin | 60 |
| Permethrin | 60 |
| Chlorpyrifos | 60 |
| Fenitrothion | 30 |
| Malathion | 60 |
| Bendiocarb | 30 |
| Propoxur | 30 |
| DDT | 120 |
The sand flies were captured after insecticide exposure via mechanical aspiration, released into 1-pint cardboard containers with a fine mesh screen top, and kept under the same temperature, light, and humidity environment as the main untreated colonies. A cotton ball saturated with 30% sugar–water was placed on the top of each container as an energy/water source. Using procedures established for mosquitoes, sand flies were held in these containers for 24 h prior to mortality being recorded (Saavedra-Rodriguez et al. 2008). Mortality was scored as a complete cessation of movement (Perea et al. 2009). A 24-h holding period was used because in some preliminary experiments, many of the sand flies that appeared physically affected, and would have been scored as dead at the end of a 30-, 60-, or 120-min exposure period as described in Brogdon and McAllister (1998b), recovered after this 24-h period.
If mortality in the control group ranged between 5 and 20%, mortalities in the experimental bottles of that test group were corrected using Abbott’s formula (CDC 2010). Abbott’s formula was not used to correct experimental mortalities if the control group mortality was <5%. If control group mortalities exceeded 20%, the entire testing replicate was not used (Saeidi et al. 2012).
Survival Curves
Using the QCal software, a dose–response survival curve was created for each insecticide (Lozano-Fuentes et al. 2012). This software can be used for any insect vector with data from insecticide bioassays. The QCal software also uses a logistic regression model to generate LC50s, LC90s, and LC95s for each insecticide. Mortalities corrected with Abbott’s formula were rounded to the nearest whole fly. For example, a cohort of 30 flies had an empirical mortality of 80% (24 flies died). If 80% was Abbott’s-corrected to 78.1% mortality, then 23.43 flies died. In QCal, a mortality of 23 flies of 30 was recorded.
Results
Physical Observations
Both L. longipalpis and P. papatasi sand fly species shed their legs when exposed to cypermethrin, deltamethrin, lambda-cyhalothrin, and permethrin during and after exposure. This was observed predominantly at the higher concentrations of each insecticide. Neither species shed its legs when exposed to organophosphates, carbamates, or DDT. In addition, for the pyrethroids, both L. longipalpis and P. papatasi experienced the “knockdown effect,” evident by involuntary movements and muscle spasms, during insecticide exposure and during the initial recovery time in the holding containers (Martins et al. 2009). At lower concentrations of the four pyrethroids, many sand flies were able to recover from the knockdown (no convulsions or erratic movements) by the completion of the 24-h holding period. At higher pyrethroid concentrations, sand flies succumbed to muscle spasms, convulsions, and paralysis.
It was also observed that the time required for the carbamates, organophosphates, and organochlorine (DDT) to cause mortality differed. The carbamates were lethal very quickly, causing death only a few minutes after the sand flies were aspirated into the bottles. This quick lethality necessitated a reduction in the exposure time of both sand fly species to the carbamates (Table 2). On the other hand, the three organophosphates and DDT caused delayed mortality. Many sand flies appeared physically healthy after exposure to these insecticides, but died during the 24-h holding period.
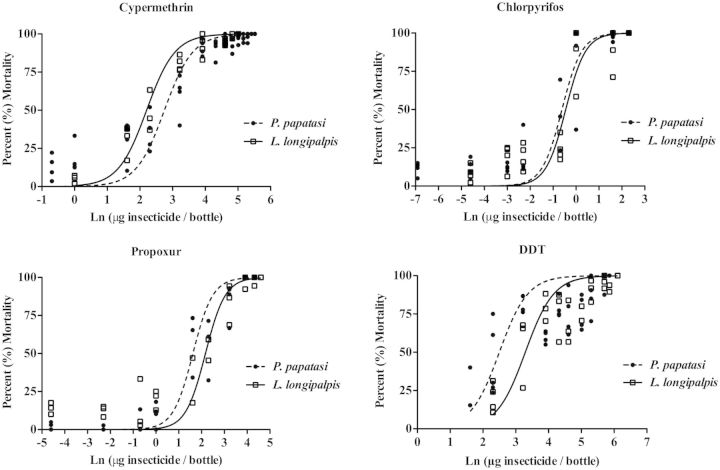
Survival Curves
A dose–response survival regression analysis was performed for L. longipalpis and P. papatasi to estimate LC50, LC90, and LC95 for all 10 insecticides. Figure 1 shows each species’ survival curve for cypermethrin (pyrethroid), chlorpyrifos (organophosphate), propoxur (carbamate), and DDT (organochlorine). These graphs were produced in GraphPad Prism (version 6.0, GraphPad Software Inc., San Diego, CA). Table 3 shows the QCal logistic regression parameters and the extrapolated LC50, LC90, and LC95 values for each insecticide for both species. For many insecticides, the LC95 was substantially greater than the LC90 (e.g., P. papatasi’s LC90 for cypermethrin was 73.279 µg cypermethrin per bottle, while its LC95 for cypermethrin was 150.010 µg cypermethrin per bottle), which may be attributed to the sigmoidal shape of the logistic curve, where it takes much higher doses to reach a smaller percentage change in mortality (i.e., LC90 to LC95) nearing the 100% mortality asymptote.
