Abstract
FTY720, an analogue of sphingosine-1-phosphate, is cardioprotective during acute injury. Whether long-term FTY720 affords cardioprotection is unknown. Here we report the effects of oral FTY720 on ischemia/reperfusion (I/R) injury and in HypoE mice deficient in SR-BI receptor expression (ApoeR61h/h/SRB1–/– mice), a model of diet-induced coronary atherosclerosis and heart failure. We added FTY720 (0.3mg/kg/day) to the drinking water of C57BL/6J mice. After ex vivo cardiac I/R injury these mice had significantly improved left ventricular (LV) developed pressure and reduced infarct size compared with controls. Subsequently, ApoeR61h/h/SRB1–/– mice fed a high fat diet (HFD) for 4 weeks were treated or not with oral FTY720 (0.05mg/kg/day). This sharply reduced mortality (P<0.02) and resulted in better LV function and less LV remodeling compared with controls without reducing hypercholesterolemia and atherosclerosis. Oral FTY720 reduced the number of blood lymphocytes and increased the percentage of CD4+Foxp3+ T cells (Tregs) in the circulation, spleen and lymph nodes. FTY720-treated mice exhibited increased TGF-β and reduced IFN-γ expression in the heart. Also, CD4 expression was increased and strongly correlated with molecules involved in natural Treg activity, such as TGF-β and GITR. Our data suggest that long-term FTY720-treatment enhances LV function and increases longevity in mice with heart failure. These benefits resulted not from atheroprotection but from systemic immunosuppression and a moderate reduction of inflammation in the heart.
Keywords: FTY720, S1P, ApoE, coronary atherosclerosis, heart failure, cardioprotection, immunosuppression
Introduction
Sphingosine-1-phosphate (S1P) is a natural sphingolipid present at high nanomolar concentrations in serum (1). It couples via G-proteins to S1P receptors which are widely expressed on different cell types (2). These receptors activate a multitude of physiological and pathological processes such as immunity, angiogenesis, cell migration, and inflammation (3). Signaling pathways involving S1P1 and S1P3 receptors mediate the cytoprotective actions of S1P in a range of tissues, including the heart (2, 4). Recent studies, including those from our laboratory, have examined effects of S1P in ex vivo mouse hearts subjected to ischemia/reperfusion (I/R) injury as well as pre- and post-conditioning regimens (2, 5-8). Physiological concentrations (10nmol/L) of extracellular S1P infused either before ischemia or at the onset of reperfusion reduced infarct size and augmented myocardial contractile recovery during reperfusion (6). Activation of PI3K-AKT and ERK pro-survival signaling pathways and inactivation of their downstream target GSK-3β (7, 9, 10) is part of the well-described SAFE and RISK pathways (11, 12), of which S1P release (13) is a component. Thus, in different cell types, including cardiac myocytes, acute S1P receptor agonism has resulted in activation of AKT, ERK and inactivation of GSK-3β (2, 4, 14, 15).
FTY720 is derived from myriocin, a component of the Chinese herb Iscaria sinclarii, which has been used as a treatment for asthma. FTY720 is a structural homologue of S1P. After endogenous phosphorylation, FTY720 serves as a potent agonist of S1P1 receptors, and binds less avidly to S1P3 receptors (16). We and others have demonstrated that FTY720 enhances cardiac myocyte survival during oxidative stress (2, 14). Additional studies showed that acute treatment with FTY720 prevents I/R injury-associated arrhythmias, and improves the recovery of left ventricular developed pressure (LVDP) in an ex vivo rat heart model (14, 15).
Beyond its effects in acute cardioprotection, FTY720 is also known to modulate lymphocyte trafficking from blood and peripheral tissues to lymph nodes resulting in profound immunosuppression (17, 18). Currently, FTY720 is approved to treat an autoimmune disease, multiple sclerosis. FTY720 has also been studied in atherosclerosis, which is a recognized chronic inflammatory process and a leading cause of heart disease. Previous studies have reported that FTY720 is protective in two atherosclerosis models: Apoe–/– and Ldlr–/– mice fed an atherogenic diet (19, 20). However, in neither of these reports was heart failure or mortality noted as these genetically altered mice do not develop severe coronary atherosclerosis. Although effects of FTY720 in acute cardioprotection have been reported, the influence of long-term FTY720 treatment on cardiac responses is unknown.
In the present study we investigated the cardioprotective effects of FTY720 in a mouse model of diet-induced occlusive coronary atherosclerosis and chronic heart failure. These mice were developed using our previously described hypomorphic apoE or “HypoE” mouse model of reduced apoE expression that display 2-5% of normal plasma apoE levels due to the hypomorphic Apoeh/h allele (21). HypoE mice were bred to mice deficient in SR-BI receptor expression. The unique feature of diet inducible hyperlipidemia and atherosclerosis inherent to HypoE mice (21, 22) allows the double gene targeted HypoE mice deficient in SR-BI, also known as ApoeR61h/h/SRB1–/– mice, to display a normal phenotype when they are on a conventional chow diet (23). However, when fed a diet containing high amounts of fat and cholesterol (HFD), ApoeR61h/h/SRB1–/– mice develop severe hyperlipidemia and occlusive coronary atherosclerosis, and after 4-7 weeks die prematurely from myocardial infarction (MI) and ischemic heart failure (23). We hypothesized that long-term FTY720-treatment would ameliorate the responses to ex vivo I/R injury in normal C57BL/6J mice and reduce the extent of coronary atherosclerosis, MI, and subsequent deterioration of cardiac function and death in ApoeR61h/h/SRB1–/– mice fed a HFD.
Methods
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academic Press, Washington DC, 1996). All procedures were approved by the Animal Care Subcommittee of the San Francisco VA Medical Center, and conform to NIH Guidelines.
Animal Models and Diet
This study made use of male C57BL/6J and hypomorphic Apoeh/h mice, also termed HypoE mice (21) that were bred to mice deficient in SR-BI receptor expression to create ApoeR61h/h/SRB1–/– mice. The latter are of mixed background (C57BL/6Jx129), as we previously described (23) and all experiments with this strain of mice were conducted with littermate control mice. At 3 months of age, C57BL/6J mice fed a chow diet (2916, Harlan Teklad, Madison, WI) received FTY720 (Calbiochem, San Diego, CA) in the drinking water at a dose of 0.3 mg/kg/day for 1 or 3 weeks and were used for ex vivo Langendorff studies. Mice that received regular drinking water served as controls. The dose of 0.3 mg/kg/day was arrived at as follows: we initially used several different concentrations of oral FTY720 ranging from 0.1 - 10 mg/kg/day for 1-3 weeks. We then harvested the hearts and mounted them on a Langendorff rig and performed ischemia/reperfusion experiments. We found that the optimal reduction in infarct size and hemodynamic recovery occurred with the 0.3 mg/kg/day dose.
