Summary
Background
A systematic assessment of potential disease-modifying compounds for Parkinson's disease concluded that pioglitazone could hold promise for the treatment of patients with this disease. We assessed the effect of pioglitazone on the progression of Parkinson's disease in a multicentre, double-blind, placebo-controlled, futility clinical trial.
Methods
Participants with the diagnosis of early Parkinson's disease on a stable regimen of 1 mg/day rasagiline or 10 mg/day selegiline were randomly assigned (1:1:1) to 15 mg/day pioglitazone, 45 mg/day pioglitazone, or placebo. Investigators were masked to the treatment assignment. Only the statistical centre and the central pharmacy knew the treatment name associated with the randomisation number. The primary outcome was the change in the total Unified Parkinson's Disease Rating Scale (UPDRS) score between the baseline and 44 weeks, analysed by intention to treat. The primary null hypothesis for each dose group was that the mean change in UPDRS was 3 points less than the mean change in the placebo group. The alternative hypothesis (of futility) was that pioglitazone is not meaningfully different from placebo. We rejected the null if there was significant evidence of futility at the one-sided alpha level of 0.10. The study is registered at ClinicalTrials.gov, number NCT01280123.
Findings
210 patients from 35 sites in the USA were enrolled between May 10, 2011, and July 31, 2013. The primary analysis included 72 patients in the 15 mg group, 67 in the 45 mg group, and 71 in the placebo group. The mean total UPDRS change at 44 weeks was 4.42 (95% CI 2.55–6.28) for 15 mg pioglitazone, 5.13 (95% CI 3.17–7.08) for 45 mg pioglitazone, and 6.25 (95% CI 4.35–8.15) for placebo (higher change scores are worse). The mean difference between the 15 mg and placebo groups was −1.83 (80% CI −3.56 to −0.10) and the null hypothesis could not be rejected (p=0.19). The mean difference between the 45 mg and placebo groups was −1.12 (80% CI −2.93 to 0.69) and the null hypothesis was rejected in favour of futility (p=0.09). Planned sensitivity analyses of the primary outcome, using last value carried forward (LVCF) to handle missing data and using the completers' only sample, suggested that the 15 mg dose is also futile (p=0.09 for LVCF, p=0.09 for completers) but failed to reject the null hypothesis for the 45 mg dose (p=0.12 for LVCF, p=0.19 for completers). Six serious adverse events occurred in the 15 mg group, nine in the 45 mg group, and three in the placebo group; none were thought to be definitely or probably related to the study interventions.
Interpretation
These findings suggest that pioglitazone at the doses studied here is unlikely to modify progression in early Parkinson's disease. Further study of pioglitazone in a larger trial in patients with Parkinson's disease is not recommended.
Funding
National Institute of Neurological Disorders and Stroke.
Introduction
Parkinson's disease affects nearly 1% of the population aged over 60 years.1 Despite effective therapies to treat symptoms of Parkinson's disease and many clinical trials,2,3 no interventions have been proven to slow progression of disability (ie, achieve disease modification). In 2001, the National Institute of Neurological Disorders and Stroke (NINDS) created the Neuroprotection Exploratory Trials of Parkinson's Disease (NET-PD) programme to assess therapies to slow progression of disability in Parkinson's disease (based on recommendations by the Committee to Identify Neuroprotective Agents in Parkinson's [CINAPS]).4 Pioglitazone was selected through a rigorous systematic review of agents to be tested by the NET-PD network as a potential disease-modifying agent in early Parkinson's disease.4 Pioglitazone is approved by the US Food and Drug Administration (FDA) for the treatment of type 2 diabetes and acts to reduce insulin resistance; it belongs to the class of thiazolidinediones, the peroxisome proliferator-activated receptor γ (PPAR-γ) agonists. Preclinical and early clinical evidence suggests that thiazolidinediones might have neuroprotective effects in Parkinson's disease and other neurodegenerative diseases.5–9 Although the precise mechanisms through which PPAR-γ agonists might provide neuroprotection are still unclear, they inhibit the activation of microglia and astrocytes and production of pro-inflammatory cytokines and nitric oxide.10 PPAR-γ coactivator 1-α (PGC-1α) is a transcriptional coactivator that controls mitochondrial biogenesis and oxidative stress.11 Preclinical data in rodent and primate Parkinson's disease models showed good CNS penetration of pioglitazone and neuroprotective effects at a dose in animals that is the equivalent of the FDA-approved dose for use in human beings.11–14
Our primary objective was to assess the effect of pioglitazone on the progression of Parkinson's disease to establish whether further study of this agent is futile. The study known as FS-ZONE was based on the futility design (non-superiority) that has been used in two phase 2 studies done by NET-PD.15,16 Futility studies are phase 2 clinical trials designed to identify and eliminate compounds that have low likelihood of being efficacious in definitive efficacy studies by comparing the primary outcome measure in the treatment group versus placebo to a prespecified threshold value.17,18 Progression of Parkinson's disease was measured by change in total Unified Parkinson Disease Rating Scale score (UPDRS parts I–III)19 between the baseline and the 44 week visit. If both doses of pioglitazone were non-futile, the plan was to select the dose that was associated with the smallest (better) change of the UPDRS score. A final decision about whether to continue studying that dose had also to take into account tolerability, toxicity, and other safety issues.
Methods
Study design and participants
The trial was a multicentre, three-group, double-blind, placebo-controlled parallel group study. The trial was organised by the Clinical Trials Coordination Center (CTCC) at the University of Rochester, NY, USA, the Statistical Center at the Division of Biostatistics, University of Texas School of Public Health, TX, USA, and the NINDS.
Participants were men and women aged 30 years or older with idiopathic Parkinson's disease based on UK Brain Bank diagnostic criteria20 diagnosed within 5 years of enrolment with a Hoehn and Yahr score of 2 or less. Participants had to be on a stable dosage of rasagiline 1 mg/day or selegiline 10 mg/day for at least 8 weeks but not more than 8 months and were expected to remain on that dose for the duration of the study. The rationale for enrolling patients on monoamine oxidase type B inhibitor (MAO-BI) therapy was to reduce the number of participants needing additional dopaminergic therapy during the study. Key exclusion criteria (the full list is in the appendix) included exposure to other dopaminergic Parkinson's disease therapy or amantadine within 60 days before baseline visit or for 90 days or more at any point in the past. Patients with diabetes (glycated haemoglobin [HgbA1c] ≥6% at screening) were excluded. All participants signed a written informed consent before entry into the study. The study was done in accordance with the Good Clinical Practice guidelines.
