Abstract
Breast cancer-resistance protein (BCRP1), encoded by Abcg2 mRNA, limits the penetration of a spectrum of compounds into the brain. The fetal brain is a primary target for many BCRP1 substrates; however, the developmental expression, function, and regulation of Abcg2/BCRP1 in the mouse fetal brain are unknown. Synthetic glucocorticoids (e.g., dexamethasone [DEX]) increase Abcg2/BCRP1 expression and function in vitro in endothelial cells derived from brain microvessels. A regulatory role of glucocorticoids on Abcg2/BCRP1 in the fetal brain is of importance given that approximately 10% of pregnant women are treated with synthetic glucocorticoid for threatened preterm labor. We hypothesized the following: 1) Abcg2 mRNA and BCRP1 protein expression increases with development (from Embryonic Day [E] 15.5 to E18.5), corresponding to decreased accumulation of BCRP1 substrate in the fetal brain. 2) Maternal treatment with DEX will up-regulate Abcg2 mRNA and BCRP1 protein expression in the fetal brain, resulting in decreased BCRP1 substrate accumulation. Pregnant FVB dams were euthanized on E15.5 or E18.5, and fetal brains were collected and analyzed for [3H]mitoxantrone (BCRP1-specific substrate) accumulation and Abcg2/BCRP1 expression. In another six groups (n = 4–5/group), pregnant mice were treated with DEX (0.1 or 1 mg/kg) or vehicle (saline) from either E9.5 to E15.5 (midgestation) or E12.5 to E18.5 (late gestation) and then injected with [3H]mitoxantrone. In conclusion, Abcg2 mRNA expression significantly decreases with advancing gestation, while BCRP1-mediated neuroprotection increases. Furthermore, there is a dose-, sex-, and age-dependent effect of DEX on Abcg2 mRNA in the fetal brain in vivo, indicating a complex regulatory role of glucocorticoid during development.
Keywords: Abcg2, BCRP1, blood-brain barrier, breast cancer-resistance protein, developmental biology, dexamethasone, fetal blood-brain barrier, gene regulation, [3H]mitoxantrone, multidrug resistance, neuroprotection, pregnancy
Abcg2/BCRP1-mediated protection increases in the fetal brain with advancing gestation, and Abcg2 mRNA is regulated by synthetic glucocorticoid in a dose-, sex-, and age-dependent manner.
INTRODUCTION
Breast cancer-resistance protein (BCRP1), encoded by the Abcg2 gene, was first identified in MCF-7 cells (a human breast carcinoma subline), where it resulted in chemoresistance [1]. In normal tissues, BCRP1 limits absorption and facilitates the excretion of a wide range of substrates. Substrates of BCRP1 include hormones such as 17-β estradiol or therapeutic agents such as nitrofurantoin (antibiotic), mitoxantrone (antineoplastic), cimetidine (histamine H2-receptor antagonist), and glyburide (antidiabetic) [2–8]. As a result of maternal use of therapeutic drugs, the developing fetus may be inadvertently exposed to teratogenic factors present in maternal circulation, with the fetal brain being particularly susceptible to many of these BCRP1 substrates.
The blood-brain barrier (BBB), composed of capillary endothelial cells, is a dynamic structure that regulates the entry of endogenous and exogenous substrates into the brain. In the adult brain, BCRP1 is localized on the luminal surface of capillary endothelial cells [9, 10]. Here, BCRP1 has an important role in neuroprotection by limiting entry of BCRP1 substrates into the brain [11]. For example, in the presence of a BCRP1 inhibitor, uptake of prazosin and mitoxantrone (prototypical substrates of Abcg2/BCRP1) increased in both wild-type and CF1 mice (Abcb1a/P-gp knockout mutant strain) [11], demonstrating that Abcg2/BCRP1 restricts brain uptake of these substrates.
Abcg2/BCRP1 in the fetal brain has not been extensively investigated. In a recent study [4], the fetal brain:body ratio of genistein (phytoestrogen BCRP1 substrate) increased 1.4-fold in Abcg2/BCRP1 knockout mice compared with wild type at 2 wk of gestation, suggesting that BCRP1 in the fetal brain has an important role in limiting the entry of substrates into the fetal brain. Mapping neurodevelopment of the mouse brain onto that of the human is complex given that brain development is not linear [12, 13]. In general, the first half and second half of gestation and the first 7 days after birth in the mouse brain are considered to be equivalent to the first, second, and third trimesters, respectively, in the human fetal brain [14–16]. BCRP1 expression has been reported as early as 22 wk of gestation (in the human) and Embryonic Day (E) 13 (in the rat) in fetal brain capillary endothelial cells [17, 18]. We have previously shown that BCRP1 is localized to capillary endothelial cells in the fetal mouse brain on E18.5 [19]. However, the developmental expression and function of Abcg2/BCRP1 in the fetal brain remain to be determined.
Nothing is known with regard to regulation of Abcg2/BCRP1 in normal fetal tissues. In the breast cancer cell line MCF-7/MX, dexamethasone (DEX) treatment decreased Abcg2 mRNA and BCRP1 protein expression in a dose- and time-dependent manner [20, 21]. Furthermore, in isolated adult rat brain endothelial cells, DEX treatment increased BCRP1 activity and expression [22]. Clinically, synthetic glucocorticoids are prescribed to women at risk of premature delivery (approximately 10% of all pregnancies) to mature the fetal lungs and other organs [23, 24]. However, the consequences of synthetic glucocorticoid exposure on Abcg2/BCRP1 expression and function in the fetal brain are unknown.