Fig. 1.
L. longipalpis and P. papatasi dose–response survival curves to cypermethrin (pyrethroid), chlorpyrifos (organophosphate), propoxur (carbamate), and DDT (organochlorine).
Table 3.
QCal logistic regression parameters and lethal concentration (LC) values causing 50, 90, and 100% mortality in L. longipalpis and P. papatasi exposure to 10 insecticides with the CDC bottle bioassay
| Insecticide | Species | LC50 (µg insecticide per bottle) [LL, UL]* | LC90 (µg insecticide per bottle) [LL, UL]* | LC95 (µg insecticide per bottle) [LL, UL]* |
|---|---|---|---|---|
| Cypermethrin | L. longipalpis | 8.955 [7.888, 10.167] | 41.851 [35.499, 49.338] | 70.704 [57.530, 86.886] |
| P. papatasi | 8.897 [7.499, 10.556] | 73.279 [61.313, 87.584] | 150.010 [120.265, 187.354] | |
| Deltamethrin | L. longipalpis | 0.922 [0.637, 1.334] | 28.707 [18.291, 45.056] | 92.434 [51.594, 165.571] |
| P. papatasi | 9.907 [8.165, 12.020] | 90.244 [67.938, 119.869] | 191.290 [130.804, 279.779] | |
| λ-Cyhalothrin | L. longipalpis | 0.232 [0.189, 0.284] | 5.001 [3.627, 6.895] | 14.215 [9.487, 21.298] |
| P. papatasi | 0.269 [0.217, 0.334] | 3.654 [2.625, 5.087] | 8.873 [5.863, 13.430] | |
| Permethrin | L. longipalpis | 17.069 [14.889, 19.570] | 82.402 [65.957, 102.946] | 140.752 [105.890, 187.073] |
| P. papatasi | 41.344 [37.233, 45.906] | 188.579 [162.796, 218, 438] | 315.955 [261.648, 381.572] | |
| Chlorpyrifos | L. longipalpis | 0.458 [0.377, 0.557] | 5.734 [4.058, 8.099] | 13.538 [11.695, 29.020] |
| P. papatasi | 0.327 [0.256, 0.419] | 6.417 [4.102, 10.037] | 17.653 [10.135, 30.774] | |
| Fenitrothion | L. longipalpis | 0.347 [0.277, 0.434] | 2.655 [1.933, 3.647] | 5.306 [3.549, 7.934] |
| P. papatasi | 1.368 [1.173, 1.595] | 7.334 [5.684, 9.489] | 13.007 [9.478, 17.850] | |
| Malathion | L. longipalpis | 8.432 [8.004, 8.883] | 13.815 [12.914, 14.779] | 16.340 [14.957, 17.852] |
| P. papatasi | 20.011 [17.277, 23.176] | 77.008 [63.459, 93.447] | 121.778 [94.869, 156.319] | |
| Bendiocarb | L. longipalpis | 0.986 [0.737, 1.318] | 38.961 [29.312, 52.159] | 136.047 [94.292, 196.311] |
| P. papatasi | 0.289 [0.232, 0.359] | 2.507 [1.875, 3.353] | 5.229 [3.632, 7.529] | |
| Propoxur | L. longipalpis | 3.837 [2.860, 5.148] | 75.446 [46.150, 123.347] | 207.763 [112.101, 385.060] |
| P. papatasi | 5.502 [4.524, 6.692] | 39.135 [28.126, 54.451] | 76.264 [50.729, 114.652] | |
| DDT | L. longipalpis | 28.364 [23.173, 34.716] | 218.581 [166.052, 287.724] | 437.685 [303.506, 631.249] |
| P. papatasi | 15.047 [11.321, 19.997] | 295.979 [196.684, 445.412] | 815.173 [463.219, 1434.541] |
*LL, lower 95% confidence limit; UL, upper 95% confidence limit.