The steering committee developed the protocol and consent forms, and guided the implementation of the trial. The protocol and consent forms were approved by a NINDS-appointed oversight board, an independent data safety monitoring board (DSMB), and the institutional review boards of each of the participating sites. The DSMB monitored the safety, data integrity, and progress of the trial.
Randomisation and masking
Eligible patients with Parkinson's disease were randomly assigned (1:1:1) to one of three study groups: 15 mg/day pioglitazone, 45 mg/day pioglitazone, or placebo. Dose selection was based on the preclinical data showing a neuroprotective effect within 15–45 mg/kg human dose equivalent11–14 and these doses falling within the FDA-approved range for use in the human diabetic population. Participants and investigators were masked to treatment group. The Statistical Coordination Center generated the random allocation sequence using a permuted block randomisation scheme, and the sites accessed the masked treatment assignment via a secure webpage.
Pioglitazone was purchased from the manufacturer (Takeda Pharmaceuticals America, Deerfield, IL, USA). University of Iowa Pharmaceuticals over-encapsulated the active tablets and created the placebo to match in accordance with current Good Manufacturing Practice regulations. The University of Rochester Clinical Materials Services Unit provided packaging, labelling, and distribution of the study drug in participant-specific kits. Active study drug capsules contained 15 mg pioglitazone. Identical in appearance and taste, placebo contained microcrystalline cellulose.
Procedures
At the screening visit, participants had a baseline medical history interview, physical and neurological examination, electrocardiogram, UPDRS, and assessments of mood, cognition, and disability. Blood and urine were obtained for clinical laboratory tests, a pregnancy test for women of childbearing potential, and a test for HgbA1c. Blood and urine samples were also collected from consenting participants for measurement of exploratory biomarkers. After the baseline visit, participants were reassessed at 2 weeks (within 3 days) by telephone and at 4, 16, 28, and 44 weeks (within 5 days) in person.
Study drug was given orally, three capsules once daily. Titration of pioglitazone to 45 mg/day target dose occurred in 15 mg increments, once every 2 weeks. Dose reduction for intolerability was allowed at any point during the study and participants were maintained on the highest tolerated dose up to their assigned dose.
Outcomes
The primary outcome was change in total UPDRS score between the baseline visit and 44 weeks. Data for participants needing additional symptomatic therapy before 44 weeks were imputed for the primary analysis, but these participants continued to be followed for secondary analyses. Secondary outcome measures were the change in individual parts of the UPDRS, change in ambulatory capacity,21 change in Schwab and England Activities of Daily Living (SEADL) scale,22 change in Parkinson's Disease Questionnaire 39 (PDQ-39),23 change in Mattis Dementia Rating Scale (DRS),24 and change in the Geriatric Depression Scale (GDS-15).25 Secondary measures of safety and tolerability were the proportion of participants who completed the study on their assigned dose of study drug, the adverse event frequency and severity, changes in vital signs, and clinical laboratory values. Exploratory outcome measures included the effect of pioglitazone on blood and urine biomarkers and the association of concentrations of these biomarkers with change in total UPDRS score. Results of the biomarkers analyses will be reported separately. The University of Pennsylvania Smell Identification Test (UPSIT) was included in the initial protocol as an exploratory measure, but owing to budgetary constraints no UPSIT data were collected at follow-up.
Statistical analysis
For each dose group, the primary null hypothesis was that pioglitazone reduces the mean UPDRS decline over 44 weeks by 3 points or more compared with placebo. The alternative hypothesis (of futility) was that pioglitazone is not meaningfully different from placebo. The statistical hypotheses were H0: μ ≤ μp – 3 versus HA: μ > μp – 3, where μ was the expected total UPDRS change score from baseline to 44 weeks for the active treatment group and μp was the expected placebo total UPDRS change score. If the null hypothesis was rejected at a significance level of 0.10, then the drug would be unlikely to be effective and not considered for further investigation.
To estimate the sample size, we used the UPDRS change for patients treated with rasagiline (1 mg daily) in the controlled trial of efficacy of rasagiline in early Parkinson's disease (TEMPO).26 The approximate mean UPDRS change from 8 weeks to 52 weeks (ie, 44 weeks) was 4.5 (SD 8.0), which provided an estimate of μp and was the rationale for the 44 week duration of our study. Under the null hypothesis we assumed μ to be μp – 3, which is 1.5. Under these assumptions (H0: μ=1.5 and HA: μ=4.5), with 65 patients per group, a two-sample t-test at 0.10 one-sided significance level had 80% power to reject the null hypothesis and declare futility if a true difference existed. Assuming a dropout rate of 5%, the sample size was inflated from 65 to 72 patients per treatment group.27
Under the intention-to-treat principle all randomly assigned patients were included in the primary analyses. Patients who needed additional symptomatic therapy before 44 weeks were considered missing primary outcome data. Missing 44 week UPDRS assessments were imputed using multiple imputation28 by using all available data for each individual (before the add-on of additional symptomatic therapy) and adjusting for the need for additional symptomatic therapy (indicator variable), the length of time on rasagiline or selegiline at baseline, and treatment group. The multiple imputation procedure assumed a monotone missing mechanism, missing at random, and used 20 imputed datasets. The primary analysis was adjusted for length of time on rasagiline or selegiline at baseline as a fixed effect and enrolling site as a random effect in a mixed-effects linear model. Since this was a phase 2 study, the type I error rate was relaxed, and there was no correction for multiplicity for the two dose group comparisons. Planned secondary analyses of the primary outcome were last value carried forward (LVCF), in which the last observation before additional symptomatic therapy was carried forward to 44 weeks, and an analysis of completers, which included participants who had a 44 week visit including ones who started additional symptomatic therapy. As a post-hoc sensitivity analysis, a mixed-effects model was estimated that included the repeated UPDRS measures available for all patients (assuming a first-order autoregressive correlation structure for repeated measures) and was adjusted for baseline UPDRS, clinical site (as a random effect), and time on rasagiline or selegiline at baseline. For the secondary outcomes, the means and 95% CIs were reported by treatment group. A non-parametric global statistical test was the prespecified analysis for the secondary outcomes, to test whether each active treatment group had less progression compared with placebo as measured by SEADL, PDQ-39, ambulatory capacity, and Mattis-DRS. Missing data were imputed via multiple imputation. Patients were ranked on each outcome, and then the ranks were summed. Higher ranks were worse. The study is registered at ClinicalTrials.gov, number NCT01280123.
Role of the funding source
The study sponsor did not participate in the design of the study, but a representative of NINDS (D Babcock, NINDS) participated in data interpretation and in the writing of the report. NINDS approved the study protocol, had an oversight role in the data collection and analysis, and reviewed and approved the decision to submit the paper for publication. All authors had full access to all of the data in the study, and TS had responsibility for the final decision to submit the report for publication.
