Abstract
Appearance of mouse fetal Leydig cells requires activation of the Hedgehog pathway. Upon binding to the membrane-bound receptor patched, Hedgehog ligands induce intracellular responses via a combined effect of Gli transcription factors. Szczepny et al. (Biol Reprod 2009; 80:258–263) found that Gli1, one of the three Gli transcription factors, is present in the fetal testis and that its expression is suppressed by the Hedgehog inhibitor cyclopamine. In this study, we investigated the involvement of the Gli1 and Gli2 factors in mouse fetal Leydig cell differentiation. The Gli1 and Gli2 transcription factors showed an overlapping expression pattern in the testis interstitium at the time when fetal Leydig cells appear. Despite their similar expression, Gli1 and Gli2 patterns were differentially regulated. Initial Gli1 and Gli2 expression depends upon an active Hedgehog pathway; however, maintenance of only Gli1, but not Gli2, expression requires activation of the pathway. Inactivation of either the Gli1 or Gli2 gene did not affect fetal Leydig cell development and testis morphology, suggesting a functional redundancy. When the transcriptional activity of both GLI1 and GLI2 was suppressed by a selective inhibitor, GANT61, in cultured fetal testes before the appearance of fetal Leydig cells, Gli1 and Gli2 expression and steroidogenic marker activity were completely abolished. However at later stages when Leydig cells were already present, GANT61 treatment inhibited Gli1 expression but had no effects on Gli2 expression and fetal Leydig cell appearance. Our results reveal overlapping and redundant Gli1 and Gli2 roles in fetal Leydig cell differentiation and a novel regulation of Gli2 expression in the fetal testis.
Keywords: developmental biology, Gli, hedgehog, Leydig cells, steroidogenesis, testis
The transcription factors Gli1 and Gli2 are redundant initiators of fetal Leydig cell differentiation, while Gli2 maintains fetal Leydig cells in a Hedgehog-independent manner.
INTRODUCTION
In mammals, the Hedgehog (Hh) pathway is known to regulate patterning of fetal organs, and its aberrant activation has been linked to the development of cancers in adult life [1]. Three mammalian Hh genes have been identified: desert hedgehog (DHH), Indian hedgehog (IHH), and Sonic hedgehog (SHH) [2]. Intracellular signaling pathway induced by SHH is the most well-characterized among the three mammalian HH genes [1]. Once SHH is processed and secreted, it binds to its receptor, patched (PTCH), in a concentration-dependent manner [3]. In mammals, PTCH, a 12-span transmembrane receptor, is encoded by two different genes, PTCH1 and PTCH2 [4]. PTCH1 has been shown to be the dominant receptor [4]. In the absence of SHH, the PTCH1 receptor is expressed at a basal level, which is sufficient to inhibit the activity of the seven-transmembrane-span SMO protein [5]. The binding of SHH to PTCH1 results in the loss of the ability of PTCH1 to inhibit SMO. The consequent activation of SMO transduces the Hh signal to the cytoplasm, leading to the activation/inhibition of the Gli family of transcription factors [6]. Both GLI1 and GLI2 act primarily as transcriptional activators, whereas GLI3 is a transcriptional repressor [7]. Evidence suggests that GLI2, but not GLI1, is cleaved to generate repressor forms of the protein, dependent upon the tissue context [8]. The immediate molecular action in response to Hh activation is the up-regulation of Gli1 and Ptch1 [2]. Thus, Gli1 or Ptch1 up-regulation is used as an indicator of an active Hh pathway.
The Hh pathway has been implicated in the development and differentiation of the fetal testis [9] and postnatal and adult ovary [10, 11]. In the fetal testis, Sertoli cell-derived DHH is responsible for differentiation of the fetal Leydig cells, the primary steroidogenic cell type in the developing testis [12]. At embryonic Day 11.5 (E11.5) in mouse embryos, Sertoli cells start to synthesize DHH, which triggers the differentiation of fetal Leydig cells in the testis interstitium [13, 14]. When Dhh was inactivated, numbers of fetal Leydig cells were significantly reduced, leading to lowered androgen production and consequent underdevelopment of androgen-dependent male accessory organs [14, 15]. On the other hand, when E11.5 testes were cultured for 48 h in the presence of cyclopamine, a general inhibitor of SMO [16], the fetal Leydig cell population was completely abolished [14, 17]. These observations together suggest the possibility of compensation by other Hh ligands in addition to Dhh in the fetal testis. Indeed, the ability of Hh ligands to compensate for one another has been reported in other tissues such as the prostate [18], where loss of Shh is accompanied by an up-regulation of Ihh expression.