In the present study, we hypothesized the following: 1) Abcg2 mRNA and BCRP1 protein expression increases in the fetal brain with advancing gestation (from E15.5 to E18.5), and this corresponds to decreased accumulation of BCRP1 substrate in the fetal brain. 2) Maternal treatment with synthetic glucocorticoid (DEX) will up-regulate Abcg2 mRNA and BCRP1 protein expression and function in the fetal brain.
MATERIALS AND METHODS
Female FVB mice (Charles River, Germantown, NY) were bred in our colony. Pregnancy was defined by the presence of a vaginal plug and was designated as E0.5 (average gestation period, approximately 19.5 days). All experiments were carried out in our facility at 0900 h. These studies were performed using protocols approved by the University of Toronto Animal Care Committee in accord with the Canadian Council for Animal Care.
In Vivo Drug Distribution Studies
Functional changes of fetal BBB Abcg2/BCRP1 across gestation.
On either E15.5 (n = 4 dams) or E18.5 (n = 5 dams), pregnant FVB dams were injected (i.v.) with [3H]mitoxantrone (prototypical substrate for assessing BCRP1 function; prepared with 5 mg/kg of unlabeled mitoxantrone [Sigma Chemical Co., St. Louis, MO] and 1 μCi/animal of [3H]mitoxantrone [Moravek Biochemicals, Brea, CA]) and euthanized 30 min following the mitoxantrone injection using isoflurane (AErrane, USP; Baxter Corporation, Mississauga, ON, Canada) [2, 11, 25]. E15.5 and E18.5 were chosen to investigate Abcg2/BCRP1 expression and function in the fetal brain because they correspond to findings in our previous study [19] examining BCRP1 expression in the placenta, the primary barrier between maternal circulation and the fetus. Fetal brains and bodies (excluding the fetal head) were collected and stored at −80°C until further use. Homogenized whole fetal brain and body (200 μl) were dissolved in SOLVABLE (1 ml; PerkinElmer Inc., Boston, MA), and hydrogen peroxide (30%, 100 μl) was added to decolorize samples and optimize counting efficiency. Following addition of scintillation fluid (Ultima-Gold; PerkinElmer Inc.), radioactivity (disintegrations per minute [DPM]) in the fetal brain and body was determined on a Tri-Carb Beta-Counter (PerkinElmer Inc.). Levels of [3H]mitoxantrone were standardized as a drug equivalent per weight of tissue. Drug distribution was expressed as the fetal brain tissue concentration:fetal body tissue concentration ratio, designated “drug ratio.” [3H]Mitoxantrone accumulation in the fetal body was used as an index of substrate availability for the fetal brain, as fetal blood collection is impossible in the mouse. Fetal body substrate accumulation may vary with fetal growth. However, accumulation of [3H]mitoxantrone in the fetal body was standardized to weight, thus correcting for differences in fetal growth. Fetal tails were collected and stored at −20°C for sex determination.
Glucocorticoid regulation of Abcg2 and BCRP1 expression and function.
Glucocorticoid regulation was examined in two groups of pregnant FVB mice. In the first group, pregnant dams were injected (s.c.) daily (at 0900 h) with either DEX (pharmacological doses of 0.1 mg/kg [n = 4 dams/group] or 1 mg/kg [n = 5 dams/group]) or vehicle (saline [n = 5 dams/group]) from E9.5 to E15.5 (midgestation). In the second group, pregnant dams were injected (s.c.) daily (at 0900 h) with DEX (0.1 mg/kg [n = 4 dams/group] or 1 mg/kg [n = 5 dams/group]) or vehicle (saline [n = 4 dams/group]) from E12.5 to E18.5 (late gestation). The dose of DEX utilized in clinical management of threatened preterm labor is 0.2 mg/kg [26]. Glucocorticoid treatment during the last week of gestation (from E12.5 to E18.5) has been used in murine models to mimic antenatal glucocorticoid treatment [27, 28]. We chose an equivalent treatment regimen (from E9.5 to E15.5) earlier in gestation based on the ontogenic profile and because fetal brain sensitivity to glucocorticoids is lower at this time [29]. On the last day (E15.5 or E18.5) at 2 h after final treatment of DEX or vehicle injection, [3H]mitoxantrone (1 μCi/animal and 5 mg/kg of unlabeled mitoxantrone) was injected (i.v.), and dams were then euthanized 30 min later with isoflurane (AErrane, USP; Baxter Corporation). Maternal blood and fetal tissues were collected and processed as already described. Fetuses were separated by sex, and data were averaged per litter per treatment group for analysis. Averages of 3.0 and 4.4 male and female fetuses, respectively, were used per dam per treatment group for analysis of mitoxantrone transfer. Absolute (DPM/g) [3H]mitoxantrone accumulation in the fetal body (excluding the head) did not significantly differ with treatment.
Sex Determination
DNA was extracted from fetal tails utilizing a REDExtract-N-AMP Tissue PCR kit (Sigma Chemical Co.), and PCR was performed to determine fetal sex using Sry forward (5′ TCATGAGACTGCCAACCACAG 3′) and Sry reverse (5′ CATGACCACCACCACCACCAA 3′) primers [30] according to the manufacturer's guidelines. Amplification product was detected by 1% gel electrophoresis.