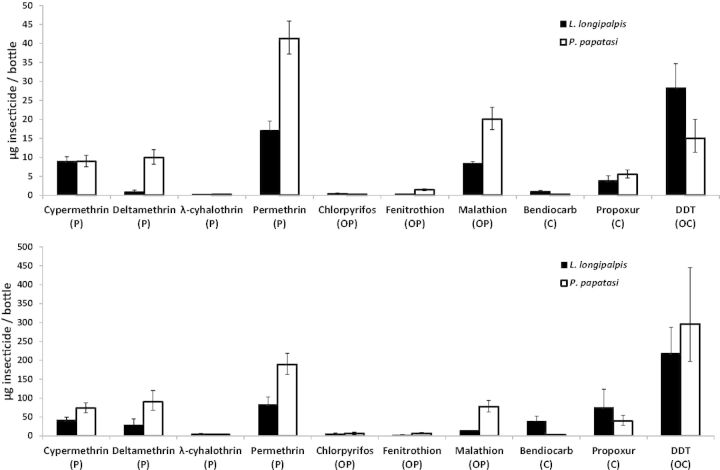
Pyrethroids
L. longipalpis and P. papatasi have very similar LC50’s for cypermethrin, roughly 9.0 µg cypermethrin per bottle; however, P. papatasi has an LC95 more than twice as large as L. longipalpis (Table 3). For deltamethrin, L. longipalpis has a 10-fold lower LC50 than P. papatasi (Fig. 2A) and a much lower LC90 and LC95 than P. papatasi (Fig. 2B; Table 3). L. longipalpis and P. papatasi have very similar lethal concentration values for lambda-cyhalothrin, and both species are very susceptible as their LC50, LC90, and LC95 values are <20.0 µg lambda-cyhalothrin per bottle, which are the lowest LC95 values for of the four pyrethroid insecticides (Table 3). For permethrin, P. papatasi has a LC50, LC90, and LC95 that are at least twice as large compared with those same LC values of L. longipalpis.
Fig. 2.
Bar graphs of L. longipalpis and P. papatasi lethal concentrations causing 50% mortality (LC50) (2A) and 90% mortality (LC90) (2B) for 10 insecticides. Error bars represent the lower and upper 95% CIs, as determined by QCal. Letters in parentheses below each insecticide represent the insecticide class: P, pyrethroid; OP, organophosphate; C, carbamate; OC, organochlorine.
Organophosphates
Both sand fly species are highly susceptible to chlorpyrifos and fenitrothion. The LC95’s for both L. longipalpis and P. papatasi are <20.0 µg per bottle. Besides P. papatasi’s LC95 for bendiocarb and both species LC95’s for lambda-cyhalothrin, these are the lowest LC95’s for all 10 insecticides (Table 3). In addition, the LC50’s for both species to chlorpyrifos are <0.5 µg chlorpyrifos per bottle. Like chlorpyrifos, both L. longipalpis and P. papatasi are highly susceptible to fenitrothion (Table 3) even with exposure times of 30 min. P. papatasi has a LC95 malathion that is approximately eight times larger than L. longipalpis’ LC95 for malathion.
Carbamates
L. longipalpis has a smaller LC95 than P. papatasi to all of the pyrethroids and to all of the organophosphates except lambda-cyhalothrin. For the carbamates, P. papatasi is more susceptible than L. longipalpis to bendiocarb and propoxur. The exposure time for both species is 30 min. In preliminary tests for the carbamates, ∼100% mortality was observed for all of the insecticide doses with a 60-min exposure time. Therefore, the duration of exposure was reduced to 30 min, which was a sufficient amount of time to obtain 50, 90, and 95% mortality (Table 3). P. papatasi has a LC95 for bendiocarb that is 26 times lower than L. longipalpis’ bendiocarb LC95 (Table 3), and P. papatasi has a LC90 of bendiocarb that is ∼15 times lower than L. longipalpis’ bendiocarb LC90 (Fig. 2B). Both species have a LC50 <1.0 µg bendiocarb per bottle (Fig. 2A). P. papatasi has a much lower LC95 for propoxur (LC95 = 76.264 µg propoxur per bottle) than L. longipalpis (LC95 = 207.763 µg propoxur per bottle). However, P. papatasi does have a greater LC50 to propoxur than does L. longipalpis (Fig. 2A).
Organochlorine
In preliminary tests with DDT, 60 min was insufficient to quantify 50, 90, and 95% mortality with all of the insecticide doses. Therefore, the duration of exposure was increased to 120 min to allow sufficient time to obtain these values for both L. Longipalpis and P. papatasi. Even with this extended exposure period, both species have very high LC95’s (437.729 µg DDT per bottle and 815.173 µg DDT per bottle for L. longipalpis and P. papatasi, respectively). These are the highest LC95’s for any of the 10 insecticides evaluated in this study (Table 2).
Discussion
The objective of this study was to quantify insecticide susceptibility in laboratory L. longipalpis and P. papatasi to 10 insecticides comprising four chemical classes using a modified version of the CDC bottle bioassay. It was demonstrated that this modified CDC bottle bioassay is an effective tool for measuring the susceptibility of these two sand fly species to pyrethroid, organophosphate, carbamate, and organochlorine insecticides.