In addition to the redundant action of Hh ligands, a second tier of compensation has been reported at the level of the Gli transcriptional factors. It was found that replacement of the Gli2 with the Gli1 gene was able to restore the function of the Hh signaling pathway in the Gli2 knockout mice [19]. Additionally, Gli1 knockout mice were viable, whereas Gli1 knockout mice heterozygous for the Gli2 knockout allele (Gli1ZFD/ZFD;Gli2ZFD/+) died perinatally due to severe developmental defects, indicating gene dosage-dependent compensation of Gli2 in the absence of Gli1 [20]. Taking into account the complexity of the mammalian Hh pathway and its different levels of compensation and redundancy, we investigated the roles of Gli1 and Gli2 in mediating Hh-induced differentiation of fetal Leydig cells in the mouse embryos. We did so by first identifying the Gli genes targeted by SMO and then we sought to determine their functional roles in the establishment of fetal Leydig cells, using ex vivo testis culture and knockout models.
MATERIALS AND METHODS
Animals
Mouse colonies were maintained following the University of Illinois Institutional Animal Care and Use Committee regulations and in accordance with the Animal Welfare Act and Public Health Service Policy. Both Gli1-LacZ and Gli2-LacZ mouse lines were generously provided by Dr. Alex Joyner (Sloan-Kettering Institute, New York City, NY) and were maintained in a mixed genetic background [19, 21, 22]. Mice heterozygous for the LacZ allele were used as reporter lines, and mice homozygous for the LacZ allele were used for knockout analysis.
X-Gal Staining
Gonads with the mesonephroi attached were isolated from embryos and placed in X-gal substrate (0.25–1 mg/ml in final concentration of 2 mM MgCl2 plus 0.02% NP-40 in PBS plus 3.5 mM K ferrocyanide plus 3.5 mM K ferricyanide). Once color development had occurred, samples were fixed in 4% paraformaldehyde for 30 min, dehydrated through a sucrose gradient, and embedded in a sucrose-OCT embedding compound (Bayer) medium at a ratio of 1:3. Samples were cryosectioned at 8–10 μm on treated glass slides. FastRed nuclear counterstain (Vector Laboratories, CA) was applied to the slides for 30 sec to 1 min. The slides were rinsed in double-distilled water and then dehydrated through an ethanol-xylene gradient and mounted.
Ex Vivo Organ Culture
E11.5 and E12.5 testis and mesonephros complexes from CD1 or LacZ reporter embryos were isolated. The complexes were cultured in 35-mm cell culture dishes (Greiner Bio-One, Germany) floating in drops of Dulbecco modified Eagle medium with high glucose, supplemented with 10% fetal calf serum (Hyclone, UT) and 50 μg/ml ampicillin, at 37°C with 5% CO2/95% air flow. Cyclopamine (25 μM; Sigma Aldrich, MO), GANT61 (15 μM; Alexis Biochemicals, CA), or an equal volume of vehicle, dimethyl sulfoxide (DMSO), was added to the culture medium [16, 23]. The concentrations of cyclopamine and GANT61 represent the minimal concentrations that result in effects on the Hh pathway in the fetal testes in our studies. All culture experiments were repeated three times, and in each experiment, at least three gonads were exposed to the same treatment.
Whole-Mount In Situ Hybridization
Tissues were collected and fixed overnight in 4% paraformaldehyde in PBS at 4°C and dehydrated through a methanol gradient in PTW (0.1% Tween 20 in diethyl pyrocarbonate-PBS). Samples were rehydrated though a methanol gradient and digested in 10 μg/ml proteinase K solution at 37°C for 5–7 min, followed by immediate fixation in 4% paraformaldehyde-0.1% glutaraldehyde for 20 min at room temperature. Samples were washed three times for 5 min each in PTW, rinsed in PTW-hybridization buffer (1:1), and washed once in hybridization buffer at room temperature. Samples were incubated in the hybridization buffer consisting of 5× SSC (1× SSC is 0.15 M sodium chloride and 0.015 M sodium citrate), pH 5, 50% formamide, 0.1% CHAPS, 0.1% Tween 20, 1 mg/ml yeast tRNA, 50 ng/ml heparin, and 5 mM EDTA (pH 8) for 2 h at 65°C. Digoxigenin-labeled RNA probe was added to the solution, and samples were incubated in an oven at 65°C overnight (12–16 h). On the following day, samples were washed with prewarmed hybridization buffer and then washed with MABTL (5% maleic acid buffer [MAB], 0.1% Tween 20, and 0.05% Levamisol) at room temperature. Samples were then incubated at room temperature in 20% heat-inactivated sheep serum in MABTL blocking (20 mg/ml, blocking powder; Boehringer) for 2–4 h prior to the addition of alkaline phosphatase-conjugated anti-digoxigenin antibody (1:1000 dilution). Samples were incubated on a shaker at 4°C overnight. After three 1-h washes with MABTL, samples were incubated in 20 μl/ml alkaline phosphatase substrate (NBT/BCIP; Roche Diagnostics) in alkaline phosphatase buffer for color development. The reaction was stopped at the appropriate color intensity by washing the samples in PTW, and this was followed by fixation in 4% paraformaldehyde for 20 min at room temperature.