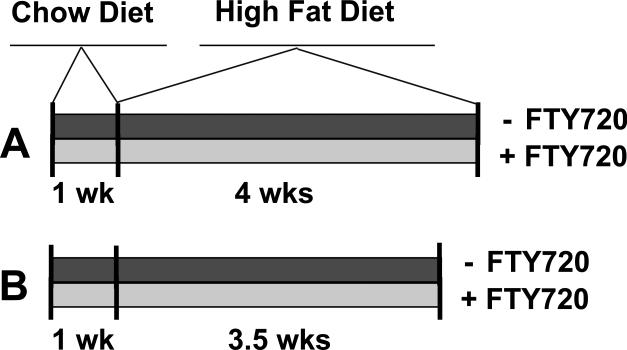
In the ApoeR61h/h/SRB1–/– mice fed a high fat, high cholesterol diet, we found that this dose was toxic and led to extensive mortality, possibly because of congestive hepatopathy resulting in altered liver metabolism of FTY720 that occurred as the mice developed heart failure. We then progressively reduced the dose until no deaths attributable to the drug occurred. This dose was 0.05 mg/kg/day. Accordingly, for the mortality study, at 3 months of age chow fed ApoeR61h/h/SRB1–/– mice were randomly allocated to receive FTY720-treatment at a dose of 0.05 mg/kg/day in the drinking water (n=22) or not (n=29). The group receiving FTY720 were fed this agent one week prior and during 4 weeks of a high-fat, high-cholesterol diet (HFD). The control group received only plain drinking water for the duration of the study (Figure 1A). For the pathology study, ApoeR61h/h/SRB1–/– mice were treated identically but received 3.5 weeks of the HFD instead of 4 weeks (Figure 1B). This was to limit the mortality in the control group. The HFD consisted of 16% fat and 1.25% cholesterol (D12336, Research Diets Inc., New Brunswick, NJ). All mice were maintained with a 12-h light/12-h dark cycle in the San Francisco VA Medical Center animal facility.
Figure 1.
Design of the mortality study (A). Design of the pathology study (B). High fat diet = High fat, high cholesterol diet.
Langendorff perfused Heart Preparation and Ex Vivo Ischemia/Reperfusion (I/R)
Mice were heparinized (500 U/kg, IP) and anesthetized with sodium pentobarbital (60 mg/kg, IP). Hearts were rapidly excised, washed in ice-cold arresting solution (NaCl 120 mmol/L, KCl 30mmol/L), and cannulated via the aorta on a 20 gauge stainless steel blunt needle. Hearts were perfused at 70 mmHg on a modified Langendorff apparatus using Krebs–Henseleit solution at 37°C as previously described (5, 6). The protocol consisted of a 20min equilibration period after mounting the ex vivo hearts on the Langendorff apparatus. Sustained global ischemia was then induced by halting perfusion for 50min. This was followed by 50min of reperfusion. Left ventricular developed pressure (LVDP) was recorded continuously as previously described (5, 6).
Infarct Size Measurement
After I/R, hearts were then removed from the cannula, sectioned and stained with 1% triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline. The infarct area was determined by image analysis and compared with the area at risk, which is the entire heart in this global ischemia model, as previously described (5, 6).
Echocardiography
Transthoracic echocardiography was performed with a commercially available system (Acuson Sequoia c256, Acuson, Siemens) using a 15-MHz linear array transducer. After the anterior chest was shaved, mice were inserted into a plastic cone and maneuvered into a prone position. The cone was fixed with adhesive tape, and warm ultrasound transmission gel was used to fill the gap between the chest and the cone, so that two-dimensional imaging could be performed through the cone. Care was taken to avoid excessive pressure on the thorax, which can induce bradycardia. Two-dimensional long-axis images of the LV were obtained at the plane of the aortic and mitral valves where the LV cavity is largest and a short-axis image was recorded at the level of the papillary muscles. M-mode echocardiograms were recorded through the anterior and posterior walls at a sweep speed of 200mm/s using two-dimensional guidance. Images were acquired digitally and stored on a magneto-optical disk. All measurements were made from digital images captured on cine loops at the time of study with the use of a specialized software package (Acuson Sequoia). The LV end-diastolic dimension (LVEDD) and end-systolic dimension (LVESD) were determined as the largest and smallest dimensions of the LV, respectively, on M-mode images, and fractional shortening (FS) was derived from the following equation: FS=(LVEDD−LVESD)/LVEDD. The LV end-diastolic volume (LVEDV) was calculated using the following two-dimensional area–length method: LVEDV=(5/6)A−L, where A is the endocardial parasternal short-axis area at end-diastole and L is the parasternal long-axis length. Analogous methods were used to calculate end-systolic volume. Ejection fraction (EF) in percent was calculated as the ratio of stroke volume [LVEDVLVESV] to LVEDV.
Plasma Lipid Measurements and Lipoprotein Fractionation
Blood was collected from ApoeR61h/h/SRB1–/– cohorts before and after 3.5 weeks of HFD feeding. After mice had been fasted for 4hrs, they were anesthetized by isoflurane inhalation and plasma was obtained from blood drawn by retro-orbital puncture. Plasma was pooled from 4-7 mice and lipoproteins were fractionated by fast protein liquid chromatography (FPLC) on a Superose 6 GL 10/30 column (GE Healthcare, NJ). Colorimetric assays were used to measure cholesterol and triglyceride levels in plasma and FPLC fractions according to the manufacturer's instructions (Cholesterol E, L-type TG M; Wako, VA) using a VersaMax microplate reader (Molecular Devices Corporation, Sunnyvale, CA).
Morphologic and Histologic Analyses
Tissue collection
ApoeR61h/h/SRB1–/– mice fed the HFD with FTY720-treatment or not for 3.5 weeks were weighed and anesthetized with 2.5% tribromoethanol (Avertin). After thoracotomy, mice were perfused via heart puncture with 10ml ice-cold PBS for 1min. Hearts were collected, weighed and cut in half. Both portions were embedded in tissue freezing medium (OCT) that was flash frozen in an isopentane bath cooled with liquid nitrogen. In a separate group of experiments, the apical portion of the heart was flash frozen in liquid nitrogen for RNA analysis.
Cryosectioning and Staining
Atherosclerosis and ischemic pathology were assessed by obtaining simultaneous serial sections of the basal portion of the heart subdivided into 2 segments. Serial 10μm sections were collected on 5 different slides from each segment. Serial 10μm sections were cut from the root of the aorta through the aortic sinuses encompassing a total length of 500-600μm (3 sections per slide). Slides from each segment were used for immuno-staining or were stained with hematoxylin and eosin (H&E), Oil Red O (ORO) and Sirius Red counterstained with Fast Green.
Quantification of Occlusive Coronary Atherosclerosis
The extent and severity of coronary atherosclerosis was quantified in a blinded fashion by counting vessels in twenty four Sirius Red heart sections per mouse. Scoring the level of occlusion in vessels was done by visual inspection of the slides at a power of 100X. Vessels were defined as non-occluded (NO) with a 0-5% burden, partially occluded (PO) with a 5-50% burden, severely occluded (SO) with a 50-95% burden, and completely occluded (CO) with a 95-100% burden. Calculating the average percent of obstructed vessels in each category provided an index of vessel occlusion (2).
Quantification of Aortic Atherosclerosis
For morphologic analysis, sections of the aortic sinuses were stained with Sirius Red and counterstained with Fast-Green. The atherosclerotic burden in the aortic root was quantified in a blinded fashion using six sections per mouse. Metamorph software (Molecular Devices Inc., Sunnyvale, CA) was used to quantify the lesion area per cross section, which was then averaged to provide mean lesion area per mouse.
Quantification of Myocardial Collagen and Lipid Deposition
Myocardial collagen and lipid area were quantified in a blinded fashion using six or more heart sections per mouse stained with Sirius Red and ORO, respectively. Metamorph software was used to quantify the collagen and lipid area per cross section, which was then averaged to provide mean lesion area per mouse.
Flow Cytometry
Blood leukocytes, spleen and lymph node cell suspension were stained with pre-mixed combination of antibodies (FITC-conjugated anti-Ly6C, anti-CD4, and anti-CD45R/B220; PE-conjugated anti-CD33, anti-Ly6G, and anti-CD44; PERCP-Cy5.5-conjugated anti-CD45 and anti-CD11b; PE-Cy7-conjugated anti-NK1.1 and CD8α; APC-conjugated anti-CD45 and anti-CD62L, all purchased from BD Pharmigen). For intracellular staining of FoxP3, antibodies (PE-conjugated anti-Foxp3, FITC-conjugated anti-CD4 and APC-conjugated anti-CD25) and Foxp3 staining buffer from eBioscience was used according to the manufacturer's recommended protocol. All samples were analyzed using a C6 Flow Cytometer (BD Cytometers Inc., Ann Arbor, MI). The absolute cell counts or cell concentration per μl was determined with the CFlow Plus software (BD Cytometers Inc.) and other analyses were performed with FlowJo software (Tree Star Inc., OR).