One important observation of this study was that different insecticide classes have different LTs. Organophosphate insecticides caused delayed mortality, while carbamate insecticides caused mortality extremely quickly, although both insecticide classes have similar modes of action: inhibiting the acetylcholinesterase enzyme from hydrolyzing acetylcholine (Fukuto 1990). Despite the differences in kill rates for carbamates and organophosphates, L. longipalpis and P. papatasi are most susceptible to the carbamates bendiocarb and propoxur and to the organophosphate fenitrothion. A 30-min exposure to these insecticides is sufficient to cause 100% mortality in these sand fly species. Aedes and Anopheles mosquitoes both have diagnostic LTs of 30 min for bendiocarb and fenitrothion using the CDC bottle bioassay (CDC 2010). For vector control programs aimed at targeting sand flies with synthetic insecticides, bendiocarb, propoxur, and fenitrothion deserve attention for their efficacy.
Conversely, of the 10 insecticides tested, both L. longipalpis and P. papatasi are least susceptible to DDT. Even with an exposure time of 120 min, the longest exposure time of the 10 insecticides, both species’ LC95’s are very large: at least 400 µg DDT per bottle. Unlike pyrethroids, which inhibit the sodium channels involved in action potential propagation in the central nervous system and in the peripheral nervous system, DDT only blocks the sodium channels in the peripheral nervous system (Davies et al. 2007). Only affecting the peripheral nervous system requires more time and higher doses to cause excitatory paralysis that leads to death (Davies et al. 2007). Similar results have been found in insecticide-susceptible Italian P. perniciosus and P. papatasi, where the LT50’s and LT90’s for DDT were longer compared with permethrin and lambda-cyhalothrin (Maroli et al. 2002). Also, Saeidi et al. (2012) found both insecticide-susceptible male and female P. papatasi to have much longer LT50’s and LT90’s to DDT than to permethrin, deltamethrin, cyfluthrin, and lambda-cyhalothrin.
For many years, DDT has been used worldwide to control sand flies by direct intervention or inadvertently as a collateral benefit of antimalaria campaigns (Kaul et al. 1994, Alexander and Maroli 2003, Surendran et al. 2005, Kishore et al. 2006, Dinesh et al. 2010, Afshar et al. 2011, Faraj et al. 2012, Saeidi et al. 2012). Our results suggest that laboratory colonies of insecticide-susceptible sand flies are not very susceptible to DDT. Despite reports of sand fly tolerance and resistance to DDT in India, Iran, Nepal, and Turkey (WHO 1986, Kaul et al. 1994, Yaghoobi-Ershadi and Javadian 1995, Dinesh et al. 2010, Afshar et al. 2011), DDT’s use for indoor residual spraying is still permitted (WHO 2007). The data from this study suggest that large doses of DDT are required, which may produce strong selection pressure for resistance if it not applied correctly or at appropriate times (Maharaj 2011). Compounded with years of DDT use, and the potential for underlying low levels of tolerance and resistance, field populations of sand flies may be able to develop resistance to DDT more quickly than to other insecticides.
The shedding of legs in response to exposure to the four pyrethroids and DDT used in this study was evident for both L. longipalpis and P. papatasi. A similar phenomenon was observed in L. longipalpis from Brazil when exposed to permethrin, deltamethrin, and lambda-cyhalothrin (Alexander et al. 2009). It is suggested that sand flies lacking one or more legs will be unable to blood-feed effectively, which could subsequently reduce the potential to vector Leishmania parasites (Alexander et al. 2009). However, we have consistently observed that laboratory L. longipalpis and P. papatasi exposed to pyrethroids that have shed one or more legs are still capable of blood-feeding on anesthetized mice (unpublished data). Female sand flies with shed legs, and with a mature Leishmania infection, which probe the skin of a vertebrate host, have also been shown to transmit Leishmania parasites without a complete blood-meal. During probing, Leishmania metacyclic promastigotes are regurgitated in attempt of the female sand fly to clear her alimentary canal of the Leishmania-secreted promastigote secretory gel (PSG) (“blocked-fly hypothesis”) (Bates 2007).
One future study could quantify and evaluate the ability of surviving sand flies, with shed legs that have been routinely exposed to pyrethroids or DDT, to persist with probing vertebrate hosts. Rogers and Bates (2007) demonstrated that female sand flies infected with Leishmania metacyclic promastigotes are manipulated by the Leishmania to increase their biting persistence, leading to an increase in the number of parasites transmitted to the vertebrate host. We have observed that a loss of legs is a potential physical challenge for the female sand fly. When other sand flies are in the vicinity of the female with shed legs during a blood feeding event, the female with shed legs would often lose her balance and would need to relocate to find a suitable position to probe and blood-feed. Increased probing because of a physical challenge, in combination with Leishmania manipulation, could theoretically increase probing and the number of parasites vectored to a host. These hypothetical scenarios apply to pyrethroid and DDT insecticides. Future studies with organophosphate and carbamate insecticides, which do not cause sand flies to shed their legs, and their effect on surviving flies’ ability to probe and transmit Leishmania warrant investigation as well.