Real-Time PCR
Total RNA was extracted from frozen brains (one male and one female brain were arbitrarily selected per litter per treatment group) using TRIzol (Invitrogen Canada Inc., Burlington, ON, Canada) per the manufacturer's instructions. Total RNA was subjected to reverse transcription using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) as we have described previously [31].
Real-time PCR was performed using FAM-labeled TaqMan gene expression assays for Abcg2 mRNA and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA (Mm00496364_m1 and 4352932E; Applied Biosystems) and TaqMan Universal PCR mix (Applied Biosystems) [31]. Abcg2 mRNA expression was quantified on a Chromo4 real-time PCR detector (Bio-Rad Laboratories, Hercules, CA) with activation at 50°C for 2 min and initial denaturation at 95°C for 10 min. Thirty-five cycles were then performed of denaturation at 95°C for 15 sec, annealing and extension at 60°C for 60 sec, and plate reading. All samples were run in triplicate. Data were analyzed using Opticon software (Bio-Rad Laboratories), and relative quantification was calculated using the ΔΔCT method with Gapdh as the endogenous control [32]. Selection of an appropriate endogenous control for mouse studies was performed by analyzing the expression of placental Gapdh and with treatment. Similar amplification efficiencies and threshold cycle (CT) values were obtained for Gapdh with treatment (vehicle versus DEX [data not shown]). The stability of Gapdh expression validated the use of this gene for normalization. Furthermore, similar amplification efficiencies were obtained for both the target gene (Abcg2) and our housekeeping gene (data not shown).
Western Blot Analysis
BCRP1 protein expression was assessed by Western blot analysis as described previously [19]. Briefly, frozen brain (one male and one female were arbitrarily selected per litter per treatment group [n = 4–5 litters/treatment]) was prepared for electrophoresis. Samples (50 μg of protein total) were subjected to SDS-PAGE electrophoresis (8% resolving gel) and transferred to a nitrocellulose membrane. Nitrocellulose membranes were blocked overnight at 4°C in skim milk (5% wt/vol of PBS with Tween 20/PBS-T). Membranes were washed with PBS-T and cut at the 50-kDa mark. The upper half was then incubated with BXP-21 mouse monoclonal antibody (1:250 dilution for 1 h at 23°C, catalog No. OP191; Calbiochem, La Jolla, CA), and the lower half was incubated with β-actin (1:5000 dilution, rabbit polyclonal A2066; Sigma Chemical Co.). The membranes were then washed and incubated with anti-mouse and anti-rabbit IgG, respectively (1:5000 dilution for 1 h at 23°C; NEN Life Science Products, Boston, MA), followed by Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Inc.). Bands were visualized by exposure to Kodak Blue X-OMAT film (PerkinElmer Inc.). The relative optical density of the bands was measured using computerized image analysis and was standardized against the β-actin signal (MCID TM Core 7.0; Imaging Research Inc., Interfocus Imaging Ltd., Cambridge, England). Western blot analysis was performed in duplicate for each fetal brain. The specificity of the antibody that detects the 72-kDa band has previously been confirmed in our laboratory [19].
Statistical Analysis
Group data are presented as the mean ± SEM and are analyzed using Prism (GraphPad Software Inc., San Diego, CA). Abcg2 mRNA expression was normalized to the endogenous control Gapdh mRNA. Analysis was undertaken on data obtained from sex-separated fetal brains averaged per dam. For comparison of treatment with vehicle, data were expressed as percentage vehicle and then log10 transformed. One-sample t-test against a hypothetical value of 2 (log100 = 2 represents vehicle value) was performed, followed by Holm modification of Bonferroni correction [33]. For comparison of treatment with vehicle drug ratios, treatment was normalized to corresponding control as the percentage vehicle mean. Data were log10 transformed and analyzed with one-sample t-test for differences between treatment and vehicle. Unpaired Student t-test for all column combinations using Holm modification of Bonferroni correction to control for multiplicity was then performed. Two-way ANOVA was utilized to determine differences among treatment doses, sex, and gestational age, and where significance was demonstrated, post hoc analysis was undertaken using Student t-test for all column combinations using Holm modification of Bonferroni correction. Significance was set at P < 0.05.
RESULTS
Development of Abcg2/BCRP1 Expression and Function in the Fetal Brain
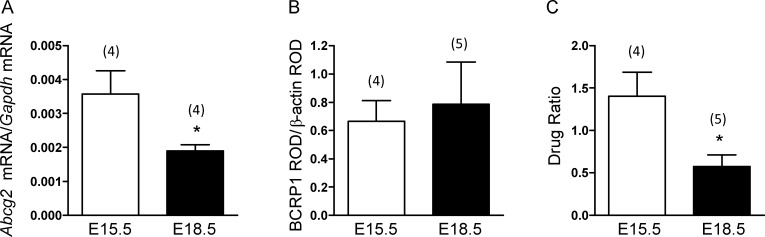
There were no sex differences in the expression of Abcg2 mRNA or BCRP1 protein or in function with advancing gestation. As such, data were combined from male and female fetal brains for the developmental assessment of expression and function. Abcg2 mRNA significantly decreased on E18.5 compared with E15.5 (P < 0.05) (Fig. 1A). However, there was no corresponding decrease in BCRP1 protein (Fig. 1B). [3H]Mitoxantrone accumulation in the fetal brain significantly decreased with advancing gestation (P < 0.05) (Fig. 1C), indicating increased BCRP1 function with advancing gestation in the fetal brain.