Results
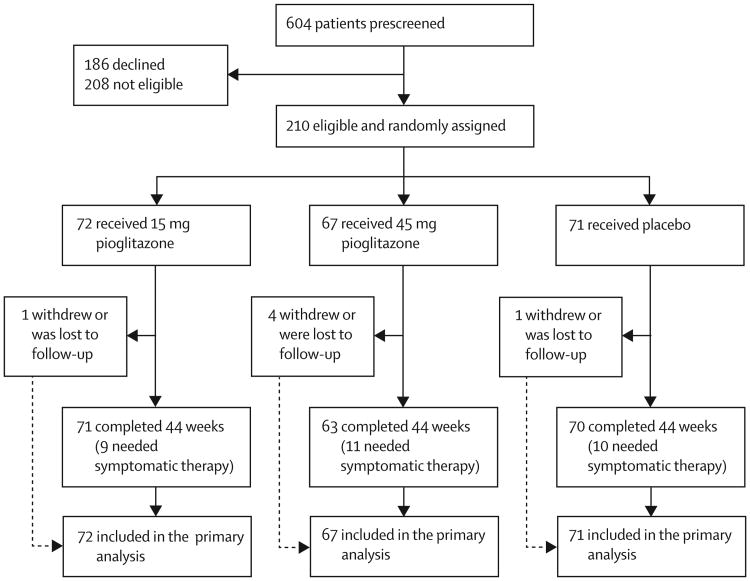
Between May 10, 2011, and July 31, 2013, 604 potential participants were identified from pre-screening chart review. Of these, 208 were ineligible, 186 declined study participation, and the remaining 210 eligible patients were randomly assigned to the three treatment groups at 35 sites (figure 1). Baseline demographics and clinical characteristics were similar across the three treatment groups except for a difference in GDS-15 (table 1).
Figure 1. Trial profile.
Table 1. Baseline demographics and clinical characteristics.
| 15 mg pioglitazone (n=72) | 45 mg pioglitazone (n=67) | Placebo (n=71) | |
|---|---|---|---|
| Age (years) | 61.3 (10.6) | 58.8 (9.2) | 59.0 (9.9) |
| Years of education | 17.1 (3.1) | 16.2 (3.3) | 16.8 (3.0) |
| Males | 53 (74%) | 47 (70%) | 48 (68%) |
| Non-latino whites | 58 (81%) | 63 (94%) | 63 (89%) |
| Right-handed | 62 (86%) | 62 (93%) | 62 (87%) |
| Duration of PD symptoms (years) | 2.3 (1.9) | 2.0 (1.2) | 2.3 (2.3) |
| Time since PD diagnosis (years) | 0.8 (07) | 0.7 (0.7) | 0.8 (0.7) |
| UPDRS total | 23.8 (9.9) | 21.2 (8.8) | 21.7 (8.7) |
| UPDRS mental | 0.8 (0.9) | 0.8 (0.9) | 0.9 (1.1) |
| UPDRS motor | 17.1 (7.7) | 15.0 (7.1) | 15.3 (6.5) |
| UPDRS ADL | 5.9 (3.2) | 5.5 (2.9) | 5.5 (3.0) |
| Ambulatory capacity | 1.1 (0.9) | 0.8 (0.8) | 1.1 (0.9) |
| SEADL* | 93.8 (4.9) | 94.1 (5.0) | 93.9 (5.0) |
| PDQ-39 Summary Index | 8.5 (8.1) | 8.1 (5.9) | 10.6 (7.9) |
| GDS-15 | 1.4 (1.4) | 1.1 (1.3) | 1.8 (1.9) |
| Mattis-DRS* | 138.6 (8.2) | 138.8 (10.2) | 138.0 (11.4) |
| Months on rasagiline or selegiline | 4.1 (2.2) | 4.0 (2.0) | 3.7 (1.9) |
| GDS-15 ≥5 | 2 (2.8%) | 1 (1.5%) | 10 (14.1%) |
| Rasagiline use | 60 (83.3%) | 57 (85.1%) | 60 (84.5%) |
| Selegiline use | 12 (16.7%) | 10 (14.9%) | 11 (15.5%) |
Data are mean (SD) or n (%). UPDRS=Unified Parkinson's Disease Rating Scale. ADL=activities of daily living. SEADL=Schwab and England Activities of Daily Living scale. PDQ-39=Parkinson's Disease Questionnaire 39. GDS-15=15-item Geriatric Depression Scale. DRS=Dementia Rating Scale.
Higher scores are associated with less severe clinical presentation; for all other clinical symptoms, higher scores are associated with more severe presentation.
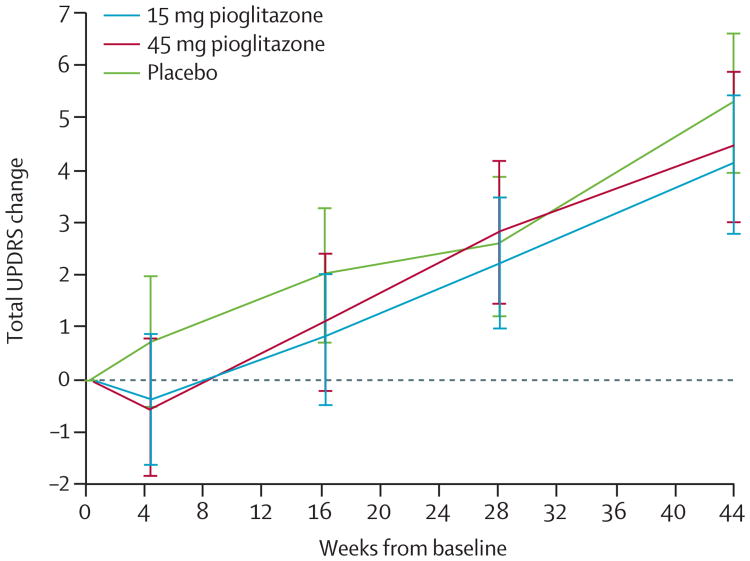
The primary analysis suggested that the 45 mg treatment was futile: the mean difference between the 45 mg and placebo groups was −1.12 (80% CI −2.93 to 0.69), and we rejected the null hypothesis that the 45 mg group was 3 or more points better than the placebo group (p=0.09). The primary analysis did not indicate futility for the 15 mg group: the mean difference between the 15 mg and placebo groups was −1.83 (80% CI −3.56 to −0.10), and the null hypothesis could not be rejected (p=0.19; table 2). Change in UPDRS from baseline to week 44 for each treatment group is presented in figure 2. The planned sensitivity analyses of the primary outcome suggested that the 15 mg treatment was futile in the last value carried forward (p=0.09) and completers only (p=0.09) analyses, and this was supported by the post-hoc repeated measures mixed model analyses (p=0.05). The planned analyses indicated that the null hypothesis could not be rejected for the 45 mg group (p=0.12 for the last value carried forward, p=0.19 for the completers) but the post-hoc repeated measures mixed model did not (p=0.04; table 2). For secondary efficacy outcomes, the mean changes from baseline to 44 weeks for the treatment groups were similar to placebo (table 3).