Another observation of this study is the difference between the LC values of the Type I and Type II pyrethroid insecticides. Type I pyrethroids, including permethrin, have been described to cause sodium channel modifications that can last up to tens of milliseconds and are better at causing knockdown in insects. Whereas Type II pyrethroids, including cypermethrin, deltamethrin, and lambda-cyhalothrin, cause sodium channel modifications that can last for many seconds and are better at causing mortality in insects (Davies et al. 2007). In this study, permethrin LC50’s for both L. longipalpis and P. papatasi were greater than cypermethrin, deltamethrin, and lambda-cyhalothrin LC50’s (Fig. 2A; Table 3). These findings at the LC50 support previous research and are consistent with the physiological differences between the two types of pyrethroids in that it takes a higher concentrations of permethrin (Type I pyrethroid) to cause 50% mortality than it does cypermethrin, deltamethrin, or lambda-cyhalothrin (Type II pyrethroids; Fletcher and Axtell 1993, Jirakanjanakit et al. 2007).
One potential limitation of this study is that we used well-established, laboratory-adapted strains of L. longipalpis and P. papatasi. All the female sand flies used in this experiment were nulliparous. Comparisons of the efficacy of the 10 insecticides between parous and nulliparous females would be extremely difficult. Through several years of laboratory observation, the percent survival of gravid females after oviposition is extremely low. This low survivorship presents a challenge to replicate this experiment in parous females. In addition, lethal concentrations and lethal times from insecticide-susceptible laboratory and field-collected sand flies may differ. This is why determining LCs and LTs for susceptible laboratory strains are imperative for using a bioassay on field populations. Due to the highly variable conditions in nature, wild sand flies may exhibit different development times, body sizes, longevity, behaviors, and physiologies that make them more or less susceptible to insecticides (Rivero et al. 2010).
In the initial development of the bottle bioassay by Brogdon and McAllister (1998b), 250-ml Wheaton bottles were used. These sized glass bottles are now recommended for all bottle assays (CDC 2010), although Alexander et al. (2009) used 200-ml Wheaton glass bottles. Another potential limitation of this study is that owing to availability, 0.5-gallon and 1,000-ml glass bottles were used. For both L. longipalpis and P. papatasi, the deltamethrin, fenitrothion, chlorpyrifos, propoxur, and DDT exposure trials were completed using both the 0.5-gallon and the 1,000-ml bottles. In these situations, when the bottles of one size were temporarily unavailable (e.g., being cleaned for reuse), the other size bottles were used. Therefore, the survival curves for these insecticides were generated by combining the mortalities from the 0.5-gallon and from the 1,000-ml bottles. Comparatively, the mortalities between the bottle sizes were similar, but often the percent mortality was higher in the smaller 1,000-ml bottles than in the 0.5-gallon bottles. Despite an equal concentration of insecticide and the even coating of insecticide, an unequal density of sand flies exposed, 20–30 and 40–50 in the 1,000-ml and 0.5-gallon bottles, respectively, or potential differences in air volume to bottle surface area may explain the differing mortalities.
Using a modified bioassay that combines aspects of the CDC bottle bioassay and the WHO exposure kit bioassay allowed us to manipulate insecticide concentrations to collect dose–response survival curve data and to determine LCs and LTs. In our experiments, to determine LCs and LTs, a 24-h holding period was incorporated for all 10 insecticides after insecticide exposure (Saavedra-Rodriguez et al. 2008, Norris and Norris 2011). A 24-h holding period was used because many of the sand flies that scored as dead following the insecticide exposure were able to completely recover. We suggest that the additional 24 h of recovery time provided more precise susceptibility data than seen immediately at the end of the insecticide exposure period. Using the data from this study, a future direction could still be to determine diagnostic doses and diagnostic times for L. longipalpis and P. papatasi using the CDC bottle bioassay for these same 10 insecticides. With these future data, researchers and public health administrators will have diagnostic doses and diagnostic times comparable with what is available for Aedes and Anopheles mosquitoes (CDC 2010). Having diagnostic doses and diagnostic times for phlebotomine sand flies will enable field researchers to assess the insecticide susceptibility status of sand fly populations in the wild using the CDC bottle bioassay.