Immunohistochemistry
Fixed samples were rehydrated from methanol back to PBS. Samples were washed with a sucrose gradient in PBS (10%, 15%, and 20% in a 20% sucrose-OCT medium at 1:1 ratio) and embedded in a 20% sucrose-OCT medium (at 1:3 ratio). The blocks were cryosectioned into 8- to 10-μm sections, which were mounted on glass slides. Sections were blocked for 1 h at room temperature in blocking solution (5% donkey serum and 0.1% Triton X-100 in PBS). Sections were then incubated overnight at 4°C in different concentrations of the following primary antibodies: anti-CYP17 (1:100 dilution; from Dr. Buck Hales, University of Illinois, Chicago), anti-laminin (1:200 dilution; Sigma), anti-anti-Müllerian hormone (anti-AMH; 1:100 dilution; Santa Cruz Biotechnology), and anti-TRA98 (1:1000 dilution; from Dr. Tanaka, Osaka University, Japan). After overnight incubation, slides were washed three times for 10 min each in washing solution (0.5% donkey serum and 0.1% Triton X-100 in PBS) and treated with either fluorescein isothiocyanate or rhodamine-conjugated secondary antibody at 1:500 concentration for 1 h at room temperature. Then, slides were washed three times for 10 min each in PBS, dried, and mounted with Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole nuclear counterstain (Vector Labs).
RESULTS
Gli1 and Gli2 Expression in the Fetal Gonads
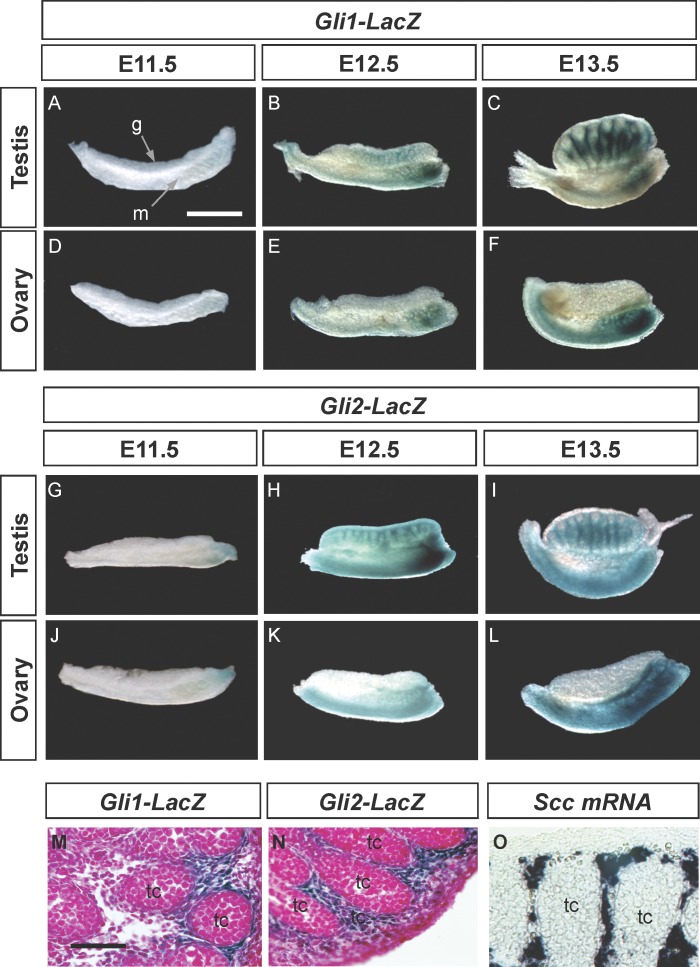
We first characterized the Gli1 and Gli2 expression patterns in gonads obtained from Gli1-LacZ and Gli2-LacZ embryos, where the LacZ reporter gene is inserted into the endogenous Gli1 or Gli2 locus [19, 21]. At E11.5, no Gli1-LacZ or Gli2-LacZ gene was detected in the male or female gonads (Fig. 1, A, D, G, and J). At E12.5 and E13.5, when steroidogenic fetal Leydig cells start to appear, both Gli1-LacZ and Gli2-LacZ expression became positive in the fetal testis (Fig. 1, B, C, H, and I). In contrast, the fetal ovary remained devoid of Gli1-LacZ and Gli2-LacZ expression (Fig. 1, E, F, K, and L). In the testis, both Gli1-LacZ and Gli2-LacZ expression was confined to the interstitium outside of the testis cords (Fig. 1, M and N), overlapping with that of fetal Leydig cell marker P450 side chain cleavage (Scc) mRNA (Fig. 1). We also obtained identical Gli1 and Gli2 expression patterns by using mRNA in situ hybridization (data not shown), confirming the fact that the LacZ activity faithfully reflects endogenous Gli1 and Gli2 expression. These results indicate that both the Gli1 and Gli2 genes are expressed in the fetal Leydig cells. In the mesonephros, Gli1-LacZ and Gli2-LacZ staining started to appear in the anterior tip of the mesonephros in both sexes at E11.5 and spread posteriorly afterward.