Table 2. Change in total Unified Parkinson's Disease Rating Scale from baseline to 44 weeks.
| Patients enrolled (n) | Patients analysed* (n) | Mean (95% CI) | H0:μ≤μi−3 t statistic† | p value (one sided) | |
|---|---|---|---|---|---|
| Intention-to-treat sample, multiple imputation‡ | |||||
|
| |||||
| 15 mg pioglitazone | 72 | 72 | 4.42 (2.55–6.28) | 0.88 | 0.19 |
| 45 mg pioglitazone | 67 | 67 | 5.13 (3.17–7.08) | 1.34 | 0.09 |
| Placebo | 71 | 71 | 6.25 (4.35–8.15) | ‥ | ‥ |
|
| |||||
| Last value carried forward (unadjusted)§ | |||||
|
| |||||
| 15 mg pioglitazone | 72 | 72 | 4.03 (2.14–5.92) | 1.35 | 0.09 |
| 45 mg pioglitazone | 67 | 67 | 3.87 (2.08–5.65) | 1.2 | 0.12 |
| Placebo | 71 | 71 | 5.34 (3.79–6.88) | ‥ | ‥ |
|
| |||||
| Completers only, actual value at 44 weeks (includes visits on additional dopaminergic therapy; unadjusted)§ | |||||
|
| |||||
| 15 mg pioglitazone | 72 | 71 | 3.35 (1.48–5.23) | 1.35 | 0.09 |
| 45 mg pioglitazone | 67 | 63 | 2.79 (0.55–5.03) | 0.89 | 0.19 |
| Placebo | 71 | 70 | 4.47 (2.78–6.17) | ‥ | ‥ |
|
| |||||
| Mixed model repeated measures¶ | |||||
|
| |||||
| 15 mg pioglitazone | 72 | 62 | 4.25 (2.75–5.75) | 1.69 | 0.05 |
| 45 mg pioglitazone | 67 | 53 | 4.42 (2.80–6.03) | 1.77 | 0.04 |
| Placebo | 71 | 60 | 5.39 (3.86–6.91) | ‥ | ‥ |
Number of patients contributing a 44 week measure.
For each dose group, the primary null hypothesis was that pioglitazone reduces the mean Unified Parkinson's Disease Rating Scale (UPDRS) decline over 44 weeks by 3 points or more compared with placebo; the alternative hypothesis (of futility) is that pioglitazone is not meaningfully different from placebo; except where indicated, the mean changes in UPDRS scores were adjusted for site (random effect) and time on rasagiline or selegiline at baseline.
Prespecified primary analysis.
Prespecified secondary analyses of the primary outcome.
Post-hoc sensitivity analysis (no imputation at 44 weeks; uses repeated measures for all 210 patients; patients without a 44 week assessment contributed to earlier timpoints only).
Figure 2. Change in total Unified Parkinson's Disease Rating Scale (UPDRS) over time by treatment group.
Means (95% CI) are adjusted for site, time on rasagiline or selegiline at baseline, and baseline UPDRS (from repeated measures mixed model). Assessments on participants taking additional symptomatic therapy were excluded.
Table 3. Secondary outcomes: change from baseline to 44 weeks.
| 15 mg pioglitazone (n=72) | 45 mg pioglitazone (n=67) | Placebo (n=71) | |
|---|---|---|---|
| UPDRS mental | 0.10 (−0.16 to 0.36) | 0.09 (−0.18 to 0.36) | 0.18 (−0.13 to 0.49) |
| UPDRS motor | 3.12 (1.79 to 4.46) | 3.10 (1.29 to 4.90) | 3.86 (2.47 to 5.26) |
| UPDRS ADL | 1.44 (0.76 to 2.12) | 1.44 (0.72 to 2.17) | 1.73 (0.9 to 2.56) |
| Ambulatory capacity | 0.39 (0.16 to 0.61) | 0.38 (0.07 to 0.70) | 0.40 (0.17 to 0.64) |
| SEADL* | −2.12 (−3.47 to −0.78) | −2.52 (−3.95 to −1.09) | −1.84 (−3.49 to −0.18) |
| PDQ-39 Summary Index | 2.03 (0.47 to 3.59) | 2.08 (0.32 to 3.84) | 0.08 (−1.59 to 1.76) |
| GDS-15 | 0.13 (−0.33 to 0.58) | 0.38 (−0.10 to 0.85) | 0.18 (−0.37 to 0.72) |
| Mattis-DRS* | 1.16 (−1.26 to 3.58) | 2.11 (−0.41 to 4.63) | 3.16 (0.66 to 5.65) |
Data are mean (95% CI). Secondary outcomes analyses include all patients enrolled and are change from baseline to 44 weeks. Missing data imputed with multiple imputation. Means are adjusted for site. UPDRS=Unified Parkinson's Disease Rating Scale. ADL=activities of daily living. SEADL=Schwab and England Activities of Daily Living scale. PDQ-39=Parkinson's Disease Questionnaire 39. GDS=Geriatric Depression Scale. DRS=Dementia Rating Scale.
Higher scores are associated with less severe clinical presentation; for all other clinical symptoms, higher scores are associated with more severe presentation.
In the non-parametric global statistical test, to assess the change from baseline to 44 weeks in SEADL, PDQ-39, ambulatory capacity, and Mattis-DRS, the mean (SD) summed rank was 435 (137) for the 15 mg group, 427 (139) for the 45 mg group, and 404 (141) for the placebo group (15 mg vs placebo, p=0.20; 45 mg vs placebo, p=0.37).
Of the 210 participants enrolled, 204 (97%) remained in the study for 44 weeks, and 195 (93%) remained active and on study drug. There were no deaths or treatment unmasking. Overall, 30 (14%) participants needed additional symptomatic therapy before 44 weeks (nine [13%] in the 15 mg group, 11 [16%] in the 45 mg group, and ten [14%] in the placebo group). Missing data occurred owing to the need for additional symptomatic therapy, withdrawal of consent, or loss to follow-up; these data were imputed for the total UPDRS at 44 weeks for 35 (17%) patients (ten in the 15 mg group, 14 in the 45 mg group, and 11 in the placebo group). Tolerability, defined as the proportion of the participants taking the assigned dose for 44 weeks, was slightly lower in the pioglitazone groups (62 of 72 in the 15 mg group [86%, 95% CI 78–94], 54 of 67 in the 45 mg group [81%, 71–90], and 67 of 71 in the placebo group [94%, 89–100]).