The CDC recommends determining diagnostic concentrations and diagnostic times from time–response mortality curves (CDC 2010). To assess an insect populations’ insecticide susceptibility status, diagnostic concentrations and diagnostic times are used (CDC 2010). A diagnostic dose is the dose of an insecticide that kills 100% of susceptible insects within a given time, the diagnostic time. Because we used our assays to produce dose–response survival curves, we were insufficiently able to determine diagnostic doses and diagnostic times, even though doses causing 100% mortality were discovered. QCal cannot determine LC100 values (diagnostic doses) because an insecticide concentration causing empirical 100% mortality cannot be determined with a logistic regression because 100% mortality is the upper asymptote. When put into the model, doses causing 100% mortality empirically are adjusted to causes mortality <100%. In time–response mortality curves, mortality from an insecticide dose is measured at distinct time intervals during the exposure test. Percent mortality is then plotted at each time interval (Brogdon and McAllister 1998b). A time–response diagnostic dose is the lowest concentration of insecticide that causes 100% mortality in a specified exposure time period, between 30 and 60 min (CDC 2010). A diagnostic dose and diagnostic time can both serve as reference points to understand the insecticide susceptibility of a population of insects (WHO 1998).
The baseline LCs and LTs for each insecticide were determined for laboratory L. longipalpis and P. papatasi and can now be incorporated as comparative reference points in field assays measuring the insecticide susceptibility of sand flies. The CDC recommends determining diagnostic doses and diagnostic times for an insecticide for each vector species in a specific geographic region (CDC 2010). Similarly, the LCs and LTs from this experiment should not be considered universal for L. longipalpis or P. papatasi. The data from this study should be used only as a reference point for future determinations of diagnostic doses and diagnostic times for different populations of Phlebotomus and Lutzomyia around the world.
Insecticide resistance management requires control programs to monitor for resistance (Surendran et al. 2005, Badolo et al. 2012). Insecticide resistance resulting from poor timing of insecticide application or from incorrect dosage applications can lead to ineffective vector control programs. Where insecticides are used, resistance monitoring will ensure that appropriate insecticides and dosages are applied at times when they will most effectively control the target vectors (Maharaj 2011). This modified version of the CDC bottle bioassay and the WHO exposure kit assay can help to inform researchers and epidemiologists of sand fly populations that are resistant to specific insecticides or to entire insecticide classes. It is vital to continue to further develop integrated public health management programs that include effective vector surveillance and control.
Acknowledgments
We are grateful to J.C. McAllister and W.G. Brogdon (CDC) for their careful review and constructive critique of the manuscript. We thank the many undergraduate research students in the Bernhardt laboratory for their assistance with maintaining and rearing the sand fly colonies. The maintenance of SKH1 hairless mice (Charles River, Wilmington MA) and the experimental animal-use protocol was approved by Utah State University’s Institutional Animal-Care and Use Committee. This work was supported by Utah State University’s Office of Research and Graduate Studies.
References Cited
- Afshar A. A., Rassi Y., Sharifi I., Abai M. R., Oshaghi M. A., Yaghoobi-Ershadi M. R., Vatandoost H. 2011. Susceptibility status of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) to DDT and deltamethrin in a focus of cutaneous leishmaniasis after earthquake strike in Bam, Iran. Iran J. Arthropod-Borne Dis. 5: 32–41. [PMC free article] [PubMed] [Google Scholar]
- Aïzoun N., Ossè R., Azondekon R., Alia R., Oussou O., Gnanguenon V., Aikpon R., Padonou G. G., Akbogbéto M. 2013. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, West Africa. Parasit Vectors 6: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B., Maroli M. 2003. Control of phlebotomine sand flies. Med. Vet. Entomol. 17: 1–18. [DOI] [PubMed] [Google Scholar]
- Alexander B., Barros V. C., Souza S. F. de, Barros S. S., Teodoro L. P., Soares Z. R., Gontijo N. F., Reithinger R. 2009. Susceptibility to chemical insecticides of two Brazilian populations of the visceral leishmaniasis vector Lutzomyia longipalpis (Diptera: Psychodidae). Trop. Med. Int. Health 14: 1272–1277. [DOI] [PubMed] [Google Scholar]