Quantitative RT-PCR
Total RNA was extracted from hearts using RNeasy Mini kit including a DNase step according to the manufacturer's instruction (Qiagen, CA). One microgram of total RNA was used to generate complimentary DNA (cDNA) using iScript (Bio-Rad Laboratories, Life Sciences Research, CA). Assay-On-Demand primers (Applied Biosystems) were used to determine gene expression. Variability in loading different amounts of cDNA was normalized using the average of 2 housekeeping genes, TATA box binding protein (Tbp) and hypoxanthine phosphoribosyl transferase (hprt). mRNA levels were calculated according to the 2-ΔCt method.
Statistical Analysis
Data are expressed as the mean ± SEM. Numerical data were compared using Student's t test for paired observations. A p value of less than 0.05 was considered significant.
Results
FTY720-treatment improves cardiac function and infarct size in C57BL/6J mouse hearts after ex vivo I/R injury
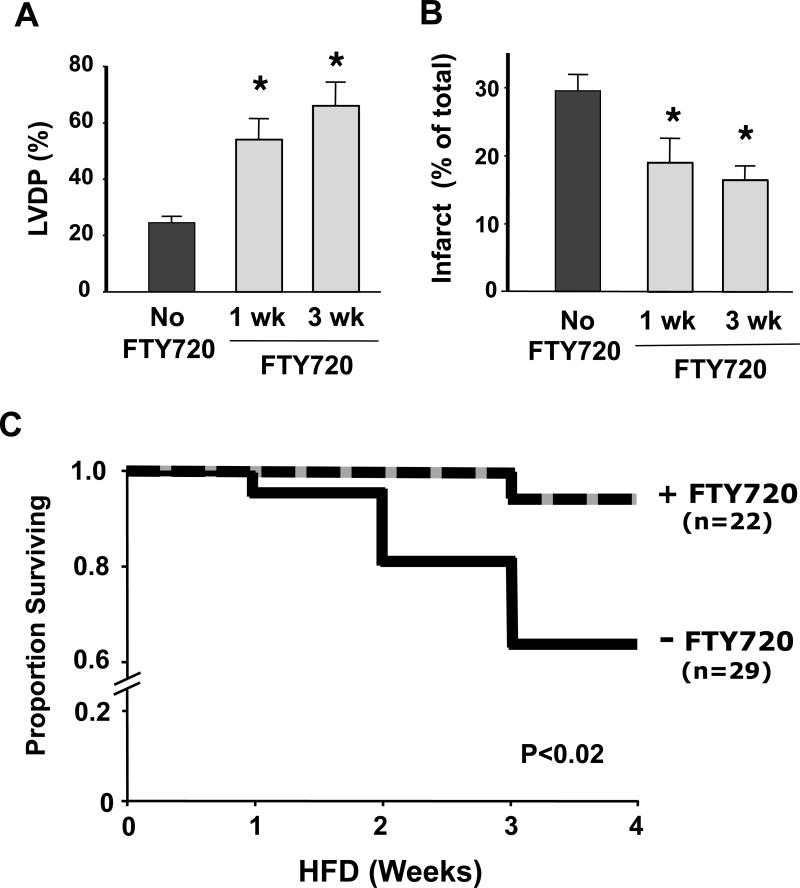
To determine if long-term treatment with FTY720 could improve cardiac function and infarct size in response to I/R injury, we added this agent to the drinking water of C57BL/6J mice at a concentration of 0.3mg/kg/day. This concentration was chosen as optimal after testing a range from 0.3-10mg/kg/day. After treatment for either 1 or 3 weeks, the isolated heart was subjected to 50min ischemia and 50min reperfusion using the Langendorff technique. Left ventricular developed pressure (LVDP) was continuously monitored and infarct size was measured at the end of the experiment. As shown in Figure 2A, recovery of LVDP was significantly improved by an average of 2.7-fold at the end of reperfusion in hearts from mice treated with FTY720 for 3 weeks compared with control mice (24.6 ± 2.2 vs 66.1± 8.2 mmHg, p<0.05). Infarct size was also significantly reduced by almost half at 3 weeks of treatment with FTY720 compared with the control group (Figure 2B, 29.6 ± 2.4% to 16.5 ± 2.1%, p<0.05). Similar results were found after one week of FTY720-treatment (Figure 2A and B).
Figure 2.
FTY720-treatment in wildtype C57BL/6J mice improved recovery of left ventricular developed pressure (LVDP) (A) and reduced infarct size (B) after ex vivo ischemia/reperfusion (I/R) injury. The % at the end of the ischemia/reperfusion period was calculated relative to the baseline LVDP expressed in mmHg for each heart. Data are mean ± SEM, n=6/group, * P<0.05 vs control. FTY=FTY720; Con=Control. (C) Kaplan–Meier survival curve for ApoeR61h/h/SRB1–/– mice fed the HFD and treated or not with FTY720 (FTY720 and Control) for 4 weeks. The FTY720-treated mice exhibited a markedly lower mortality rate (P<0.02). HFD = high fat high cholesterol diet.
FTY720-treatment reduces mortality of HFD-fed ApoeR61h/h/SRB1–/– mice
Based on the efficacy of FTY720-treatment as a cardioprotective intervention described above and its effects on atherosclerosis in previous studies (19, 20), we hypothesized that adding FTY720 to the drinking water would protect high fat high cholesterol diet (HFD) fedApoeR61h/h/SRB1–/– mice from heart failure and premature death caused by occlusive coronary atherosclerosis (23). As shown in Figure 2C, a concentration of 0.05mg/kg/day of FTY720, markedly reduced the 4 week mortality rate of mice fed the HFD. During this period, only 1 of 22 FTY720-treated mice died, while 10 of 29 control mice succumbed from fatal myocardial infarctions caused by occlusive coronary atherosclerosis (p<0.02 by log rank test).
FTY720-treated HFD-fed ApoeR61h/h/SRB1–/– mice exhibit less impairment of LV function and display less LV remodeling
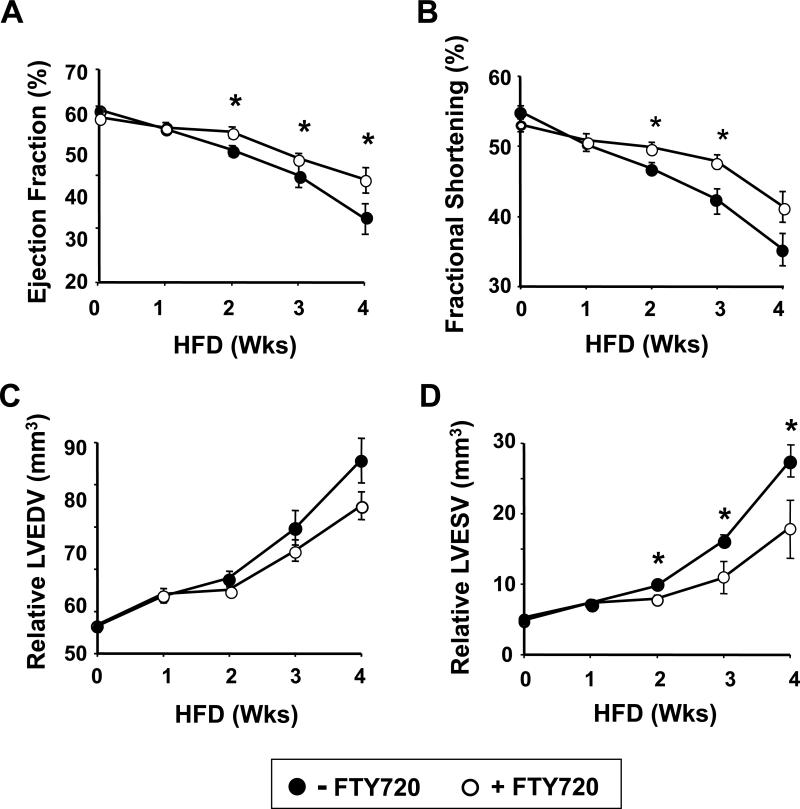
To directly determine the effects of FTY720 on cardiac performance in ApoeR61h/h/SRB1–/– mice fed the HFD, we performed serial echocardiography in conscious mice. As shown in Figure 3A-B, LV function measured by ejection fraction and fractional shortening was less depressed starting by the second week of HFD feeding in the FTY720-treated mice. Similarly, LV end-diastolic volume was less after 2 weeks of HFD feeding (Figure 3C), while LV end-systolic volume, a clinically relevant predictor of an adverse prognosis in humans (24), was significantly less after 2 weeks of HFD feeding in the FTY720-treated mice (Figure 3D). It should be noted that all of these measures were presumably worse in the mice that had already died, so the beneficial effect of FTY720 may in fact be an underestimate.