18 patients had serious adverse events. Six occurred in the 15 mg group (ovarian cyst ruptured, ankle fracture, atrial flutter, intestinal obstruction, and two cases of intervertebral disc protrusion or degeneration). Nine occurred in the 45 mg group (one each of spondylolisthesis, osteoarthritis requiring surgery, transient ischaemic attack, dehydration, myocardial infarction, intestinal obstruction, and dyspnoea, hypoxia, and respiratory failure, and two cases of confusion state). Three occurred in the placebo group (knee replacement, atrial fibrillation and hypertension, and coronary artery stenosis). None of the serious adverse events was judged as definitely or probably related to the study interventions by the masked investigators. The frequency of non-serious adverse events was similar across groups: 63 (88%) in the 15 mg group, 51 (76%) in the 45 mg group, and 59 (83%) in the placebo group. Expected adverse events included the spectrum of adverse events in the diabetic population, as summarised on the package insert. Table 4 shows the most frequently occurring adverse events. Cardiovascular events occurred most frequently in the placebo group (6% in 15 mg group, 9% in the 45 mg group, 11% in the placebo). Although a difference in the proportion of oedema events was detected between all three groups (Fisher's exact test, p=0.047), there was no significant pairwise difference versus placebo (Fisher's exact test, 45 mg group vs placebo, p=0.35; 15 mg vs placebo, p=0.16). No other statistically significant differences were noted.
Table 4. Frequently occurring adverse events.
| Placebo | 15 mg pioglitazone | 45 mg pioglitazone | Total | |
|---|---|---|---|---|
| Oedema | 9 (13%) | 4 (6%) | 13 (19%) | 26 (12%) |
| Cardiovascular events | 8 (11%) | 4 (6%) | 6 (9%) | 18 (9%) |
| Diarrhoea | 3 (4%) | 7 (10%) | 3 (4%) | 13 (6%) |
| Nausea | 3 (4%) | 6 (8%) | 7 (10%) | 16 (8%) |
| Raised blood creatine phosphokinase | 11 (15%) | 12 (17%) | 7 (10%) | 30 (14%) |
| Dizziness | 6 (8%) | 5 (7%) | 6 (9%) | 17 (8%) |
| Fatigue | 3 (4%) | 8 (11%) | 2 (3%) | 13 (6%) |
Data are n (%) of patients.
Weight gain differed by treatment group: the 45 mg group had an adjusted mean increase of 1.6 kg (SD 2.15), compared with a decrease of −0.25 kg (2.13) for placebo and −0.02 kg (2.13) for the 15 mg groups (all adjusted for time). Repeated measures analysis of weight change over time indicated a significant difference between treatment groups (F2,207=14.9, p<0.0001).
Monitoring of depression was prespecified in the safety monitoring plan, although no specific statistical criteria for stopping were established. Depression as measured by a GDS-15 score of 5 or greater occurred more frequently at baseline in the placebo group (2 [3%] for 15 mg group, 1 [1%] for 45 mg group, and 10 [14%] for placebo), but the mean GDS change at 44 weeks was similar by treatment group (table 3). Depressed mood adverse events occurred similarly across treatment groups (2 [3%] for 15 mg group, 3 [5%] for 45 mg group, 3 [4%] for placebo). There were no significant differences in laboratory values by treatment group over time or body-mass index (data not shown).
Discussion
Our results suggest that both doses of pioglitazone are unlikely to be effective as interventions to slow progression of disability in early Parkinson's disease and we do not recommend that they are considered for further study. Although 15 mg pioglitazone was not futile in the primary analysis, absence of efficacy on all preplanned sensitivity analyses and the secondary outcome measures suggest this dose is futile as well.
Pioglitazone was chosen by the CINAPS panel of experts for testing based on the results of well conducted preclinical studies showing reproducible neuroprotective effects in tissue culture and animal models.4,29,30 Unfortunately, this is another study in which animal models were not predictive of efficacy in human beings. A possible explanation for negative outcomes is that toxin animal models are not reflective of Parkinson's disease pathogenesis. Another possibility is that pioglitazone failed to reach the target nigral neurons and achieve sufficient drug exposure in this study, although good CNS penetration and target engagement were shown in primate studies.13
Alternatively, it is possible that the beneficial effect of pioglitazone was missed owing to pitfalls of the study design, including the choice of the primary outcome measure. Although whether UPDRS is the best outcome measure for the assessment of disease-modifying benefit in early Parkinson's disease remains to be proven, it is the best validated measure and the one that has extensive data showing its sensitivity to change in early Parkinson's disease.31 Consistent findings for all secondary outcomes, which included a spectrum of validated measures of quality of life, disability, and cognitive impairment, support an absence of biological effect rather than a failure to capture and measure an effect. The biomarkers also failed to show a separation of the active treatment groups from placebo and as such are concordant with the conclusions drawn from the clinical study (these results will be reported separately). If serum and urine biomarkers had shown a shift in the predicted direction, then an argument could have been made for a biological effect that was not captured by the clinical measures. The finding that both clinical and biological markers failed to move is disappointing, but solidifies the conclusion that pioglitazone is not promising for further testing in early Parkinson's disease.
Another consideration is the short duration of this and other Parkinson's disease futility trials. 1 year or less amounts to a small proportion of the overall clinical course of Parkinson's disease. The major objective of futility studies is to screen out quickly compounds that do not work. Studies of such short duration might miss important disease-modifying effects that could be shown if patients were followed up for longer. Longer studies impose a greater burden on patients and higher cost. Ideally, future phase 2 studies of disease modification in Parkinson's disease will rely on biomarkers that will increase the sensitivity of the analysis over shorter follow-up. Another important consideration for future studies is how early Parkinson's disease is defined, as the onset of classic motor symptoms might be late biologically. Neuroprotection might not be feasible unless we intervene at the premotor stage of the disease. Of the other 12 agents recommended by CINAPS for clinical testing, most have entered phase 2 studies and four have completed phase 3 studies. Unfortunately, all studies have been negative so far.15,32,33
A novel aspect of this study is that the participants were required to be treated with MAO-BI therapy at the time of enrolment. The rationale for this inclusion criterion was to reduce the number of patients who would need other dopaminergic treatment during the trial. Untreated Parkinson's disease patients are often enrolled to assess short-term (1 year or less) effects of a potential disease-modifying drug in exploratory trials. However, nearly half of the participants might need dopaminergic therapy before the end of follow-up.34 This poses a major problem for the primary analysis because these patients' outcomes have to be carried forward from the last observed visit before initiation of dopaminergic therapy, or these data must be imputed. Treatment of de novo Parkinson's disease participants with low-dose MAO-BI therapy might delay the time to initiation of additional dopaminergic therapy by providing symptomatic benefit that might be mild enough to allow for detection of improvement due to a study intervention, should one exist. Indeed, only 30 (14%) participants needed additional dopaminergic therapy in this study, which is a two-to-three-fold reduction compared with the previously completed trials in similar populations.34 MAO-BIs are unlikely to have masked a beneficial effect of pioglitazone because each treatment group in this study worsened similarly to those treated with 1 mg rasagiline in the TEMPO study,26 which was used to estimate the placebo effect for the power calculations of this trial. On the basis of these data we propose to consider enrolment of patients on a stable regimen of a mild symptomatic therapy such as MAO-BIs in future phase 2 disease modification trials.