- Badolo A., Traore A., Jones C. M., Sanou A., Flood L., Guelbeogo W. M., Ranson H., Sagnon N. 2012. Three years of insecticide resistance monitoring in Anopheles gambiae in Burkina Faso: resistane on the rise. Malar. J. 11: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. A. 2007. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int. J. Parasitol. 37: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman Y., Chizov-Ginzburg A., Pener H., Wilamowski A. 2004. Susceptibility and repellency of Culicoides imicola and Culex pipiens to lambda-cyhalothrin. Vet. Ital. 40: 336–339. [PubMed] [Google Scholar]
- Brogdon W. G., McAllister J. C. 1998a. Insecticide resistance and vector control. Emerg. Infect. Dis. 4: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon W. G., McAllister J. C. 1998b. Simplification of adult mosquito bioassays through the use of time-mortality determinations in glass bottles. J. Am. Mosq. Control Assoc. 14: 159–164. [PubMed] [Google Scholar]
- (CDC) Centers for Disease Control and Prevention. 2010. Methods in Anopheles Research. Second Edn, CDC Technical Report; CDC, Atlanta, GA. [Google Scholar]
- Chen C. D., Low V. L., Lau K. W., Lee H. L., Nazni W. A., Heo C. C., Azidah A. A., Sofian-Azirun M. 2013. First report on adulticide susceptibility status of Aedes albopictus, Culex quinquefasciatus, and Culex vishnui from a pig farm in Tanjung Sepat, Selangor, Malaysia. J. Am. Mosq. Control 29: 243–250. [DOI] [PubMed] [Google Scholar]
- Davies T.G.E., Field L. M., Usherwood P.N.R., Williamson M. S. 2007. DDT, pyrethrin, pyrethroids, and insect sodium channels. IUBMB Life 59: 151–162. [DOI] [PubMed] [Google Scholar]
- Dinesh D. S., Das M. L., Picado A., Roy L., Rijal S., Singh S. P., Das P., Boelaert M., Coosemans M. 2010. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl. Trop. Dis. 4: e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj C., Ouahabi S., Adlaoui E. B., Elkohli M. E., Lakraa L., El Rhazi M., Ameur B. 2012. Insecticide susceptibility of Phlebotomus (Paraphlebotomus) sergenti and Phlebotomus (Phlebotomus) papatasi in endemic foci of cutaneous leishmaniasis in Morocco. Parasit. Vectors 5: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyereisen R. 1995. Molecular biology of insecticide resistance. Toxicol. Lett. 82/83: 83–90. [DOI] [PubMed] [Google Scholar]
- Fletcher M. G., Axtell R. C. 1993. Susceptibility of the bedbug, Cimex lectularis, to selected insecticides and various treated surfaces. Med. Vet. Entomol. 7: 69–72. [DOI] [PubMed] [Google Scholar]
- Fukuto T. R. 1990. Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 87: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin P. J., Olliaro P., Sundar S., Boelaert M., Croft S. L., Desjeux P., Wasunna M. K., Bryceson D. M. 2002. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2: 494–501. [DOI] [PubMed] [Google Scholar]
- Hassan M. M., Widaa S. O., Osman O. M., Numiary M.S.M., Ibrahim M. A., Abushama H. M. 2012. Insecticide resistance in the sand fly, Phlebotomus papatasi from Khartoum State, Sudan. Parasit. Vectors 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. 2000. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45: 371–391. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Field L., Vontas J. 2002. An overview of insecticide resistance. Science 298: 96–97. [DOI] [PubMed] [Google Scholar]
- Henriquez C., Pereira Y., Cochero S., Bejarano E. E. 2009. Dosis diagnóstica y umbral de resistencia de Lutzomyia evansi (Diptera: Psychodidae), a dos insecticidas utilizados en salud pública en Colombia: Deltametrina y lambdacihalotrina. Rev. Entomol. Soc. Argent. Mendoza 68: 287–294. [Google Scholar]
- Jirakanjanakit N., Rongnoparut P., Saengtharatip S., Chareonviriyaphap T., Duchon S., Bellec C., Yoksan S. 2007. Insecticide susceptible/resistant status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J. Econ. Entomol. 100: 545–550. [DOI] [PubMed] [Google Scholar]
- Kaul S. M., Sharma R. S., Dey K. P., Rai R. N., Verghese T. 1994. Impact of DDT indoor residual spraying on Phlebotomus argentipes in a kala-azar endemic village in eastern Uttar Pradesh. Bull. World Health Organ. 72: 79–81. [PMC free article] [PubMed] [Google Scholar]
- Kishore K., Kumar V., Kesari S., Dinesh D. S., Kumar A. J., Das P., Bhattacharya S. K. 2006. Vector control in leishmaniasis. Indian J. Med. Res. 123: 467–472. [PubMed] [Google Scholar]
- Lawyer P. G., Rowton E. D., Perkins P. V., Johnson R. N., Young D. G. 1991. Recent advances in laboratory mass rearing of phlebotomine sand flies. Parassitologia 33: 361–364. [PubMed] [Google Scholar]
- Lozano-Fuentes S., Saavedra-Rodriguez K., Black W. C., IV, Eisen L. 2012. QCal: A software application for the calculation of dose-response curves in insecticide resistance bioassays. J. Am. Mosq. Control Assoc. 28: 59–61. [DOI] [PubMed] [Google Scholar]
- Maharaj R. 2011. Global trends in insecticide resistance and impact on disease vector control measures. OAIP 3: 27–33. [Google Scholar]