FIG. 1.
Developmental expression of fetal brain (A) Abcg2 mRNA on E15.5 and E18.5. The error bar represents the mean ± SEM expressed as relative units of mRNA standardized against Gapdh. B) Relative levels of BCRP1 protein expressed on E15.5 and E18.5. The error bar represents the mean ± SEM BCRP1:β-actin ratio. C) Function of fetal brain BCRP1 on E15.5 and E18.5 determined by accumulation of transporter-specific substrate and accumulation of [3H]mitoxantrone (5 mg/kg) in whole fetal brain on E15.5 and E18.5. The error bar represents the mean ± SEM drug ratio (DPM/g fetal brain:DPM/g fetal body). The number in parentheses represents the number of dams in each group. *P < 0.05 versus E15.5. ROD indicates relative optical density.
Glucocorticoid Regulation
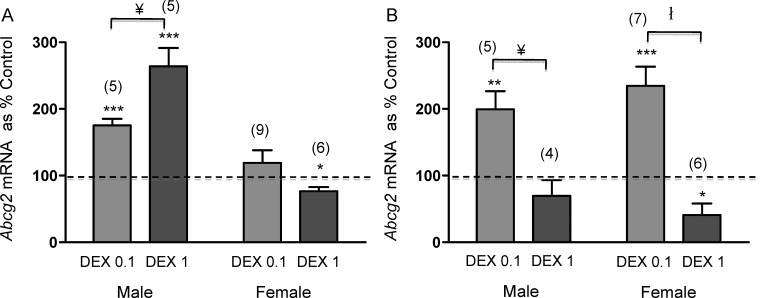
Two-way ANOVA revealed a significant interaction between treatment and gestational age in the expression of Abcg2 mRNA in the male brain (P = 0.0005). Subsequent post hoc analysis revealed a significant dose-dependent effect of DEX on Abcg2 mRNA expression on E15.5 and E18.5 (P < 0.05). Furthermore, a significant age-dependent effect was observed for high-dose DEX (1 mg/kg) in the male brain (P < 0.01) between E15.5 and E18.5. In the female brain, two-way ANOVA also revealed a significant interaction between treatment and gestational age (P = 0.0018). Subsequent post hoc analysis demonstrated a significant effect of treatment on E18.5 (P < 0.001). Furthermore, there was a significant effect of gestational age on Abcg2 mRNA expression with low-dose DEX (0.1 mg/kg) (P < 0.05).
In the male fetal brain, low-dose DEX (0.1 mg/kg) treatment significantly increased Abcg2 mRNA expression on both E15.5 and E18.5 (P < 0.001 and P < 0.01, respectively) (Fig. 2). At E15.5, high-dose DEX increased Abcg2 mRNA (P < 0.001), whereas at E18.5, the same dose of DEX had no effect on Abcg2 expression (Fig. 2). In the female fetal brain, low-dose DEX (0.1 mg/kg) treatment during midgestation (between E9.5 and E15.5) had no significant effect on Abcg2 mRNA at E15.5 (Fig. 2A) but on E18.5 resulted in a significant increase in Abcg2 mRNA (P < 0.001) (Fig. 2B). In contrast, high-dose DEX (1 mg/kg) treatment during midgestation (between E9.5 and E15.5) and late gestation significantly decreased Abcg2 mRNA on E15.5 and E18.5 (P < 0.05) in the female fetal brain (Fig. 2).
FIG. 2.
Abcg2 mRNA on E15.5 (A) and E18.5 (B) from male and female brains following DEX treatment (0.1 and 1 mg/kg). The error bar represents the mean ± SEM expressed as relative units of mRNA standardized against Gapdh. Treatment data were then normalized to respective vehicle and presented as percentage vehicle. The dotted line represents the control. The number in parentheses represents the number of dams per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001 versus control, two-way ANOVA, followed by post hoc analysis comparing dose. ¥P < 0.05, łP < 0.001.