Our data show that pioglitazone is unlikely to be efficacious as a disease-modifying intervention in early Parkinson's disease and therefore is not recommended for further testing for that indication. Although our negative results are disappointing, the design of this futility study is an example of a useful and efficient study design that can exclude a compound unlikely to be successful in larger and more costly phase 3 studies. Unfortunately, as was seen in the recent NET-PD study of creatine,35 even compounds deemed non-futile in futility studies might also fail in longer studies. Accordingly, much attention has shifted to discovery and validation of biomarkers of disease progression, which we hope will accelerate the development of disease-modifying or curative agents.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published in English before Jan 15, 2015, using the terms “Parkinson's disease”, “disease modification”, “clinical trials”, and “pioglitazone”. As of that date, no agents had proven to be disease-modifying agents (ie, to slow progression) in patients with Parkinson's disease and no studies had assessed the potential disease-modifying effects of pioglitazone.
Added value of this study
This is the first randomised controlled trial of pioglitazone, a peroxisome proliferator-activated receptor γ agonist, as a potential disease-modifying agent in patients with Parkinson's disease. The rationale for the choice of study agent was based on the robust preclinical data showing a neuroprotective effect in animal models at the doses approved for use in human beings. Although the findings of this trial do not warrant further testing of pioglitazone in patients with Parkinson's disease, the design of our study could guide that of other studies, as we have shown that this design is useful and efficient to exclude a compound unlikely to be successful in larger and more costly phase 3 trials.
Implications of all available evidence
These findings suggest that pioglitazone at the doses studied here is unlikely to modify progression in early Parkinson's disease. Further study of pioglitazone in a larger trial in Parkinson's disease is not recommended, although other peroxisome proliferator-activated receptor γ agonists might deserve further exploration as disease-modifying agents in Parkinson's disease.
Acknowledgments
We would like to thank the FS-ZONE participants for their commitment to this study and to Parkinson's disease research.
Appendix
Contributors
TS (Principal Investigator) was responsible for study design, study oversight, data collection, data analysis, data interpretation, and writing of the final manuscript. KK (Principal Investigator, Coordination Center) was responsible for study design, study oversight, data interpretation, and critical review of the manuscript. BT (Principal Investigator, Statistical Center) was responsible for study design, data analysis, data interpretation, and manuscript review. DB was responsible for study design, data interpretation, and critical review of the manuscript. JJE was responsible for study design, data collection, data analysis, data interpretation, and writing the methods and results section. ME was responsible for literature search, study design, data interpretation, and critical review of the manuscript. RH, AF, and CS were responsible for data collection, data interpretation, and critical review of the manuscript. CK was responsible for study design, study drug supply chain management, original grant writing, procurement of funding, data collection, and critical review of the manuscript. JCM, GWR, and RBD were responsible for study design, data collection, data interpretation, and critical review of the manuscript. BR was responsible for study design, study oversight, data collection, data interpretation, and critical review of the manuscript. DKS was responsible for study design, data collection, data analysis, data interpretation, and critical review of the manuscript. JBa was responsible for data collection, data analysis, and critical review of the manuscript. LB, IB-W, JBo, FC, JC, KC, CWC, RD, JF, BF, SG, JG, PLB, SLe, ML, MFL, SLo, RP, JS, BSh, KMS, SS, DT, RW, AMW, PSW, RZ, and CZ were responsible for data collection and critical review of the manuscript. ND was responsible for data collection, review of results, and critical review of the manuscript. SLu was responsible for data analysis and critical review of the manuscript. AP was responsible for data interpretation, writing, and critical review of the manuscript. BSc was responsible for data collection, data interpretation, and critical review of the manuscript. KW was responsible for data collection and review of the manuscript.
FS-ZONE Writing Committee
Tanya Simuni MD (Principal Investigator; Northwestern University, Chicago, IL, USA); Karl Kieburtz MD (Principal Investigator, Coordination Center; University of Rochester, Rochester, NY, USA); Barbara Tilley PhD (Principal Investigator, Statistical Center; University of Texas Health Science Center at Houston, Houston, TX, USA); Jordan J Elm PhD (Medical University of South Carolina, Charleston, SC, USA); Bernard Ravina MD (Voyager Therapeutics, Cambridge, MA, USA); Debra Babcock MD (NINDS, National Institutes of Health, Bethesda, MD, USA); Marina Emborg MD (University of Wisconsin, Madison, WI, USA); Robert Hauser MD (University of South Florida, Tampa, FL, USA); Cornelia Kamp MBA (University of Rochester, Rochester, NY, USA); John C Morgan MD (Georgia Regents University, Augusta, GA, USA); G Webster Ross MD (Veterans Affairs Pacific Health Care System, Honolulu, HI, USA); David K Simon MD (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA); Jacci Bainbridge PharmD (University of Colorado Denver, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Clinical Pharmacy and Neurology, Aurora, CO, USA); Liana Baker MPH (University of Rochester, Rochester, NY, USA); Ivan Bodis-Wollner MD (SUNY Downstate Medical Center, Brooklyn, NY, USA); James Boyd MD (University of Vermont, Burlington, VT, USA); Franca Cambi MD (University of Kentucky, Lexington, KY, USA); Julie Carter ANP (Oregon Health & Science University, Portland, OR, USA); Kelvin Chou MD (Departments of Neurology and Neurosurgery, University of Michigan, Ann Arbor, MI, USA); Chadwick W Christine MD (Department of Neurology, University of California, San Francisco, San Francisco, CA, USA); Nabila Dahodwala MD (University of Pennsylvania, Philadelphia, PA, USA); Richard B Dewey Jr MD (University of Texas Southwestern Medical Center, Dallas, TX, USA); Rohit Dhall MD (Barrow Neurological Institute, Phoenix, AZ, USA); John Fang MD (Vanderbilt University, Nashville, TN, USA); Buff Farrow BS (Georgia Regents University, Augusta, GA, USA); Andrew Feigin MD (The Feinstein Institute for Medical Research, North