Figure 3.
Echocardiographic measures of left ventricular performance. Ejection fraction (A) and fractional shortening (B), left ventricular end-diastolic volume (LVEDV) (C) and left ventricular end-systolic volume (LVESV) (D) in ApoeR61h/h/SRB1–/– mice fed the HFD and treated or not with FTY720 for 4 weeks. Data are mean ± SEM. * P<0.05 vs control. HFD = high fat high cholesterol diet.
FTY720-treatment does not alter hypercholesterolemia or the extent of coronary atherosclerosis, cardiac collagen and lipid deposition in HFD-fed ApoeR61h/h/SRB1–/– mice
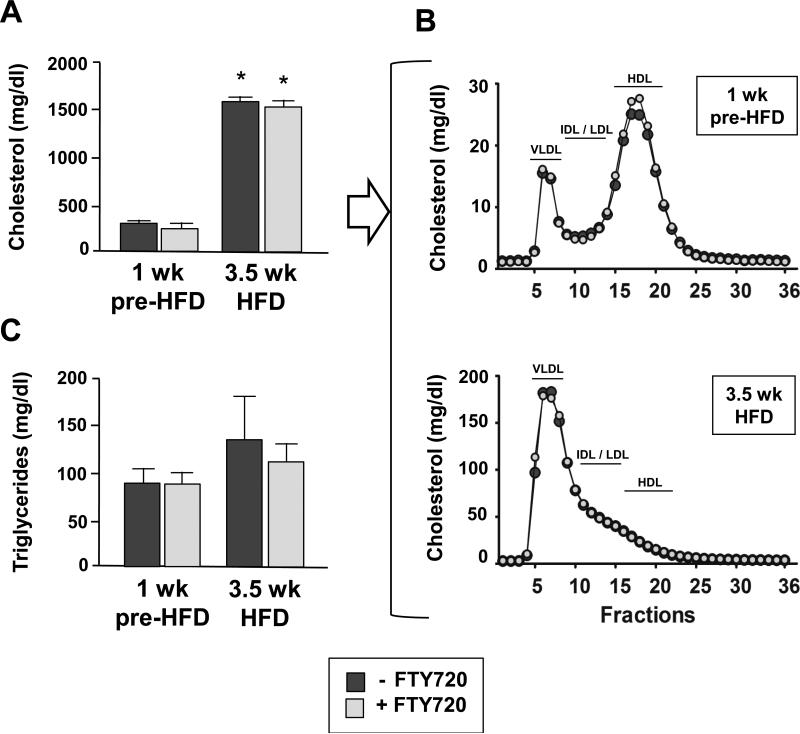
To elucidate the origin of FTY720 induced cardioprotection we determined if FTY720 ameliorates hypercholesterolemia and its pathological outcome. Additional cohorts were fed the HFD, but due to the high mortality of control mice we reduced the duration of HFD feeding to 3.5 weeks. As shown in Figure 4A and B, FTY720 did not affect the plasma cholesterol and triglyceride levels or the lipoprotein profile either after 1 week of pre-treatment with a chow diet or after 3.5 weeks of HFD-feeding with or without FTY720.
Figure 4.
(A and B) Plasma cholesterol levels (n=16-20 ApoeR61h/h/SRB1–/– mice/group) and lipoprotein profiles (n=3 pools with 3-7 mice/pool). Data are at 1wk pre-HFD and at 3.5wk after starting the HFD. Mice were fed FTY720 or not. As can be seen, the lipoprotein profiles are identical. Lipoprotein classes are indicated as VLDL (very low density lipoprotein), IDL/LDL (intermediate density lipoprotein / low density lipoprotein) and HDL (high density lipoprotein) (C) Plasma triglyceride levels obtained in the same mice also show no difference. Data are mean ± SEM. * P<0.001 (1wk pre-HFD vs 3.5wk HFD for both control and FTY720). 1wk pre-HFD = 1week ± FTY720-treatment on chow diet; 3.5wk HFD = 3.5 weeks ± FTY720-treatment with HFD feeding. HFD = high fat high cholesterol diet.
