ERAP1 controls both the repertoire and the extent of T cell responses to antigens
Keywords: adaptive immune responses, antigen presentation, endoplasmic reticulum aminopeptidase-1, memory T-cells
Abstract
Endoplasmic reticulum aminopeptidase 1 (ERAP1) is a critical component of the adaptive immune system that has been shown to increase or decrease the presentation of specific peptides on MHC class I molecules. Here, we have demonstrated that ERAP1 functions are not only important during the presentation of antigen-derived peptides, but these functions can also completely change which antigen-derived peptides ultimately become selected as immunodominant T-cell epitopes. Our results suggest that ERAP1 may do this by destroying epitopes that would otherwise become immunodominant in the absence of adequate ERAP1 functionality. We further establish that ERAP1-mediated influences on T-cell functions are both qualitative and quantitative, by demonstrating that loss of ERAP1 function redirects CTL killing toward a different set of antigen-derived epitopes and increases the percent of antigen-specific memory T cells elicited by antigen exposure. As a result, our studies suggest that normal ERAP1 activity can act to suppress the numbers of T effector memory cells that respond to a given antigen. This unique finding may shed light on why certain ERAP1 single nucleotide polymorphisms are associated with several autoimmune diseases, for example, by significantly altering the robustness and quality of CD8+ T-cell memory responses to antigen-derived peptides.
Introduction
The endoplasmic reticulum aminopeptidase 1 (ERAP1) protein has been identified as a central player in the process of antigen trimming and loading onto MHC class I molecules (MHC-I), and therefore adaptive immunity (1–3). ERAP1 is an IFN-γ-inducible, M1 zinc-binding, metalloaminopeptidase of the oxytocinase subfamily (4). ERAP1 is the only peptidase found in the ER of mice, and one of two highly homologous aminopeptidases (ERAP1 and ERAP2) found in the ER of humans (5). The N-terminal moiety of ERAP1 binds and trims the N-terminal residues from peptides present within the ER in a length- and sequence-dependent manner that results in the generation of epitopes that are 8–10 amino acids long for loading onto MHC-I (4, 6–10). Because of this function, loss of ERAP1 activity can result in an increase in the average length of all peptides found on MHC-I molecules (11). In addition to changing the length of peptides being presented to T cells by MHC-I molecules, ERAP1 functions are known to facilitate the loading of a few specific peptides (such as SIINFEKL, CMV NP396 and CMV GP34) onto MHC-I (3, 12). Additionally, and possibly as a result, peptide-MHC-I complexes generated in the absence of ERAP1 on average have a shorter half-life on the cell surface, resulting in a decreased number of peptide-MHC-I complexes on ERAP1-deficient cells (13, 14). Furthermore, these changes in MHC-I-loaded peptides can impact a cell’s immunologic identity, since induced absence of ERAP1 can cause a cell to become immunologically recognized as non-self by otherwise syngenic T cells (13).