Shore – LIJ Health System, Manhasset, NY, USA); Sofya Glazman MD (SUNY Downstate Medical Center, Brooklyn, NY, USA); John Goudreau DO (Department of Neurology, Michigan State University, East Lansing, MI, USA); Pauline LeBlanc BS (Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA); Stephen Lee MD (Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA); Maureen Leehey MD (University of Colorado Denver, Aurora, CO, USA); Mark F Lew MD (University of Southern California, Los Angeles, CA, USA); Stephanie Lowenhaupt RN (University of Virginia, Charlottesville, VA, USA); Sheng Luo PhD (The University of Texas Health Science Center at Houston, Houston, TX, USA); Rajesh Pahwa MD (University of Kansas Medical Center, Kansas City, KS, USA); Adriana Perez PhD (University of Texas Health Science Center at Houston, Austin, TX, USA); Jay Schneider PhD (Thomas Jefferson University, Department of Pathology, Anatomy and Cell Biology, Philadelphia, PA, USA); Burton Scott MD (Duke University, Durham, NC, USA); Binit Shah MD (University of Virginia, Charlottesville, VA, USA); Kathleen M Shannon MD (Rush University Medical Center, Chicago, IL, USA); Saloni Sharma MBBS (University of Rochester, Rochester, NY, USA); Carlos Singer MD (University of Miami, Miami, FL, USA); Daniel Truong MD (Parkinson's & Movement Disorder Institute, Fountain Valley, CA, USA); Renee Wagner RN (University of Kentucky, Lexington, KY, USA); Karen Williams (Northwestern University, Chicago, IL, USA); Anne Marie Wills MD (Brigham & Women's Hospital and Harvard Medical School, Boston, MA, USA); Pei Shieen Wong PharmD (University of Colorado Denver, Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO, USA; Singapore General Hospital, Singapore); Cindy Zadikoff MD (Northwestern University, Chicago, IL, USA); Richard Zweig MD (LSU Health Shreveport, Shreveport, LA, USA).
Footnotes
Declaration of interests: TS reports personal fees from Acadia, Abbvie, Allergan, Eli Lilly, Harbor, Ibsen, Merz, UCB Pharma, and US World Meds; grants, personal fees, honorarium, consulting fees, educational grant support from, and is a speaker for, GE Medical and TEVA; consulting fees from, and is an advisory board member and speaker for, IMPAX; personal fees and consulting fees from, and is a speaker for, Lundbeck; research funding from Auspex, Biotie, Civitas, NIH, and Michael J. Fox Foundation, during the conduct of the study. KK reports grants from National Institutes of Health, during the conduct of the study; grants from National Institutes of Health (NEI, NIH, NINDS); personal fees from Acorda, Astellas Pharma, AstraZeneca, Auspex, Biotie, Britannia, Cangene, CHDI, Civitas, Clearpoint Strategy Group, Clintrex, Cynapsus, INC Research, Intec, Isis, Lilly, Lundbeck, Medavante, Medivation, Melior Discovery, Neuroderm, Neurmedix, Omeros, Otsuka, Pfizer, Pharma2B, Prothena/Neotope/Elan Pharmaceutical, Raptor Pharmaceuticals, Roche/Genentech, Sage Bionetworks, Serina, Stealth Peptides, Synagile, Teikoku Pharma, Titan, Turing Pharmaceuticals, Upsher-Smith, US WorldMeds, Vaccinex, Voyager, and Weston Brain Institute; grants from Michael J Fox Foundation and Teva, outside the submitted work. BT reports personal fees from Roche, grants from MDS, and non-financial support from Michael J. Fox Foundation, outside the submitted work. DB is an employee of NINDS. JJE reports grants from NINDS, during the conduct of the study. RH reports grants from NIH, during the conduct of the study; personal fees from Teva Pharmaceuticals, USB Biosciences, AbbVie, Eli Lilly, Novartis, Biotie, Lundbeck, Pfizer, Allergan Neuroscience, Neurocrine, Chelsea, Auspex, Acadia, Michael J Fox Foundation, GLG, AstraZeneca, Acordia, and Impax Pharmaceuticals, outside the submitted work. JCM reports grants from NIH, during the conduct of the study; personal fees from Veloxis, Teva, Lundbeck, Impax Labs, and Movement Disorders Society, and grants and personal fees from National Parkinson Foundation, outside the submitted work. BR reports equity in, and is an employee of, Voyager Therapeutics. GWR reports grants from NINDS-NIH, during the conduct of the study. DKS reports grants from Lysosomal Therapeutics, outside the submitted work. JBa reports grants from National Institutes of Health (NIH), during the conduct of the study. JBo reports grants from University of Vermont, during the conduct of the study; grants and personal fees from AbbVie and Auspex, personal fees from Lundbeck, grants from Biotie, Cure HD Initiative Foundation, and MJ Fox Foundation, outside the submitted work. KC reports grants from NIH, during the conduct of the study; personal fees from Medtronic, Inc., outside the submitted work. CWC reports grants from NIH, during the conduct of this study; grants from Kinemed, Inc. and Michael J Fox Foundation, outside the submitted work. ND reports grants from NIH, National Parkinson Foundation, and PD Council, outside the submitted work. RBD reports grants from NINDS, during the conduct of the study; personal fees from Teva Neuroscience, Merz, Xenoport, UCB, Acadia, US World Meds, Impax, and Lundbeck, outside the submitted work. JF reports grants from NIH/NINDS, outside the submitted work. BF reports grants from NIH/NINDS, during the conduct of the study. AF reports personal fees from Lundbeck, US World Meds, Auspex, and Prana, grants from Voyager Therapeutics, outside the submitted work. JG reports grants from National Institutes of Health, during the conduct of the study; personal fees from Teva Neuroscience, grants from Michael J. Fox Foundation and National Institutes of Health, outside the submitted work. ML reports grants from NIH, during the conduct of the study; personal fees from Neurologic Movement Disorders Market Research Team, Giudepoint Global, Scientiae, LLC, Health Practices Research Institute, Gerson Lehman; grants from Allergan, Medtronic, Teva, US World Meds, LLC, Adamas Pharmaceuticals, Pharma2B, outside the submitted work; and grants from NINDS, NIH, and Colorado Department of Health and Environment. MFL reports grants and personal fees from Teva Pharmaceuticals, personal fees from Lundbeck, Impax, UCB, Auspex, Baxter, Acadia, and Abbvie, outside the submitted work. SLu receives research funding from NIH, Movement Disorder Society, and CHDI Foundation. RP reports grants from NIH, during the conduct of the study; grants and personal fees from Teva Neuroscience, Acadia Pharma, Adamas, UCB Pharma, Medtronic, US World Meds, Impax Pharma, Lundbeck, AbbiVie, NPF, Parkinson Study Group; personal fees from St Jude Medical; grants from Avid, Biotie, Boston Scientific, Chelesea, Civitas, Kyowa, outside the submitted work; royalties from Oxford Press and Informa Health; is a member of the data and safety monitoring committee for an ISIS study; and is co-editor-in-chief of the International Journal of Neuroscience. DT reports receiving grants for this work. RW reports grants from NINDS, during the conduct of the study; grants and personal fees from Abbvie, and grants from Teva, outside the submitted work. PSW reports grants from NIH, during the conduct of the study. CZ reports consulting fees and honoraria from Teva and Abbie, and honoraria from UCB, outside the submitted work. RZ reports grants from NINDS (NIH), during the conduct of the study. ME, CK, LB, IBW, FC, JC, RD, SG, PL, SLe, SLo, AP, JS, BSc, BSh, KMS, SS, CS, KW, and AMW declare no competing interests.