FIG. 1.
Patterns of Gli1-LacZ (A–F and M) and Gli2-LacZ (G–L and N) expression are shown in fetal mouse gonads at E11.5, E12.5, and E13.5. Gonads and mesonephroi from male or female embryos carrying the LacZ reporter gene were stained with X-gal substrate (blue color). M and N) Gli1-LacZ and Gli2-LacZ E13.5 gonads (respectively) were X-gal stained, sectioned, and counterstained with nuclear FastRed. O) E14.5 testis was stained for Scc expression by whole-mount in situ hybridization and then cryosectioned. A) g, gonad; m, mesonephros; (O) tc, testis cord. A–L) Bar = 500 μm; (M–O) bar = 200 μm.
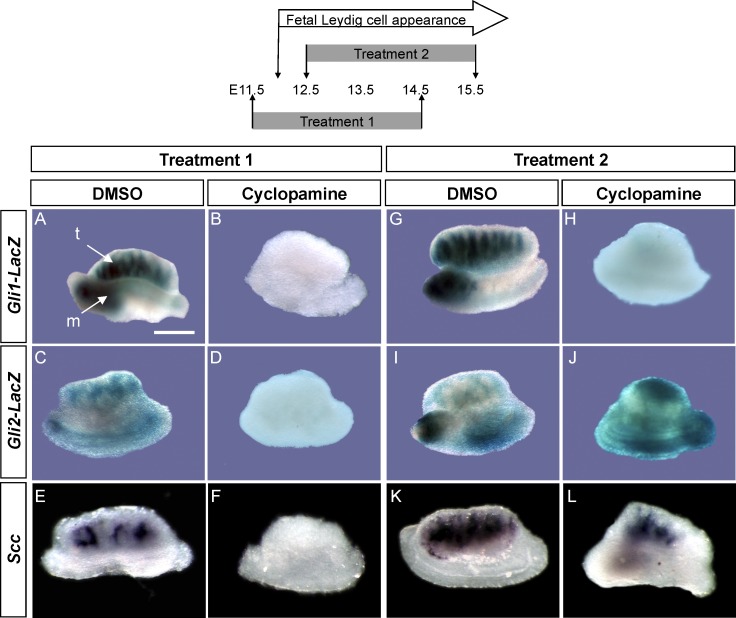
Effects of the SMO Inhibitor on Gli1 and Gli2 Expression and Appearance of Fetal Leydig Cells
To examine which Gli factors are regulated by Hh signaling in fetal testes, we cultured Gli1-LacZ or Gli2-LacZ fetal testes with cyclopamine, which inhibits the SMO activity [16]. When the organ culture started at E11.5 (Fig. 2, Treatment 1) before the time of Gli1-LacZ and Gli2-LacZ expression (Fig. 1) and fetal Leydig cell appearance, cyclopamine treatment abolished Gli1-LacZ, Gli2-LacZ, and Leydig cell marker Scc expression compared to those in the DMSO-treated control (Fig. 2, A–F, Treatment 1). On the other hand, when cyclopamine treatment was given to E12.5 testis, where Gli1-LacZ and Gli2-LacZ and fetal Leydig cells were already present, cyclopamine only suppressed Gli1-LacZ but not Gli2-LacZ and Scc expression (Fig. 2, G–L, Treatment 2). These results indicate that initial induction of both Gli1 and Gli2 and fetal Leydig cell differentiation requires an active Hh signaling via SMO. However, only maintenance of Gli1 activity, but not Gli2 and fetal Leydig cell differentiation, requires an active Hh pathway via SMO.
FIG. 2.
Effects of the Hh inhibitor cyclopamine on Gli1-LacZ (A, B, G, and H), Gli2-LacZ (C, D, I, and J), and Scc (E, F, K, and L) expression in gonad explant culture are shown. E11.5 (Treatment 1) or E12.5 (Treatment 2) testes were cultured for 72 h in the presence of either DMSO or cyclopamine. After culture, samples were processed for LacZ staining or whole-mount in situ hybridization for Scc expression as a marker for steroidogenic cells. All culture experiments were repeated three times, and in each experiment, at least three gonads were exposed to the same treatment. A) m, mesonephros; t, testis. Bar = 500 μm.