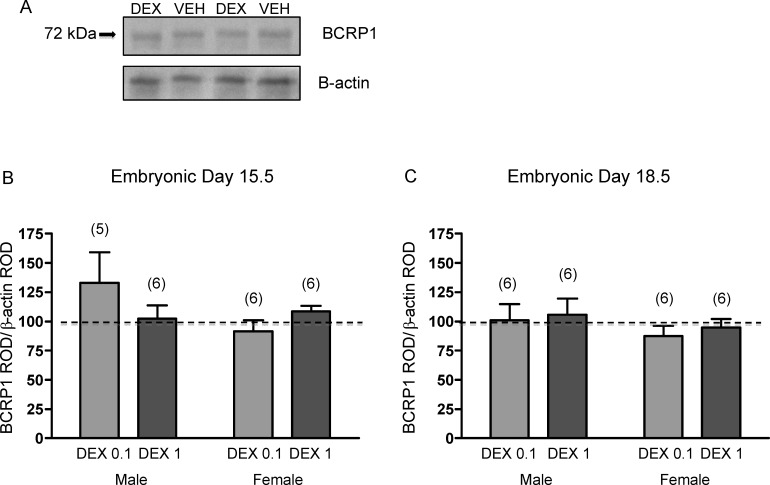
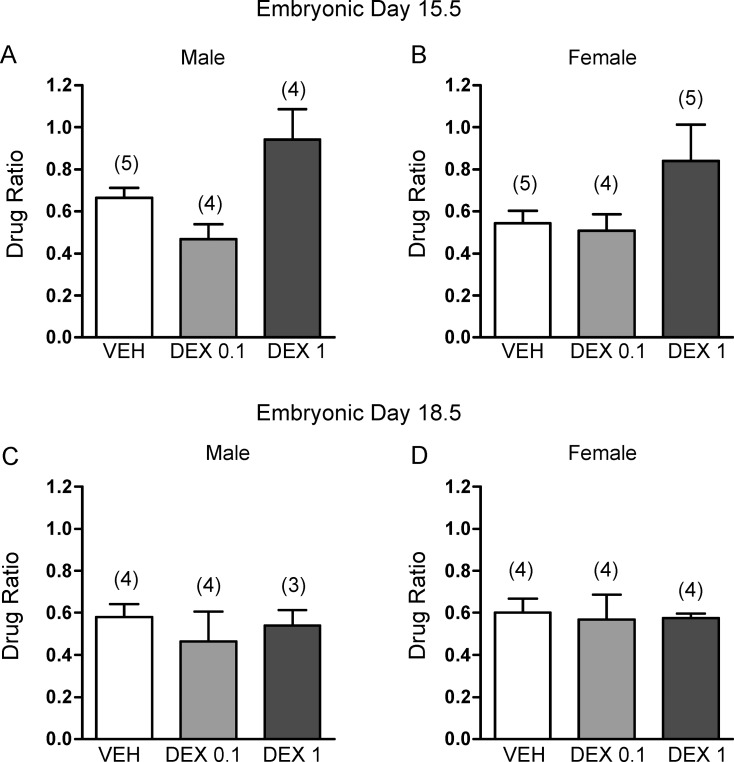
Glucocorticoid treatment during midgestation and late gestation had no significant effect on BCRP1 protein expression in the fetal brain in either sex (Fig. 3). Furthermore, there were no significant effects of glucocorticoid treatment on BCRP1 function in the fetal brain. Accumulation of [3H]mitoxantrone in the brains of fetuses exposed to DEX did not differ significantly from those exposed to vehicle at either age (Fig. 4).
FIG. 3.
A) Representative immunoblot of fetal brain BCRP1 protein following treatment with DEX (0.1 mg/kg) or vehicle (VEH). BCRP1 protein on E15.5 (B) and E18.5 (C) from male and female brains following DEX treatment (0.1 mg/kg [DEX 0.1] or 1 mg/kg [DEX 1]). The error bar represents the mean ± SEM expressed as relative optical density (ROD) of BCRP1 standardized against β-actin ROD. Treatment data were then normalized to respective vehicle. The dotted line represents the control. The number in parentheses represents the number of dams per treatment group.
FIG. 4.
[3H]Mitoxantrone (5 mg/kg) accumulation in the fetal brain in males (A, C) and females (B, D). A, B) Embryonic day (E) 15.5 after daily injections of dexamethasone (0.1 mg/kg; DEX 0.1 or 1 mg/kg; DEX 1) or vehicle (VEH) from E9.5-E15.5. C, D) E18.5 after daily injections with DEX (0.1 or 1 mg/kg) or vehicle from E12.5 to E18.5. The error bar represents the group mean ± SEM drug ratio (DPM/g fetal brain:DPM/g fetal body). The number in parentheses represents number of dams per treatment group.
DISCUSSION
The present study demonstrates a significant developmental reduction in fetal mouse brain Abcg2 mRNA expression, with no corresponding reduction in BCRP1 protein expression. Furthermore, there was a substantial decrease in BCRP1 substrate accumulation in the fetal brain, suggesting an increase in that BCRP1 efflux capacity in late gestation. Glucocorticoids appear to regulate Abcg2 mRNA in the fetal brain in a dose-, age-, and sex-dependent manner. However, there were no changes in BCRP1 protein or activity. Together, these data suggest that there is a substantial disconnect between Abcg2 gene regulation and BCRP1 function during development.
In the present study, BCRP1 protein levels remained unchanged in late gestation, despite the decrease in Abcg2 mRNA. Cygalova and colleagues [3] reported a similar decrease in fetal rat brain Abcg2 mRNA on E21 compared with E18; this group did not measure BCRP1 protein. To our knowledge, no other studies have quantified BCRP1 protein expression in the fetal brain. However, several studies [19, 34–36] have demonstrated that levels of Abcg2 mRNA and BCRP1 protein do not correlate in BeWo cells and placenta. The disparity in expression may be potentially attributed to altered posttranscriptional processing, mRNA stability, or delayed protein synthesis. A limited relationship between total protein and active protein has been previously reported [37]. In most studies, including the present study, protein is measured from total tissue extract. However, functional BCRP1 protein is localized to the membrane, and as such, protein measurements from the membrane fraction would provide a more accurate index of active protein. Further studies are required to determine the relationship between total cellular BCRP1 and that present in the membrane fraction.