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Suchowersky O, Gronseth G, Perlmutter J, Reich S, Zesiewicz T, Weiner WJ. Practice parameter: neuroprotective strategies and alternative therapies for Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:976–82. doi: 10.1212/01.wnl.0000206363.57955.1b. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Pearce LA, Ravina BM, Yaltho TC, Marler JR. Neuroprotection trials in Parkinson's disease: systematic review. Mov Disord. 2009;24:647–54. doi: 10.1002/mds.22432. [DOI] [PubMed] [Google Scholar]
- 4.Ravina BM, Fagan SC, Hart RG, et al. Neuroprotective agents for clinical trials in Parkinson's disease: a systematic assessment. Neurology. 2003;60:1234–40. doi: 10.1212/01.wnl.0000058760.13152.1a. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi RK, Beal MF. PPAR: a therapeutic target in Parkinson's disease. J Neurochem. 2008;106:506–18. doi: 10.1111/j.1471-4159.2008.05388.x. [DOI] [PubMed] [Google Scholar]
- 6.Nicolakakis N, Aboulkassim T, Ongali B, et al. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–96. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risner ME, Saunders AM, Altman JF, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenom J. 2006;6:246–54. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 8.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–29. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 9.Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–45. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 10.Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161:113–22. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Eschbach J, von Einem B, Muller K, et al. Mutual exacerbation of peroxisome proliferator-activated receptor gamma coactivator 1alpha deregulation and alpha-synuclein oligomerization. Ann Neurol. 2015;77:15–32. doi: 10.1002/ana.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson C, Emborg M. Expression of peroxisome proliferator-activated receptor-gamma in the substantia nigra of hemiparkinsonian nonhuman primates. Neurol Res. 2014;36:634–46. doi: 10.1179/1743132813Y.0000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson CR, Joers V, Bondarenko V, et al. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 15.NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 16.NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68:20–28. doi: 10.1212/01.wnl.0000250355.28474.8e. [DOI] [PubMed] [Google Scholar]
- 17.Levin B. The utility of futility. Stroke. 2005;36:2331–32. doi: 10.1161/01.STR.0000185722.99167.56. [DOI] [PubMed] [Google Scholar]
- 18.Tilley BC, Palesch YY, Kieburtz K, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–33. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton R . UPDRS Development Committee. The Unified Parkinson's Disease Rating Scale. In: Fahn SMC, Calne DB, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park, NJ: Macmillan Healthcare; 1987. pp. 153–64. [Google Scholar]
- 20.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–84. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parashos SA, Elm J, Boyd JT, et al. Validation of an ambulatory capacity measure in Parkinson disease: a construct derived from the unified Parkinson's disease rating scale. J Parkinsons Dis. 2015;5:67–73. doi: 10.3233/JPD-140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab RS, England AC., Jr . Projection technique for evaluating surgery in Parkinson's disease. In: Gillingham FJ, Donaldson IML, editors. Third symposium on Parkinson's disease. Edinburgh: E & S Livingstone; 1969. pp. 152–57. [Google Scholar]
- 23.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–48. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 24.Pirogovsky E, Schiehser DM, Litvan I, et al. The utility of the Mattis Dementia Rating Scale in Parkinson's disease mild cognitive impairment. Parkinsonism Relat Disord. 2014;20:627–31. doi: 10.1016/j.parkreldis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Meara J, Mitchelmore E, Hobson P. Use of the GDS-15 geriatric depression scale as a screening instrument for depressive symptomatology in patients with Parkinson's disease and their carers in the community. Age Ageing. 1999;28:35–38. doi: 10.1093/ageing/28.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Parkinson Study G. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol. 2002;59:1937–43. doi: 10.1001/archneur.59.12.1937. [DOI] [PubMed] [Google Scholar]
- 27.Friedman LFC, DeMets D. Fundamentals of clinical trials. 2nd. Littleton, MA: PSG Publishing Company; 1985. [Google Scholar]
- 28.Rubin DB. Multiple Imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. pp. 166–67. [Google Scholar]
- 29.Pagel-Langenickel I, Bao J, Joseph JJ, et al. PGC-1alpha integrates insulin signaling, mitochondrial regulation, and bioenergetic function in skeletal muscle. J Biol Chem. 2008;283:22 464–72. doi: 10.1074/jbc.M800842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carta AR, Simuni T. Thiazolidinediones under preclinical and early clinical development for the treatment of Parkinson's disease. Expert Opin Investig Drugs. 2014;24:1–9. doi: 10.1517/13543784.2015.963195. [DOI] [PubMed] [Google Scholar]
- 31.Parashos SA, Luo S, Biglan KM, et al. Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurol. 2014;71:710–16. doi: 10.1001/jamaneurol.2014.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang AE, Melamed E, Poewe W, Rascol O. Trial designs used to study neuroprotective therapy in Parkinson's disease. Mov Disord. 2013;28:86–95. doi: 10.1002/mds.24997. [DOI] [PubMed] [Google Scholar]
- 33.Beal MF, Oakes D, Shoulson I, et al. QE3 Investigators. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71:543–52. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- 34.Parashos SA, Swearingen CJ, Biglan KM, et al. Determinants of the timing of symptomatic treatment in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. Arch Neurol. 2009;66:1099–104. doi: 10.1001/archneurol.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kieburtz K, Tilley BC, Elm JJ, et al. Writing Group for the NINDS Exploratory Trials in Parkinson Disease (NET-PD) Investigators. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313:584–93. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.