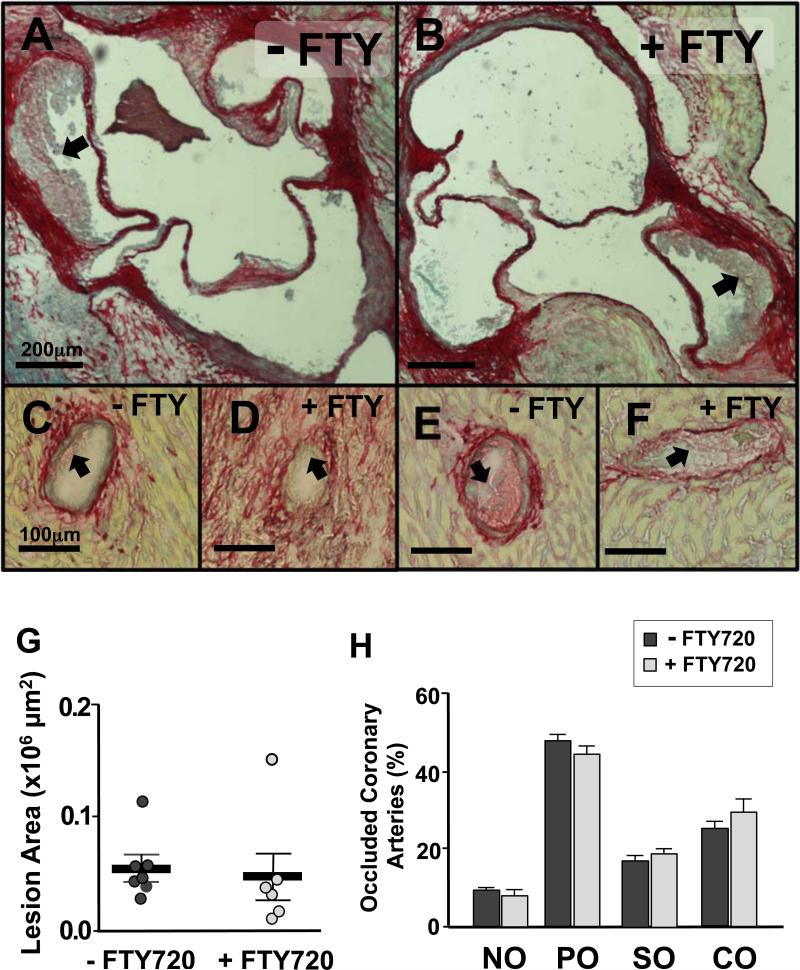
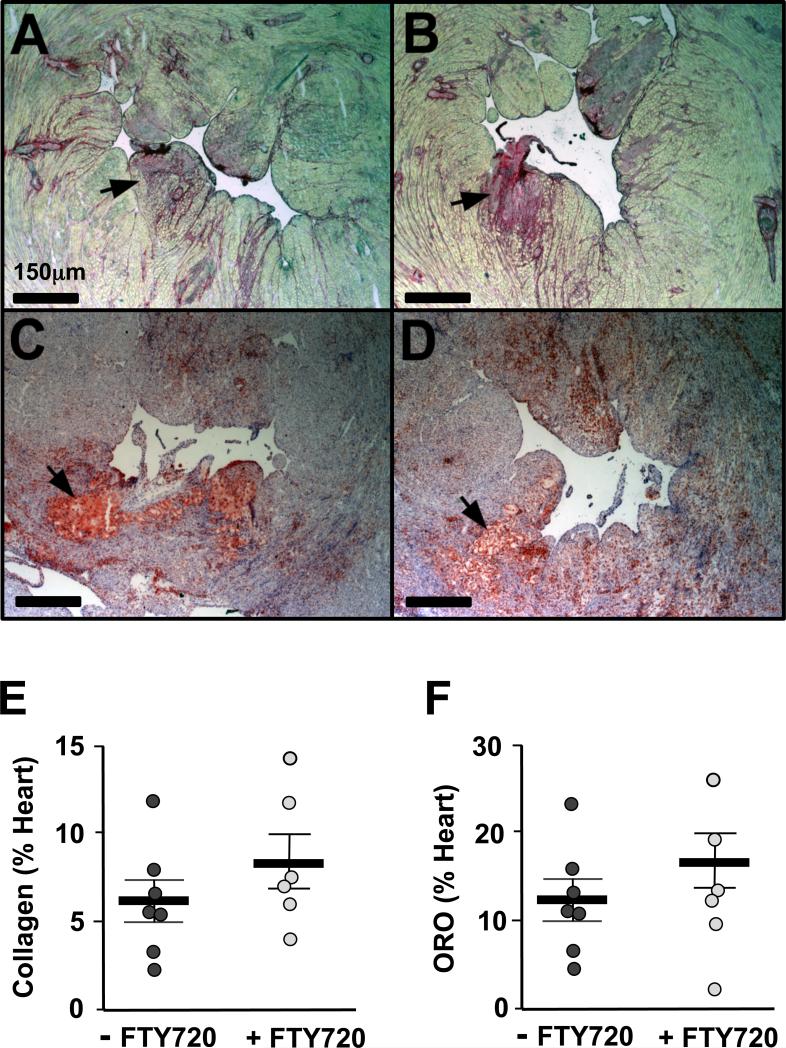
We next assessed the severity of atherosclerosis in the aortic sinuses and the coronary arteries of the mice fed the HFD for 3.5 weeks. As shown in Figure 5A, B and G, both FTY720-treated and control mice developed similar atherosclerotic lesion sizes in the aortic sinus. To measure the severity of occlusive coronary atherosclerosis we quantified the extent of obstruction via visual inspection (23). Interestingly, both groups displayed a similar and significant burden of atherosclerosis in the coronary arteries where more than 50% of all coronary arteries examined throughout the heart were severely occluded, with 20% of them showing complete occlusion (Figure 5C-F and H). We also examined the pathological consequences of hypercholesterolemia and ischemic heart disease among our cohorts of mice by quantifying the amount of collagen (Sirius red stain) and lipid (ORO staining) deposition in the heart. As shown in Figure 6, we did not observe differences in either neutral lipid or collagen surface areas in the sections collected throughout the heart. We also observed no difference in heart weights between control and FTY720 treated mice.
Figure 5.
Representative images of aortic root lesions (A and B) and partially (C and D) or severely (E and F) occluded coronary arteries stained with Sirius red from ApoeR61h/h/SRB1–/– mice fed the HFD for 3.5 weeks with FTY720-treatment or not. (n=7-8 mice/group). Quantification of aortic root lesion area (G) and occluded coronary arteries (H). Black arrows identify the plaque accumulated in the vasculature. Data are mean ± SEM. NO = non occluded (0-5%); PO = partially occluded (5-50%); SO = severely occluded (50-95%); CO = completely occluded (95-100%).
Figure 6.
Images of heart sections showing the left ventricular chamber stained with Sirius Red (A and B) and Oil red O (C and D) of ApoeR61h/h/SRB1–/– mice fed the HFD for 3.5 weeks with FTY720-treatment or not (n=7-8 mice/group). Quantification of collagen (E) and neutral lipid (F) deposited in the myocardium. Black arrows identify the area with clusters of collagen or neutral lipid. Data are mean ± SEM. ORO = oil red O
Thus, despite the similar burden of atherosclerosis in the aortic root and the coronary arteries and in the pathological consequences evidenced by collagen and lipid deposition in cardiac tissue, FTY720-treated mice fed the HFD still exhibited less impaired LV function and reduced mortality (Figures 2C and 3).
FTY720-treatment causes immunosuppression in the circulation and secondary lymphoid organs of HFD-fed ApoeR61h/h/SRB1–/– mice
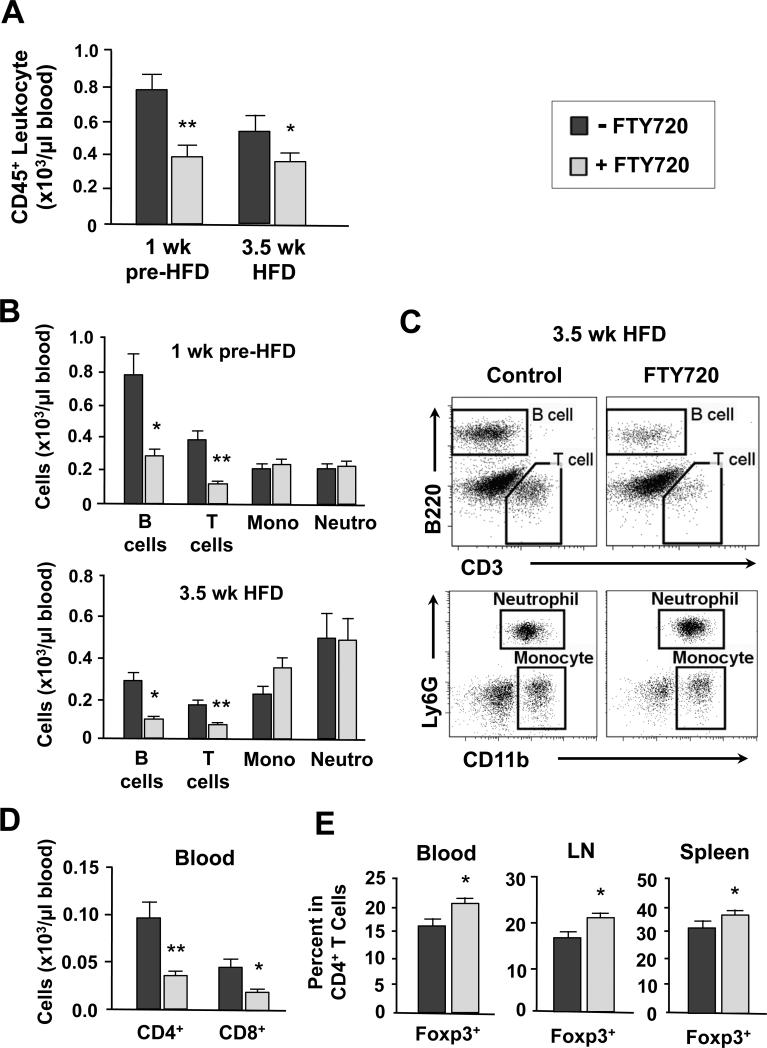
Because FTY720 did not affect hypercholesterolemia or the extent of coronary artery disease, we hypothesized that FTY720 might improve cardiac function by different mechanisms. In previous studies, the immunosuppressive effect of FTY720 was attributed to a down-regulation of lymphocyte S1P1 receptors thus preventing lymphocyte egress and recirculation from lymphoid organs to peripheral sites of inflammation (17, 18). Thus, we examined the immunosuppressive effect of FTY720 in our model. As shown in Figure 7A, FTY720-treatment induced significant and parallel immunosuppression with marked reduction of blood leukocytes both at 1wk pre-HFD and at 3.5wk of HFD feeding. Both T and B cells were most profoundly reduced and there was no difference in the number of myeloid derived cells (CD11b+) (Figure 7B), including among Ly6Chigh and Ly6Clow monocyte subsets. Representative FACS plots at 3.5 weeks of HFD feeding show the reduction of T and B cells (Figure 7C). The decline in T cells resulted from a reduction of CD4+ and CD8+ cells (Figure 7D). Interestingly, the percentage of CD4+Foxp3+ regulatory T cells (Tregs), a class of T cells involved in immunosuppression, was significantly higher among the CD4+ T cell population in the circulation both before the HFD feeding (data not shown) and after 3.5 weeks of HFD feeding (Figure 7E; left panel). Consistent with this observation, there was a significantly increased percentage of Tregs among CD4+ T cells in the spleen and lymph nodes (axillary and brachial) (Figure 7E, middle and right panels).