- Mallet J. 1989. The evolution of insecticide resistance: have the insects won? Trends Ecol. Evol. 4: 336–340. [DOI] [PubMed] [Google Scholar]
- Maroli M, Cianchi T., Bianchi R., Khoury C. 2002. Testing insecticide susceptibility of Phlebotomus perniciosus and P. papatasi (Diptera: Psychodidae) in Italy. Ann. 1st Super. Sanità. 38: 419–423. [PubMed] [Google Scholar]
- Martins A. J., Lins R.M.M.A., Linss J.G.B., Peixoto A. A., Valle D. 2009. Voltage-gated sodium channel polymorphism and metabolic resistance in pyrethroid-resistant Aedes aegypti from Brazil. Am. J. Trop. Med. Hyg. 81: 108–115. [PubMed] [Google Scholar]
- Modi G. B., Rowton E. D. 1999. Laboratory maintenance of phlebotomine sand flies, pp. 109–121. In Maramorosch K., Mahmood F. (eds.), Maintenance of human, animal, and plant pathogen vectors. Oxford & IBH Publishing Co. PVT Ltd., New Delhi, India. [Google Scholar]
- Nauen R. 2007. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 63: 628–633. [DOI] [PubMed] [Google Scholar]
- Norris L. C., Norris D. E. 2011. Insecticide resistance in Culex quinquefasciatus mosquitoes after the introduction of insecticide-treated bed nets in Macha, Zambia. J. Vector Ecol. 36: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo C. B., Salazar-Terreros M. J., Mina N. J., McAllister J., Brogdon W. 2011. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop. 118: 37–44. [DOI] [PubMed] [Google Scholar]
- Perea E. Z., León R. B., Salcedo M. P., Brogdon W. G., Devine G. J. 2009. Adaptation and evaluation of the bottle assay for monitoring insecticide resistance in disease vector mosquitoes in the Peruvian Amazon. Malar. J. 8: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A., Vézilier J., Weill M., Read A. F., Gandon S. 2010. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 6: e1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. E., Bates P. A. 2007. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 3: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge L. C., Gupta R. K. 2009. Moth flies and sand flies (Psychodidae), pp. 153–168. In Mullen G. R., Durden L. A. (eds.), Medical and veterinary entomology. Elsevier, Inc., Burlington, MA. [Google Scholar]
- Saavedra-Rodriguez K., Strode C., Suarez A. F., Salas I. F., Ranson H., Hemingway J., Black W. C., IV 2008. Quantitative trait loci mapping of genome regions controlling permethrin resistance in the mosquito Aedes aegypti. Genet. 180: 1137–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi Z., Vatandoost H., Akhavan A. A., Yaghoobi-Ershadi M. R., Rassi Y., Sheikh Z., Arandian M. H., Jafari R., Dehkordi A.R.S. 2012. Baseline susceptibility of a wild strain of Phlebotomus papatasi (Diptera: Psychodidae) to DDT and pyrethroids in an endemic focus of zoonotic cutaneous leishmaniasis in Iran. Pest Manag. Sci. 68: 669–675. [DOI] [PubMed] [Google Scholar]
- Santamaría E., Munstermann L. E., Ferro C. 2003. Aproximación al método CDC para determiner susceptibilidad a insecticidas en vectores de leishmaniasis. Biomédica 23: 115–121. [PubMed] [Google Scholar]
- Scott J. G. 1999. Cytochrome P450 and insecticide resistance. Insect Biochem. Mol. Biol. 29: 757–777. [DOI] [PubMed] [Google Scholar]
- Surendran S., Karunaratne S.H.P.P., Adams Z., Hemingway J., Hawkes N. J. 2005. Molecular and biochemical characterization of a sand fly population from Sri Lanka: Evidence for insecticide resistance due to altered esterases and insensitive acetylcholinesterase. Bull. Entomol. Res. 95: 371–380. [DOI] [PubMed] [Google Scholar]
- Volf P., Volfova V. 2011. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 36: S1–S9. [DOI] [PubMed] [Google Scholar]
- (WHO) World Health Organization. 1986. Resistance of vectors and reservoirs of disease to pesticides. WHO, Geneva, Switzerland. [Google Scholar]
- (WHO) World Health Organization. 1998. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy, and persistence of insecticide on treated surfaces. WHO, Geneva, Switzerland. [Google Scholar]
- (WHO) World Health Organization. 2006. Pesticides and their application for the control of vectors and pests of public health importance. WHO, Geneva, Switzerland. [Google Scholar]
- (WHO) World Health Organization. 2007. The use of DDT in malaria vector control: WHO position statement. WHO, Geneva, Switzerland. [Google Scholar]
- (WHO) World Health Organization. 2013. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. WHO, Geneva, Switzerland. [Google Scholar]
- Yaghoobi-Ershadi M. R., Javadian E. 1995. Susceptibility status of Phlebotomus papatasi to DDT in the most important focus of zoonotic cutaneous leishmaniasis, Isfahan Province, Iran. Iranian J. Public Health 24: 11–20. [Google Scholar]
- Young D. G., Perkins P. V., Endris R. G. 1981. A larval diet for rearing phlebotomine sand flies (Diptera: Psychodidae). J. Med. Entomol. 18: 446. [Google Scholar]