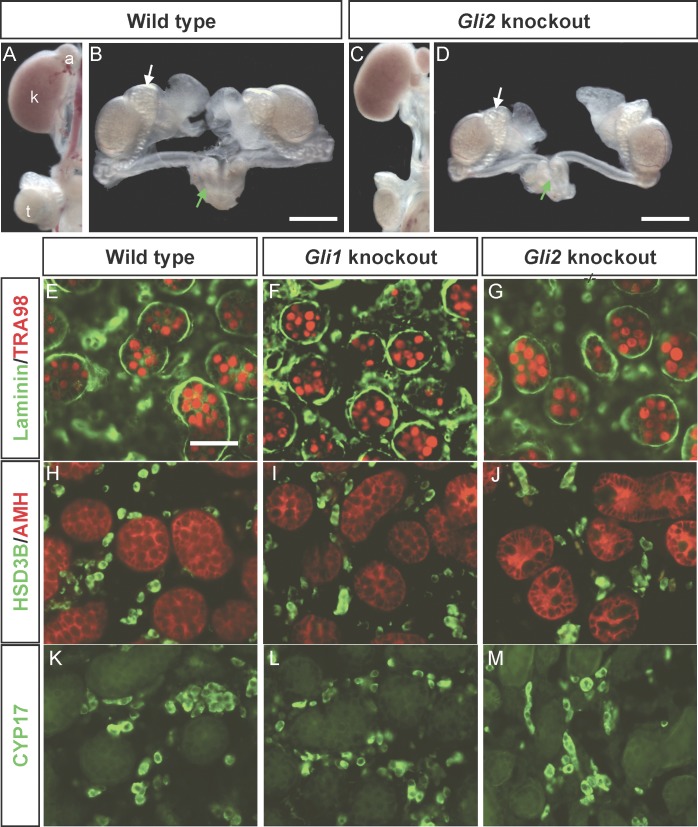
Effects of Loss of Gli1 or Gli2 on Fetal Testes
We next investigated Gli1 and Gli2 functions in the establishment of fetal Leydig cells by examining testes from embryos carrying either the Gli1 or the Gli2 knockout allele. The Gli1 knockout animals in our colony developed normally and were fertile when they reached adulthood, consistent with the original observation [24]. At the time of birth, the reproductive organs in Gli1 and Gli2 knockout newborns were indistinguishable from their respective wild-type littermates (Fig. 3, A–D [only Gli2 animals are shown]). In the Gli1 and Gli2 knockout animals, formation of the testis cords, marked by laminin staining (Fig. 3, E–G), development of germ cells (Fig. 3, E–G, TRA98 staining), differentiation of Sertoli cells (Fig. 3, H–J, anti-Müllerian hormone or AMH staining), and differentiation of fetal Leydig cells (Fig. 3, H–J and K–M, 3-beta hydroxyl steroid dehydrogenase [3βHSD] and CYP17 staining, respectively) were not different from those of the wild-type animals at birth. Gli2 knockout animals died before birth, and therefore their reproductive status in adulthood remains unknown. Loss of either Gli1 or Gli2 expression did not affect testicular morphogenesis, establishment of fetal Leydig cells, and male sexual differentiation, suggesting that the Gli1 and Gli2 genes may function redundantly.
FIG. 3.
Development of reproductive tracts and testes are shown in the wild-type and the Gli knockout newborn mice. A–D) Reproductive tracts were isolated from wild-type or Gli2 knockout embryos and processed for whole-mount imaging. B and D) White arrows and green arrows indicate epididymis and seminal vesicle, respectively. E–M) Testis sections were immunostained for the germ cell marker TRA98 (red in E–G) and laminin (green in E–G), the Sertoli cell marker AMH (red in H–J) and 3βHSD (green in H–J), and CYP17 (green in K–M). A) a, adrenal; k, kidney; t, testis. A–D) Bar = 500 μm; (E–M) bar = 100 μm.