Figure 7.
(A) Number of CD45+ leukocytes per μl of blood of ApoeR61h/h/SRB1–/– mice at 1wk pre-HFD and 3.5wk HFD time-points with FTY720-treatment or not. (B) Numbers of B cells, T cells, monocytes (mono) and neutrophils (neutro) per μl of blood at 1wk pre-HFD and 3.5wk HFD time-points. (C) Representative FACS plots of CD45+ blood leukocytes from ApoeR61h/h/SRB1–/– mice after 3.5 weeks of HFD feeding. Shown are gated populations: T cells (CD3+B220–), B cells (CD3–B220+), neutrophils (Ly6G+CD11b+) and monocytes (Ly6G–CD11b+). (D) Numbers of CD4+ and CD8+ T cells per μl of blood after 3.5 weeks of HFD feeding. (E) Percentage of Foxp3+ T cells among CD4+ T cells in the circulation, LN and spleen after 3.5 weeks of HFD feeding. Data are mean ± SEM. * P<0.05, ** P<0.01 vs Control. 1wk pre-HFD = 1week chow diet with FTY720-treatment or not; 3.5wk HFD = 3.5 weeks HFD with FTY720-treatment or not; LN = lymph nodes. HFD = high fat high cholesterol diet.
Thus, FTY720-treatment reduced the number of T and B cells in the circulation without influencing the numbers of myeloid-derived neutrophils or monocyte subsets. Moreover, FTY720 increased the proportion of Tregs among the CD4+ T cell population in the circulation, lymph nodes and spleen resulting in profound systemic immunosuppression.
FTY720-treatment ameliorates inflammation in the failing heart post-MI
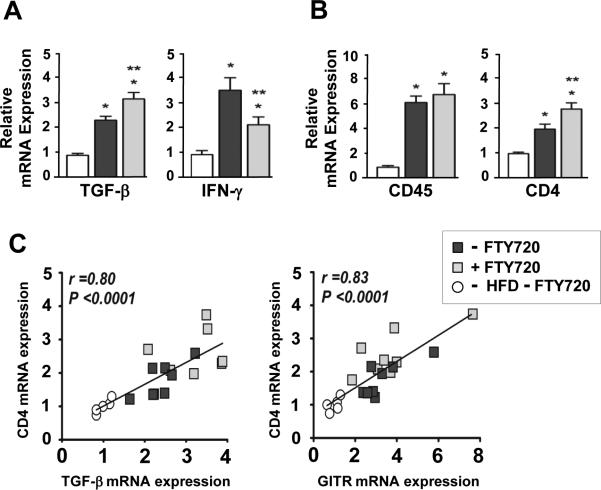
Based on these findings we next hypothesized that immunosuppression might have taken place in the heart during chronic inflammation of the coronary arteries. To determine whether the reduction of T and B cells and the increased percentage of Tregs in the circulation affected the heart during HFD feeding, we measured mRNA expression levels of pro- and anti-inflammatory cytokines and leukocyte cell surface markers. As shown in Figure 8A, in the FTY720-treated mice, the expression level of TGF-β was increased by 35% and IFN-γ was decreased by 40% compared with control mice, suggesting reduced cardiac inflammation. The mRNA expression levels of other pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 were measured, but showed no significant differences (data not shown). Interestingly, CD4 expression was significantly increased by 42% in the FTY720-treated mice, whereas CD45, a cell surface marker for all leukocytes, showed no difference (Figure 8B). Other leukocyte cell surface markers, including CD8a, CD11c, F4/80 and CD11b mRNA expression levels also were measured, but showed no significant differences (data not shown). Moreover, the expression of molecules involved in Treg activity such as GITR and TGF-β (25) showed a strong correlation with the expression of CD4 (Figure 8C). These data suggest that under chronic coronary atherosclerotic conditions the increased proportion of Tregs in the circulation of the FTY720-treated mice could have resulted in reduced inflammation and increased CD4 expression in the myocardium, which strongly correlated with Treg activity.
Figure 8.
(A) Relative mRNA expression levels of TGF-β and IFN-γ. (B) CD45+ leukocytes and CD4+ T cells in hearts from mice not fed the HFD and after 3.5 weeks of HFD feeding with FTY720-treatment or not. (C) Correlations of mRNA expression levels of CD4+ T cells with TGF-β and GITR. Amounts of cDNA were all normalized using TATA box binding protein and hypoxanthine phosphoribosyl transferase. The relative quantity of mRNA was compared to the No HFD group. Data are mean ± SEM. * p<0.05 vs No HFD; ** p<0.05 vs Control. GITR = glucocorticoid induced tumor necrosis factor receptor related gene. HFD = high fat high cholesterol diet.
Discussion
There are two principle findings of this study: first, we show for the first time that long-term oral FTY720-treatment protects the murine heart against acute I/R injury; second, we demonstrate that FTY720-treatment significantly prolongs survival in a mouse model of chronic occlusive coronary atherosclerosis and MI. In addition, the cardioprotection afforded by FTY720 occurred without affecting the underlying atherosclerotic burden but likely through substantial immunosuppression in these mice.
FTY720 is a structural analog of sphingosine. It is phosphorylated in the cytoplasm by sphingosine kinase-2 and functions as an agonist at S1P receptors. Among these receptors the S1P1 and 3 receptors are particularly pertinent for the heart, and we have previously shown that the naturally occurring compound S1P and phosphorylated FTY720 act through S1P1 receptors in isolated adult cardiac myocytes (2) although others have invoked S1P3 mediated pathways for cardioprotection (4). Overall, the cytoprotective actions of FTY720 are not limited to the heart but extend to cerebral ischemia and stroke (26, 27), hepatic I/R injury (28), and renal I/R injury (29).