The functional role of Abcg2/BCRP1 in the adult BBB remains somewhat controversial [4, 11, 38, 39]. Using Abcb1a knockout mice, Cisternino et al. [11] demonstrated reduced brain accumulation of mitoxantrone and prazosin when BCRP1 was inhibited with GF120918. However, utilizing a similar protocol, another study [38] suggested that the functional role of BCRP1 is minimal at the BBB. Knowledge pertaining to the functional role of BCRP1 in the fetal brain is even more limited. Fetal brain:body ratios of genistein concentrations (BCRP1 substrate) increased 1.4-fold in BCRP1 knockout fetus compared with wild type [4]. To date, one other study [3] has investigated BCRP1 function in the fetal rat brain during development. [3H]Cimetidine (BCRP1 substrate) accumulation was shown to significantly decrease (2.3-fold) in the fetal brain on E21 (term) compared with E18 (preterm), while accumulation in the fetal body was unchanged, suggesting an increase in BCRP1-mediated BBB function with advancing gestation. We now report a similar finding in the fetal mouse brain between E15.5 (midgestation) and E18.5 (late gestation). In both studies, Abcg2 mRNA decreased with development, demonstrating a disparity between expression and function. Given that different substrates were utilized to assess BCRP1 function, it is unlikely that this discrepancy between expression and function is due to substrate pharmacokinetics or the involvement of other transporters. Moreover, a functional BBB has been confirmed as early as E11 in the fetal mouse by the presence of tight junctions and high electrical resistance [40–42].
In addition to the BBB, the blood-cerebral-spinal fluid barrier (BCSFB), composed of capillary epithelial cells of the choroid plexus, limits entry of substrates from the cerebrospinal fluid into the brain [43]. In the choroid plexus, BCRP1 is localized to the basolateral membranes, suggesting a role in neuroprotection [18]. Recently, it has been shown in the fetal rat brain that Abcg2 mRNA expression in the lateral and fourth choroid plexus peaks on E15.5 (earliest time in gestation measured) but then decreases to term [18]. The functional significance of Abcg2/BCRP1 in the BCSFB is not understood. In the fetal mouse, determination of Abcg2/BCRP1 expression and function specifically in the BBB is difficult, as isolation of brain microvessels is impossible. As such, our studies were conducted on whole fetal brain, and measurements of Abcg2/BCRP1 expression and function relate to both the BBB and BCSFB; nonetheless, both barriers are suggested to contribute to neuroprotection. We propose that the disconnect between expression and function may relate to an interplay between BCRP1 function at the BBB and the BCSFB. Relative changes in either expression or function within these two barriers with advancing gestation could contribute to a net decrease in fetal brain exposure of xenobiotics. Studies examining the functional significance of Abcg2/BCRP1 during development in the BCSFB have not been undertaken to date. Clearly, further studies are required to investigate the relative role of BCRP1 in both the BBB and the BCSFB in adult and fetal brains.
While BCRP1 has been shown to limit the penetration of therapeutic agents into the fetal brain, there is no information concerning potential involvement of BCRP1 in normal physiological function. Dehydroepiandrosterone sulfate (DHEAS), an important precursor for estrogen synthesis, and estrone-3-sulfate (E3S), a metabolite of estrogens, are both substrates of Abcg2/BCRP1 [44], suggesting that Abcg2/BCRP1 may be involved in the elimination of these sulfated conjugates from the fetal brain. Given that Abcg2/BCRP1 may alter the dynamics of DHEAS and E3S in other tissues (placenta) [44], it is logical to assume that Abcg2/BCRP1 may regulate estrogen dynamics in the fetal brain. The physiological significance of Abcg2/BCRP1 during fetal brain development remains to be elucidated.
In the present study, fetal glucocorticoid exposure resulted in dose-, age-, and sex-dependent changes in Abcg2 mRNA. Glucocorticoids are known to exhibit biphasic effects [45]. Furthermore, dose-dependent regulation of ATP-binding cassette (ABC) transporters with DEX has been previously demonstrated in the placenta, fetal brain, liver, and small intestine [46–49]. Studies [22, 50–54] have shown that DEX activates an intracellular network of nuclear transporters, including the glucocorticoid receptor (GR) and pregnane xenobiotic transporter (PXR). The GR and PXR are expressed in endothelial cells of brain microvessels [50, 55], providing evidence for the potential involvement of the GR and PXR pathways in the regulation of Abcg2/BCRP1 in endothelial cells. Recently, both GR and PXR have been implicated in the DEX-mediated regulation of brain capillary Abcg2/BCRP1 expression using isolated endothelial cells from adult rat brain [22]. Synthetic glucocorticoids have been shown to stimulate CYP3A4 gene expression in vitro through two different pathways depending on the dose utilized. At lower doses of DEX (100 nM), CYP3A4 gene expression was modulated by GR-mediated up-regulation of PXR, whereas at higher doses (50 μM), DEX directly activated PXR [52]. Regulation of Abcg2/BCRP1 by glucocorticoids likely involves cross talk between GR and PXR.
While there were no sex differences in the expression of Abcg2/BCRP1 in the fetal brain during development, effects of fetal glucocorticoid exposure were highly sex dependent. Overall, glucocorticoid treatment significantly increased Abcg2 mRNA expression in male brains and significantly decreased expression in female brains. To our knowledge, this is the first study to show a sexually dimorphic effect of glucocorticoid on Abcg2 mRNA expression. A similar phenomenon has been previously demonstrated for the liver drug-metabolizing enzyme CYP3A4 [56, 57], where glucocorticoid treatment increased CYP3A4 expression in males but decreased expression in females [56, 57]. Further studies are required to investigate the physiological significance of this sexual dimorphic regulation of Abcg2 mRNA in late gestation.