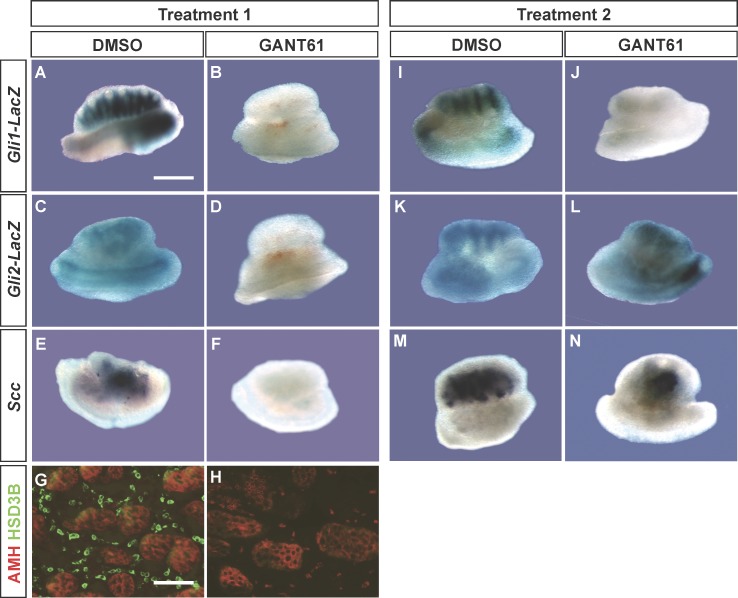
Effects of the GLI1/GLI2 Inhibitor on Fetal Leydig Cell Differentiation
We intended to generate Gli1/Gli2 double-knockout embryos; however, none of our breeding yielded viable double-knockout embryos at E11.5. We therefore utilized a chemical inhibitor, GANT61, which was shown to suppress GLI1/GLI2-mediated transcription [23, 25]. GANT61 treatment inhibited Gli1-LacZ, Gli2-LacZ, and Leydig cell marker Scc and Hsd3b expression in fetal testes when the culture started at E11.5 or before the onset of Gli1-LacZ, Gli2-Lac, and Leydig cell differentiation (Fig. 4, A–H, Treatment 1 is identical to that in Fig. 2). Sertoli cell differentiation (Fig. 4, G and H, AMH staining) was not affected by GANT61 treatment, indicating that the observed effects did not result from compromised Sertoli cell differentiation. When GANT61 treatment started at E12.5 (Fig. 2, Treatment 2) after the appearance of Gli1-LacZ, Gli2-LacZ, and fetal Leydig cell markers (Scc and Hsd3b [only Scc is shown]) expression, the treatment inhibited only Gli1-LacZ expression (Fig. 4, I and J) but not those of Gli2-LacZ (Fig. 4, K and L) and Scc (Fig. 4, M and N). These results together demonstrate that Gli1 and Gli2 transcription activity is necessary for induction of fetal Leydig cell differentiation. However, once fetal Leydig cells appear, their maintenance does not require activation of the Hh pathway via GLI1 or GLI2. In addition, Gli1 and Gli2 expression is under differential control by their own actions. Gli1 expression requires the presence of Gli transcription activity. For Gli2, on the other hand, once its expression is initiated, maintenance of its expression does not require the transcriptional activity of Gli factors.
FIG. 4.
Effects of GANT61 on Gli1-LacZ (A, B, I, and J), Gli2-LacZ (C, D, K, and L), and Scc (E, F, M, and N) expression in gonad explant culture are shown. E11.5 (Treatment 1 as shown in Fig. 2) or E12.5 (Treatment 2) testes were cultured for 72 h in the presence of either DMSO or GANT61. After culture, samples were processed for LacZ staining, whole-mount in situ hybridization for Scc, or immunohistochemistry for AMH (red) and 3βHSD (green in panels G and H). All culture experiments were repeated three times, and in each experiment, at least three gonads were exposed to the same treatment. A–F and I–N) Bar = 500 μm; (G and H) bar = 200 μm.
DISCUSSION
Steroidogenic cells are present in many organs including adrenal, ovary, testis, placenta, and brain. Gonads, in particular, synthesize sex steroids that are required for reproductive functions and fertility [26]. Steroidogenic cell function in adult gonads is regulated mainly by luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary [27, 28]; however, steroidogenic fetal Leydig cells in fetal testes do not require LH or FSH [29, 30]. Mouse fetal Leydig cells depend upon Hh signaling to acquire their steroidogenic identity [14, 15, 31, 32]. Androgen and insulin-like growth factor 3 (INSL3) produced by fetal Leydig cells are required for masculinization, testicular descent, and male reproductive tract development [13]. In this study, we examined the potential involvement of Gli1 and Gli2 transcription factors in the initial differentiation of fetal Leydig cells. Gli1 and Gli2 expression in fetal gonads is testis-specific and exclusively present in the testicular interstitium, overlapping with the Hh receptor Ptch1 expression [33] and that of the steroidogenic marker Scc. Testis-specific Gli1 and Gli2 expression indicate these two transcription factors may act downstream of the Hh pathway and play roles in fetal Leydig cell differentiation.
Initial Gli1 and Gli2 expression is sensitive to the common Hh signaling modulator, SMO, as is evident by the loss of their expression in the presence of the SMO inhibitor cyclopamine. The inhibitory effects of cyclopamine on fetal Leydig cell development and testicular structure of mouse embryo have been reported previously [14, 17]. Szczepny et al. [34] documented a similar inhibitory effect of cyclopamine on Gli1 mRNA expression in testis at 11.5 days postcoitum. However, the spatial and temporal expression of the Gli1 and Gli2 genes and their involvement in fetal Leydig cell development were not known. Our findings place both the Gli1 and Gli2 genes downstream of SMO in the Hh pathway in the fetal testes. We discovered a differential SMO activity is required to control Gli1 and Gli2 expression. Initial Gli1 and Gli2 expression is dependent on SMO and Hh signaling. On the other hand, maintenance of Gli1, but not Gli2, expression requires a constant activation of SMO in the fetal testis. Research of other organ systems such as limbs has shown, contrary to our finding, that initial Gli2 expression is usually independent of Hh signaling [19, 21, 35], whereas Gli1 expression is dependent upon the activation of SMO [2, 19, 21, 35–39]. Our findings reveal a novel regulation of Gli2 expression in the fetal testis.