FTY720 in acute heart injury
Previous studies of FTY720 in cardioprotection were mainly done in vitro or ex vivo in acute injury models. Egom et al. reported that FTY720 prevented I/R-induced cardiac arrhythmias in an ex vivo rat heart model via activation of Pak1/AKT signaling (14). The same group subsequently noted that FTY720 protected neonatal rat ventricular cardiomyocytes against CoCl2-induced hypoxic injury and triggered NO release through a pertussis toxin-sensitive Pak1/AKT/eNOS pathway (30). They also reported that the anti-hypertrophic effect of FTY720 likely acts through Pak1, thereby identifying Pak1 as a novel therapeutic target for anti-hypertrophic treatment of the heart (31). Hofmann et al. reported that treatment with FTY720 at the time of reperfusion in isolated adult rat hearts improved recovery of LVDP and reduced LV end-diastolic pressure without affecting infarct size (15). A subsequent ex vivo study from the same group using adult rats subjected to myocardial I/R injury showed that FTY720 did not reduce infarct size, and had variable effects on ventricular arrhythmias (32). In contrast, Egom et al. reported that FTY720 appears to possess anti-arrhythmic properties (14). Although we did not perform telemetry, we noted a slowing of the heart rate during echocardiography in mice with the largest hearts; the maximum decrease in heart rate was from 600 to 450 bpm, but ventricular arrhythmias were never recorded. Oral FTY720 is known to induce bradycardia in humans, but tachyarrhythmias do not seem to be one of the adverse consequences of this drug.
In our current study we found that long-term FTY720-treatment in adult C57BL/6J mice resulted in enhanced recovery of LVDP and reduced infarct size after ex-vivo I/R injury. Part of the underlying mechanism for this cardioprotection may be due to the activation of pro-survival signals, such as AKT and ERK, stimulated by FTY720 as reported by us and others (2, 10, 15, 30). We also demonstrated in adult murine cardiac myocytes that exogenous FTY720 induces reversible S1P1-mediated ERK phosphorylation, indicative of receptor recycling (10).
FTY720 in chronic inflammatory atherosclerosis
Previous studies in genetically altered mice have suggested that FTY720 could reduce the atherosclerosis burden in the setting of hyperlipidemia. Keul et al. gave Apoe–/– mice FTY720 at a concentration 1.25mg/kg/day in water for 4 weeks and then fed these mice a “Western” diet for 20 weeks (19). In that study, FTY720-treated mice had markedly reduced atherosclerotic lesion volume and macrophage and collagen content in these lesions. In another report, Nofer et al. administered FTY720 by the i.p. route to Ldlr–/– mice at a concentration of 0.04mg/kg/day (low dose) and 0.4mg/kg/day (high dose) 3 times per week for 16 weeks during high fat feeding (20). Interestingly, the aortic root lesion size and necrotic core formation were reduced only in the high dose treated mice without affecting plasma lipid levels. Similar to our findings there was a decrease in the number of lymphocytes in the circulation. In addition, they reported that FTY720 reduced plasma concentrations of IFN-γ, IL-6 and TNF-α. More recently, Blom et al. reported that FTY720 stimulates 27-hydroxycholesterol production and confers athero-protective effects in human primary macrophages (33). As such, FTY720 protects macrophages from free cholesterol-induced toxicity. Of note is that most of the doses of FTY720-treatment used in these reports were 10-30 times greater than the concentration used in our study. Also, in none of these reports was heart failure or mortality noted as Apoe–/– or Ldlr–/– mice do not develop severe coronary atherosclerosis.
HypoE mice carry the hypomorphic ApoeR61h/h allele and display low plasma Arg-61 apoE levels (21, 34). Shortly after reporting the development of HypoE mice, we demonstrated that the unique hypomorphic Apoeh/h allele endows HypoE mice with a susceptibility to diet-induced hyperlipidemia and atherosclerosis that can be rapidly reversed by returning HypoE mice to a low fat chow diet and by inducible cre-mediated gene repair of the HypoE allele (21, 22). Subsequently, HypoE mice were bred to SRB1–/– mice to generate HypoE/SRB1–/– mice, also known as ApoeR61h/h/SRB1–/– mice, which led to a model of diet-induced coronary atherosclerosis, myocardial infarction and premature death (23). Based on studies cited above in Apoe–/– and Ldlr–/– mice (19, 20) in which FTY720 reduced atherosclerosis in the aortic root, we designed a “clinical trial” of FTY720 in ApoeR61h/h/SRB1–/– mice with mortality as an end-point. Secondary end-points included the extent of LV dysfunction and remodeling determined by echocardiography. They also included the extent and proportion of occlusive coronary atherosclerosis, and collagen and lipid deposition in the myocardium. The results revealed that FTY720-treatment did indeed have a marked mortality benefit. In addition, echocardiographic heart volumes, particularly at end-systole, which are a clinical indicator of adverse outcomes in humans (24), were smaller in the FTY720-treated mice, while ejection fraction and fractional shortening were less depressed compared to the controls. Thus, cardiac function was better and there was less pathological heart remodeling in the FTY720-treated mice, consistent with the beneficial effects of FTY720-treatment after ex vivo I/R injury in the C57BL/6J mice. To our surprise, in contrast to the beneficial effects of FTY720-treatment on heart function, we observed no effects on occlusive coronary atherosclerosis or atherosclerosis burden in the aortic root. Moreover, FTY720 did not have an impact on the deposition of collagen or neutral lipid in the myocardium. Our findings are in contrast to those of Keul et al. (19) and Nofer et al. (20) who reported anti-atherosclerotic effects of FTY720. However, our findings do support those of Klingenberg et al. (35) and Poti et al. (36), which showed that FTY720 did not have an impact on reducing the extent of atherosclerosis in moderately dyslipidemic Apoe–/– and LDLr–/– mice respectively. The lack of noticeable atheroprotection from FTY720 in our mice could be explained by our use of low dose FTY720-treatment and the severity of atherosclerosis in this model (23).
Surprisingly, feeding a HFD to our ApoeR61h/h/SRB1–/– mouse model led to a dramatic expansion of spleen weight and cell number 5-10-fold above normal (data not shown). These findings are in agreement with reports showing that the level of myeloid derived cells, which are known to contribute significantly to atherosclerosis progression, increase significantly in the circulation and in the spleen in response to hyperlipidemia and myocardial infarction (37, 38).
Immunosuppression and protection from I/R injury: a role for FTY720?
Previous studies have demonstrated benefits of immunosuppression in the prevention of I/R injury in multiple organs (39). Elimination of both T and B cells using Rag–/– mice as a model demonstrated the participation of these cells in ischemic tissue injury. Thus, Rag–/– mice subjected to I/R injury display significantly smaller heart infarct areas compared to control mice, while the adoptive transfer of CD4+ T cells into Rag–/– mice led to larger areas of infarction (40). Moreover, transfusion of wild-type serum into Rag–/– mice restored I/R injury in the kidney by introducing natural autoantibodies that react to cellular neo-antigens that become exposed following ischemic injury (41).
Thus, one way in which FTY720 could have improved heart function and extended the lifespan of our ApoeR61h/h/SRB1–/– mice that displayed occlusive atherosclerosis is through promotion of immunosuppression. Indeed the five to seven-fold decrease in both T and B cells in FTY720-treated mice may likely have recapitulated aspects of protection from I/R injury described extensively in studies of Rag–/– mice (39, 40). Reduction of local cell death after ischemia/reperfusion is accompanied by diminished lymphocyte infiltration in the heart. Another mechanism for attenuation of T cell infiltration is via a reduction of cardiomyocyte apoptosis secondary to a reduction of cell death signal activation. We have previously reported that chronic S1P1 agonism reduces apopotosis in a mouse myocardial infarction model (42).