The effects of glucocorticoids on BCRP1 function in the brain have not been previously investigated to our knowledge. The present study demonstrates little effect of DEX on BCRP1 function in the fetal brain. Approximately 10% of pregnant women are treated with synthetic glucocorticoid for threatened preterm labor. Preterm labor is difficult to diagnose; as such, pregnant women until recently often received repeat treatments with synthetic glucocorticoid [58]. Given the potential protective function of BCRP1 in the fetal brain, the lack of effect of DEX may be reassuring with respect to the clinical use of glucocorticoids in the management of preterm labor.
In late gestation, there is an exponential increase in endogenous glucocorticoid (cortisol in the human and corticosterone in the rodent) in maternal and fetal circulation [59–62]. Given the lack of effect of DEX on BCRP1-mediated protection in the fetal brain, our studies also suggest that the late gestational rise in maternal and fetal glucocorticoid is unlikely to represent a major factor in mediating the profound increase in BCRP1 activity that occurs in the fetal brain in late gestation.
In conclusion, BCRP1 function increases with advancing gestation in the fetal brain, likely in preparation for life ex utero. The present study also demonstrates that synthetic glucocorticoid treatment can affect Abcg2 mRNA in a dose-, age-, and sex-dependent manner, with no corresponding change in total cellular BCRP1 protein. Furthermore, treatment with glucocorticoids does not alter BCRP1-mediated exclusion of xenobiotics from the fetal brain. The latter is reassuring given the extensive use of synthetic glucocorticoid for the management of preterm labor. The present study also suggests that the natural rise in endogenous glucocorticoids that occurs in the fetal circulation in late gestation is unlikely responsible for the dramatic rise in fetal brain BCRP1 activity observed in late gestation. The mechanisms of Abcg2/BCRP1 regulation in the fetal brain are complex and require further investigation. Understanding the regulation of Abcg2/BCRP1 expression and function in the fetal brain during normal development and under pathological conditions is critical for maintaining fetal brain health, particularly when therapeutic strategies are utilized in pregnancy.
Acknowledgment
We thank Hale Ho for her technical assistance.
Footnotes
Supported by the Canadian Institutes for Health Research (Doctoral Research Award to S.P. and FRN-84220 to W.G. and S.G.M.).
REFERENCES
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 1998; 95: 15665 15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G, Morris ME. HPLC analysis of mitoxantrone in mouse plasma and tissues: application in a pharmacokinetic study. J Pharm Biomed Anal 2010; 51: 750 753. [DOI] [PubMed] [Google Scholar]
- Cygalova L, Ceckova M, Pavek P, Staud F. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol Lett 2008; 178: 176 180. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol 2007; 72: 967 975. [DOI] [PubMed] [Google Scholar]
- Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta 2006; 27: 1096 1102. [DOI] [PubMed] [Google Scholar]
- Imai Y, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance. Jpn J Cancer Res 2002; 93: 231 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q. BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res 2008; 25: 1244 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Unadkat JD, Mao Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab Dispos 2007; 35: 2154 2158. [DOI] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 2002; 13: 2059 2063. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003; 22: 7340 7358. [DOI] [PubMed] [Google Scholar]
- Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res 2004; 64: 3296 3301. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology 2007; 28: 931 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 2001; 105: 7 17. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev 1991; 26: 61 67. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci 2002; 25: 518 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979; 3: 79 83. [DOI] [PubMed] [Google Scholar]
- Daood M, Tsai C, Ahdab-Barmada M, Watchko JF. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008; 39: 211 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek CJ, Wong A, Liddelow SA, Johansson PA, Dziegielewska KM, Saunders NR. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol Lett 2010; 197: 51 59. [DOI] [PubMed] [Google Scholar]
- Kalabis GM, Petropoulos S, Gibb W, Matthews SG. Breast cancer resistance protein (Bcrp1/Abcg2) in mouse placenta and yolk sac: ontogeny and its regulation by progesterone. Placenta 2007; 28: 1073 1081. [DOI] [PubMed] [Google Scholar]
- Elahian F, Kalalinia F, Behravan J. Evaluation of indomethacin and dexamethasone effects on BCRP-mediated drug resistance in MCF-7 parental and resistant cell lines. Drug Chem Toxicol 2010; 33: 113 119. [DOI] [PubMed] [Google Scholar]
- Elahian F, Kalalinia F, Behravan J. Dexamethasone downregulates BCRP mRNA and protein expression in breast cancer cell lines. Oncol Res 2009; 18: 9 15. [DOI] [PubMed] [Google Scholar]
- Narang VS, Fraga C, Kumar N, Shen J, Throm S, Stewart CF, Waters CM. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am J Physiol Cell Physiol 2008; 295: C440 C550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstet Gynecol 1977; 50: 186 190. [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev 2008; 57: 586 595. [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev 2009; 61: 3 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Development Conference. Effect of corticosteroids for fetal maturation and perinatal outcomes. Am J Obstet Gynecol 1995; 173: 253 344. [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 1996; 64: 412 418. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab 2004; 287: E863 E870. [DOI] [PubMed] [Google Scholar]
- Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol 2004; 181: 105 116. [DOI] [PubMed] [Google Scholar]
- McClive PJ, Sinclair AH. Rapid DNA extraction and PCR-sexing of mouse embryos. Mol Reprod Dev 2001; 60: 225 226. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Kalabis GM, Gibb W, Matthews SG. Functional changes of mouse placental multidrug resistance phosphoglycoprotein (ABCB1) with advancing gestation and regulation by progesterone. Reprod Sci 2007; 14: 321 328. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6 (2): 65 70. [Google Scholar]
- Evseenko D, Paxton JW, Keelan JA. Active transport across the human placenta: impact on drug efficacy and toxicity. Expert Opin Drug Metab Toxicol 2006; 2: 51 69. [DOI] [PubMed] [Google Scholar]
- Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 2006; 27: 602 609. [DOI] [PubMed] [Google Scholar]
- Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol 2006; 84: 1251 1258. [DOI] [PubMed] [Google Scholar]
- Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol 2009; 158: 665 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther 2005; 312: 44 52. [DOI] [PubMed] [Google Scholar]
- Zhao R, Raub TJ, Sawada GA, Kasper SC, Bacon JA, Bridges AS, Pollack GM. Breast cancer resistance protein interacts with various compounds in vitro, but plays a minor role in substrate efflux at the blood-brain barrier. Drug Metab Dispos 2009; 37: 1251 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37: 13 25. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res 2003; 314: 119 129. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell Mol Neurobiol 2000; 20: 29 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. The blood-CSF barrier explained: when development is not immaturity. Bioessays 2008; 30: 237 248. [DOI] [PubMed] [Google Scholar]
- Grube M, Reuther S, Meyer Zu Schwabedissen H, Kock K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 2007; 35: 30 35. [DOI] [PubMed] [Google Scholar]
- Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 2006; 27: 244 250. [DOI] [PubMed] [Google Scholar]
- Mark PJ, Augustus S, Lewis JL, Hewitt DP, Waddell BJ. Changes in the placental glucocorticoid barrier during rat pregnancy: impact on placental corticosterone levels and regulation by progesterone. Biol Reprod 2009; 80: 1209 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q, Richards K, Strong-Basalyga K, Fauty SE, Taylor A, Yamazaki M, Prueksaritanont T, Lin JH, Hochman J. Using real-time quantitative TaqMan RT-PCR to evaluate the role of dexamethasone in gene regulation of rat P-glycoproteins mdr1a/1b and cytochrome P450 3A1/2. J Pharm Sci 2004; 93: 2488 2496. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Gibb W, Matthews SG. Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta 2010; 31: 803 810. [DOI] [PubMed] [Google Scholar]
- Petropoulos S, Gibb W, Matthews SG. Developmental expression of multidrug resistance phosphoglycoprotein (P-gp) in the mouse fetal brain and glucocorticoid regulation. Brain Res 2010; 1357: 9 18. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 2004; 66: 413 419. [DOI] [PubMed] [Google Scholar]
- Honorat M, Mesnier A, Di Pietro A, Lin V, Cohen P, Dumontet C, Payen L. Dexamethasone down-regulates ABCG2 expression levels in breast cancer cells. Biochem Biophys Res Commun 2008; 375: 308 314. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes: sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem 2001; 268: 6346 6358. [DOI] [PubMed] [Google Scholar]
- Scheer N, Ross J, Kapelyukh Y, Rode A, Wolf CR. In vivo responses of the human and murine pregnane X receptor to dexamethasone in mice. Drug Metab Dispos 2010; 38: 1046 1053. [DOI] [PubMed] [Google Scholar]
- Shi D, Yang D, Yan B. Dexamethasone transcriptionally increases the expression of the pregnane X receptor and synergistically enhances pyrethroid esfenvalerate in the induction of cytochrome P450 3a23. Biochem Pharmacol 2010; 80: 1274 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci 1998; 18: 2570 2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakk S, Huang W, Staudinger JL, Tan K, Cole TJ, Moore DD, Strobel HW. Gender dictates the nuclear receptor-mediated regulation of CYP3A44. Drug Metab Dispos 2007; 35: 36 42. [DOI] [PubMed] [Google Scholar]
- Anakk S, Kalsotra A, Shen Q, Vu MT, Staudinger JL, Davies PJ, Strobel HW. Genomic characterization and regulation of CYP3a13: role of xenobiotics and nuclear receptors. FASEB J 2003; 17: 1736 1738. [DOI] [PubMed] [Google Scholar]
- Koenen SV, Dunn EA, Kingdom JC, Ohlsson A, Matthews SG. Overexposure to antenatal corticosteroids: a global concern. J Obstet Gynaecol Can 2007; 29: e879. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Morrison PJ, Sullivan FM. Plasma corticosterone levels during pregnancy in the mouse: the relative contributions of the adrenal glands and foeto-placental units. J Endocrinol 1974; 60: 473 483. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc 1998; 57: 113 122. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Yamaguchi H, Aoki F, Enami J, Sakai S. Corticosterone is required for the prolactin receptor gene expression in the late pregnant mouse mammary gland. Mol Cell Endocrinol 1997; 132: 177 183. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Talamantes F. Pre-parturitional changes in serum prolactin, placental lactogen, growth hormone, progesterone, and corticosterone in the C3H/HeN mouse. J Dev Physiol 1984; 6: 423 429. [PubMed] [Google Scholar]