Loss of either Gli1 or Gli2 expression did not affect fetal Leydig cell differentiation and male sexual differentiation regulated by androgens, suggesting a potential compensation between these two factors. Compensation between the Gli1 and Gli2 genes is present in other tissues in the mouse [18, 19, 21], as well as in cultured cell lines [40, 41]. For example, in the Gli2 knockout mouse, introduction of the Gli1 gene into the Gli2 knockout allele restored the Hh signaling [19]. In addition, infection of Gli2−/− mouse embryonic fibroblasts with adenovirus encoding a mouse GLI1-GFP fusion protein was able to partially restore the Hh signaling [21]. In the development of lung, nervous system, and limbs, Gli2 knockout embryos had abnormal phenotypes that were aggravated by introduction of the Gli1 knockout allele [20]. Also, in regard to optic development, Gli1 and Gli2 knockout embryos had normal optic cup length, while the Gli1/Gli2 double-knockout embryos showed significantly elongated optic cup [42]. We intended to generate the Gli1/Gli2 double-knockout embryos [20] but failed to produce any viable double-knockout embryos at or after E11.5. A possible explanation is that the mixed genetic background of the Gli1 and Gli2 mouse strains contributes to an early onset of embryonic lethality. To circumvent this problem, we used testis organ culture with GANT61 inhibitor to suppress GLI1/GLI2-moderated transcription and studied its effect on fetal Leydig cell development.
GANT61 treatment inhibited the Hh pathway and Scc and Hsd3b expression in fetal testis. To exclude the possibility that these defects were the results of nonspecific action of GANT61 in general testis development, we investigated the differentiation of Sertoli cells, the cell type responsible for testis morphogenesis, and found no apparent defects in Sertoli cells. This finding indicates that the loss of Hh signaling and fetal Leydig cells in GANT61-treated testis was not secondary to loss of functional Sertoli cells. It has been shown that Amh mRNA expression in Sertoli cells was up-regulated in response to cyclopamine treatment [34]. In our study, we used the presence of the AMH protein as an indicator of Sertoli cell development and testis cord formation. The overall effects of GANT61 were similar to those of cyclopamine, which inhibited SMO and the appearance of fetal Leydig cells. Therefore, we concluded that both GLI1 and GLI2 are downstream components of the Hh pathway that regulate the initial steroidogenic enzyme expression in fetal Leydig cells.
Similar to the effects of cyclopamine, the initial Gli1 and Gli2 expression was sensitive to GANT61 treatment, while maintenance of their expression at later stages showed a different pattern of regulation. Both initiation and maintenance of Gli1 expression in the fetal testis requires the transcriptional activation of GLI factors. Gli2 expression, once initiated, becomes insensitive to GANT61, indicating that its maintenance is independent of transcriptional activation of the GLI factors. The Hh-independent Gli2 maintenance, as shown by GANT61 and cyclopamine experiments, may partly explain the inability of cyclopamine to inhibit fetal Leydig cell differentiation once they appear in the fetal testis [14]. In summary, we demonstrate that both GLI1 and GLI2 are involved in appearance of fetal Leydig cells induced by the Hh pathway. GLI1 and GLI2 appear to work in a redundant fashion to ensure the onset of steroidogenesis and appearance of fetal Leydig cells in the testes. However, once Gli2 expression and differentiation of fetal Leydig cells are initiated, the maintenance of these two events no longer requires an active Hh pathway.
Acknowledgments
We thank Drs. Buck Hales and Ken-Ichirou Morohashi for supplying antibodies. We also thank Dr. A. Joyner for the generous supply of Gli1-LacZ and Gli2-LacZ mouse lines.