The increased percentage of CD4+Foxp3+ Tregs in the circulation and secondary lymphoid organs also may have played a significant role during chronic inflammation as these cells are well known for preventing autoimmunity (43). In a recent study, Hofmann et al. demonstrated an early relative increase in the frequencies of CD4+ T cells, especially CD4+Foxp3+ Tregs, in heart-draining lymph nodes that participate in wound healing and subsequent remodeling of the myocardium as early as 3 days after MI (44). Our study also shows an increased frequency of CD4+Foxp3+ Tregs in the draining lymph nodes, both axillary and brachial. In addition, CD4 mRNA expression levels were increased in the hearts from FTY720-treated mice which exhibited strong correlations with the expression of molecules involved in Treg activity such as GITR and TGF-β (25) in the hearts after 3.5 weeks of HFD feeding. Because FTY720 has recently been shown to enhance the conversion of conventional CD4+ T cells into CD4+Foxp3+ Tregs (45), it is possible that such an effect could explain the enhanced presence of Tregs in various tissues of our mice treated with FTY720.
We recognize that our data do not conclusively demonstrate whether FTY720 treatment resulted in changing the extent of myocardial infarction in ApoeR61h/h/SRB1–/– mice. However, we can state with certainty that the extent of collagen deposition as a consequence of myocardial infarction was not different as a result of FTY720 treatment. Despite the limitation of the histological stains we used to provide information on tissue infarction, we nonetheless recorded no differences in the extent of occlusive coronary atherosclerosis in mice given FTY720. Therefore, based on our overwhelming data demonstrating that FTY720 extended longevity and improved heart functions in these animals without altering the severity of coronary atherosclerosis, it is reasonable to infer that FTY720 did so by diminishing myocardial ischemic injury through a combination of beneficial effects on cardiomyocytes, including increased survival, reduced infarction and immunosuppression. We recognize however that FTY720 does not substitute for aggressive reduction/treatment of risk factors causing development and progression of atherosclerosis and coronary artery disease.
In summary, long-term FTY720-treatment conferred cardioprotection by reducing acute I/R injury in the normal murine heart as evidenced by a decreased infarct size and improved hemodynamic recovery after reperfusion. FTY720 also reduced the mortality of our mouse model of occlusive coronary atherosclerosis, MI and heart failure independent of the unchanged underlying atherosclerotic process and without reducing blood lipid levels. These findings point to alternative mechanisms that could be responsible for the prolonged survival of FTY720-treated mice. One explanation is that our current and prior data (2) and reports of others (14, 30) suggest that FTY720 enhances the resistance of cardiac myocytes to ischemic injury, oxidative stress and apoptosis. And another is that FTY720-treatment reduces inflammation in the heart, which likely resulted from a profound reduction of T and B cells in the circulation, and an increased proportion of CD4+Foxp3+ Tregs in the blood and secondary lymphoid organs. A focus of our future studies will be to determine whether FTY720 can reverse pathological cardiac remodeling or even normalize left ventricular function in HFD-fed ApoeR61h/h/SRB1–/– mice in which we can rapidly correct hyperlipidemia by repairing the HypoE allele using inducible cre-mediated gene repair and returning the mice to a chow diet as we previously reported (21, 22, 46). We will also explore the cardio-protective effects of FTY720 in mouse models of coronary artery ligation and in large animal models as a prelude to future human studies.
Acknowledgments
This work was supported by Grants from the National Institutes of Health HL089871 (RLR) and HL090606 (JSK), which were administered by the Northern California Institute for Research and Education, and a Merit Review grant from the Department of Veterans Affairs 5I01BX000532 to RLR, and with resources of the Veterans Affairs Medical Center, San Francisco, California.
References
- 1.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 3.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 4.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 5.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79:134–140. doi: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 6.Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- 8.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:BR318–324. [PubMed] [Google Scholar]
- 9.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Tao R, Hoover HE, Zhang J, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated extracellular signal-related kinase signaling and desensitization. J Cardiovasc Pharmacol. 2009;53:486–494. doi: 10.1097/FJC.0b013e3181a7b58a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- 13.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48:406–414. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 16.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- 17.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 19.Keul P, Tolle M, Lucke S, von Wnuck Lipinski K, Heusch G, Schuchardt M, van der Giet M, Levkau B. The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:607–613. doi: 10.1161/01.ATV.0000254679.42583.88. [DOI] [PubMed] [Google Scholar]
- 20.Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, van Berkel T, Assmann G, Biessen EA. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 21.Raffaï RL, Weisgraber KH. Hypomorphic apolipoprotein E mice. A new model of conditional gene repair to examine apolipoprotein E-mediated metabolism. J. Biol. Chem. 2002;277:11064–11068. doi: 10.1074/jbc.M111222200. [DOI] [PubMed] [Google Scholar]
- 22.Raffai RL, Loeb SM, Weisgraber KH. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M. Diet-Induced Occlusive Coronary Atherosclerosis, Myocardial Infarction, Cardiac Dysfunction, and Premature Death in Scavenger Receptor Class B Type I-Deficient, Hypomorphic Apolipoprotein ER61 Mice. Circulation. 2005 doi: 10.1161/CIRCULATIONAHA.104.523563. [DOI] [PubMed] [Google Scholar]
- 24.McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2009;22:190–197. doi: 10.1016/j.echo.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 29.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr., Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 30.Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, Stringer SE, Ke Y, Shaheen M, Wang T, et al. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301:H1487–1495. doi: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, et al. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann U, Hu K, Walter F, Burkard N, Ertl G, Bauersachs J, Ritter O, Frantz S, Bonz A. Pharmacological pre- and post-conditioning with the sphingosine-1-phosphate receptor modulator FTY720 after myocardial ischaemia-reperfusion. Br J Pharmacol. 2010;160:1243–1251. doi: 10.1111/j.1476-5381.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blom T, Back N, Mutka AL, Bittman R, Li Z, de Lera A, Kovanen PT, Diczfalusy U, Ikonen E. FTY720 stimulates 27-hydroxycholesterol production and confers atheroprotective effects in human primary macrophages. Circ Res. 2010;106:720–729. doi: 10.1161/CIRCRESAHA.109.204396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffaï RL, Dong L-M, Farese RV, Jr., Weisgraber KH. Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klingenberg R, Nofer JR, Rudling M, Bea F, Blessing E, Preusch M, Grone HJ, Katus HA, Hansson GK, Dengler TJ. Sphingosine-1-phosphate analogue FTY720 causes lymphocyte redistribution and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2392–2399. doi: 10.1161/ATVBAHA.107.149476. [DOI] [PubMed] [Google Scholar]
- 36.Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C, Simoni M, Nofer JR. Effect of sphingosine 1-phosphate (S1P) receptor agonists FTY720 and CYM5442 on atherosclerosis development in LDL receptor deficient (LDL-R(-)/(-)) mice. Vascul Pharmacol. 2012;57:56–64. doi: 10.1016/j.vph.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 41.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 42.Yeh CC, Li H, Malhotra D, Huang MC, Zhu BQ, Goetzl EJ, Vessey DA, Karliner JS, Mann MJ. Sphingolipid signaling and treatment during remodeling of the uninfarcted ventricular wall after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1193–1199. doi: 10.1152/ajpheart.01032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 45.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- 46.Eberle D, Luk FS, Kim RY, Olivas VR, Kumar N, Posada JM, Li K, Gaudreault N, Rapp JH, Raffai RL. Inducible Apoe Gene Repair in Hypomorphic ApoE Mice Deficient in the Low-Density Lipoprotein Receptor Promotes Atheroma Stabilization with a Human-Like Lipoprotein Profile. Arterioscler Thromb Vasc Biol. 2013;33:1759–1767. doi: 10.1161/ATVBAHA.112.300605. [DOI] [PMC free article] [PubMed] [Google Scholar]