Footnotes
Supported by U.S. National Institutes of Heath grants NIH-HD-46861 and HD-059961 to H.H.C.Y., March of Dimes Birth Defects Foundation to H.H.C.Y., and by a UIUC College of Veterinary Medicine Billie Field Graduate Fellowship to I.B.B. Also supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
REFERENCES
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev 2008; 22: 2454 2472. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001; 15: 3059 3087. [DOI] [PubMed] [Google Scholar]
- Stamataki D, Ulloa F, Tsoni SV, Mynett A, Briscoe J. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes Dev 2005; 19: 626 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog signaling. J Cell Sci 2007; 120: 3 6. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature 2002; 418: 892 897. [DOI] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The hedgehog response network: sensors, switches, and routers. Science 2004; 304: 1755 1759. [DOI] [PubMed] [Google Scholar]
- Koebernick K, Pieler T. Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling. Differentiation 2002; 70: 69 76. [DOI] [PubMed] [Google Scholar]
- Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol 2006; 26: 3365 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterhouse DO, Lamm MLG, Villavicencio E, Iannaccone PM. Emerging roles for Hedgehog-Patched-Gli signal transduction in reproduction. Biol Reprod 2003; 69: 8 14. [DOI] [PubMed] [Google Scholar]
- Russell MC, Cowan RG, Harman RM, Walker AL, Quirk SM. The Hedgehog signaling pathway in the mouse ovary. Biol Reprod 2007; 77: 226 236. [DOI] [PubMed] [Google Scholar]
- Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA. Hedgehog signaling in mouse ovary: Indian Hedgehog and Desert Hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 2005; 146: 3558 3566. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 2004; 5: 509 521. [DOI] [PubMed] [Google Scholar]
- Barsoum I, Yao HH. The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab 2006; 17: 223 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH-C, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 2002; 16: 1433 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert Hedgehog-null mouse testis. Biol Reprod 2001; 65: 1392 1402. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev 2002; 16: 2743 2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol 2002; 246: 356 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doles J, Cook C, Shi X, Valosky J, Lipinski R, Bushman W. Functional compensation in Hedgehog signaling during mouse prostate development. Dev Biol 2006; 295: 13 25. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development 2001; 128: 5161 5172. [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 2000; 127: 1593 1605. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 2002; 129: 4753 4761. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovi L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse Patched mutants. Science 1997; 277: 1109 1113. [DOI] [PubMed] [Google Scholar]
- Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A 2007; 104: 8455 8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi CS, Baumbusch MA, Dulay AT, Lee S, Wehrum M, Zhao G, Bahtiyar MO, Pettker CM, Ali UA, Funai EF, Buhimschi IA. The role of urinary soluble endoglin in the diagnosis of pre-eclampsia: comparison with soluble fms-like tyrosine kinase 1 to placental growth factor ratio. BJOG 2010; 117: 321 330. [DOI] [PubMed] [Google Scholar]
- Shimokawa T, Tostar U, Lauth M, Palaniswamy R, Kasper M, Toftgard R, Zaphiropoulos PG. Novel human glioma-associated oncogene 1 (GLI1) splice variants reveal distinct mechanisms in the terminal transduction of the Hedgehog signal. J Biol Chem 2008; 283: 14345 14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol 2005; 19: 2647 2659. [DOI] [PubMed] [Google Scholar]
- Rainey WE. Adrenal zonation: clues from 11[beta]-hydroxylase and aldosterone synthase. Mol Cell Endocrinol 1999; 151: 151. [DOI] [PubMed] [Google Scholar]
- Payne AH. Hormonal regulation of cytochrome P450 enzymes, cholesterol side-chain cleavage and 17 alpha-hydroxylase/C17–20 lyase in Leydig cells. Biol Reprod 1990; 42: 399 404. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 2001; 179: 47 74. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol 2004; 233: 181 241. [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult Leydig cell development. Endocrinology 2007; 148: 3704 3710. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 2000; 63: 1825 1838. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 1995; 172: 126 138. [DOI] [PubMed] [Google Scholar]
- Szczepny A, Hogarth CA, Young J, Loveland KL. Identification of Hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system. Biol Reprod 2009; 80: 258 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 1998; 125: 2533 2543. [DOI] [PubMed] [Google Scholar]
- Hynes M, Porter J, Chiang C, Chang D, Tessier-Lavigne M, Beachy P, Rosenthal A. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron 1995; 15: 35 44. [DOI] [PubMed] [Google Scholar]
- Lee J, Platt K, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 1997; 124: 2537 2552. [DOI] [PubMed] [Google Scholar]
- Grindley J, Bellusci S, Perkins D, Hogan B. Evidence for the involvement of the Gli gene family in embryonic mouse lung development. Dev Biol 1997; 188: 337 348. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci 2000; 3: 979 985. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Gipp JJ, Zhang J, Doles JD, Bushman W. Unique and complimentary activities of the Gli transcription factors in Hedgehog signaling. Exp Cell Res 2006; 312: 1925 1938. [DOI] [PubMed] [Google Scholar]
- Eichberger T, Sander V, Schnidar H, Regl G, Kasper M, Schmid C, Plamberger S, Kaser A, Aberger F, Frischauf AM. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics 2006; 87: 616 632. [DOI] [PubMed] [Google Scholar]
- Furimsky M, Wallace VA. Complementary Gli activity mediates early patterning of the mouse visual system. Dev Dyn 2006; 235: 594 605. [DOI] [PubMed] [Google Scholar]