Out of the thousands of potential epitopes present in a respective protein, only a few are maximally recognized by the cells of the adaptive immune system. This phenomenon is referred to as immunodominance and is critical for understanding and manipulating T-cell-mediated immunity (15). In previous studies of ERAP1, known immunodominant peptides were tested and found to generate reduced CD8+ T-cell responses in the absence of ERAP1 functions, indirectly suggesting that ERAP1 may play a role in immunodominance (12). However, ERAP1 functions also modulate the secretion of certain cytokines during innate immune responses, independent of its canonical role as an antigen-processing molecule (16). These latter observations complicate the interpretation of prior results that relied on cytokine secretion profiles, or reporter cell lines to measure the potency of T-cell epitopes in the presence or absence of ERAP1 functionality (2, 3, 13, 17). In light of these issues, we wished to determine if immunodominance occurred in the absence of ERAP1 and to examine the extent to which immunodominance might be altered by loss of ERAP1 functions. To test these hypotheses, we queried the role that ERAP1 might have in a mouse model. Specifically, after vaccination of wild-type (WT) or ERAP1-deficient animals with a model antigen, the immunodominance profile of the antigen was assessed. We measured antigen-specific adaptive immune responses after vaccination with an adenovirus serotype 5 (Ad5) vector expressing the non-enzymatic portion of the Toxin A (TA) protein from Clostridium difficile (Ad5-TA) (18). Surprisingly, vaccination of ERAP1−/− mice did not merely change the amount that certain peptides contributed to immunodominance, but resulted in an entirely new and distinct set of TA-derived epitopes becoming immunodominant. These changes in immunodominance selection were profound, as none of the immunodominant TA-derived epitopes, respectively, identified in Ad5-TA-vaccinated WT or ERAP−/− mice were shared between the two strains of mice. As a result, these ERAP1-mediated changes on immunodominance also had profound effects on T-cell biology, including the generation of functionally distinct CTL populations and modulating the quantity of T-cell effector memory populations present in the vaccinated animals.
Methods
Animal procedures
All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee (http://iacuc.msu.edu/). Adult C57BL/6 WT mice were purchased from Taconic Farms (Hudson, NY, USA). Ad5 vectors were injected intramuscularly (I.M.) into 8-week-old mice. A total of 1×1010 viral particles per mouse were administered I.M. in a volume of 30 μl PBS solution (pH 7. 4) into the tibialis anterior of the right hindlimb. Numbers of animals used for each experiment are specified on the corresponding figure legends. Plasma and tissue samples were collected and processed at the indicated times.
ELISpot analysis
96-Well Multiscreen high protein binding Immobilon-P membrane plates (Millipore, Billerica, MA, USA) were pretreated with ethanol, coated with mouse anti-IFN-γ (or IL-4 or IL-2) capture antibody, incubated overnight and blocked with RPMI medium [with 10% fetal bovine serum (FBS), 1% PSF (penicillin, streptomycin, fungizone)] prior to the addition of 1.0×106 splenocytes per well (19). Ex vivo stimulation included the incubation of splenocytes in 100 μl of media alone (unstimulated), or media containing 2 μg per well of a single 15-mer peptide (indicated in the figure) for 18–24h in a 37°C, 5% CO2 incubator. Ready Set Go IFN-γ, IL-2 and IL-4 mouse ELISpot kits were purchased from eBioscience (San Diego, CA, USA). Staining of plates was completed per the manufacturer’s protocol. Spots were counted and photographed by an automated ELISpot reader system (Cellular Technology, Cleveland, OH, USA).
Cell staining and flow cytometry
To evaluate intracellular cytokine responses, splenocytes from immunized mice were ex vivo stimulated with a pool of peptides that generated responses only in WT animals, WT immunodominant toxin-A-derived peptides (IDTAPs) (TGYTIINGKHFYFNT, FYFNTDGIMQIGVFK, ALTSY KIINGKHFYF, STGYTIISGKHFYFN, YTSINGKHFYFNTDG, SKMVTGVFKGPNGFE), or with a pool of peptides that generated responses only in ERAP1−/− animals, ERAP1−/− IDTAPs (AAIHLCTINNDKYYF, FEYFAPANTDANNIE, GFEYFA PANTDANNI, FAPANTDANNIEGQA, EYFAPANTDANNIEG, ANNIEGQAIRYQNRF), at a total mass of 2 μg per well. Subsequently, intracellular staining was performed as previously described (20). Briefly, cells were surface stained with allophycocyanin-Cy7-CD3, Alexa Fluor 700-CD8a and CD16/32 Fc-block antibodies, fixed with 2% formaldehyde (Polysciences, Warrington, PA, USA), permeabilized with 0.2% saponin (Sigma-Aldrich, St Louis, MO, USA) and stained for intracellular cytokines with PE-Cy7-TNF-α, allophycocyanin-granzyme B, PE-perforin, FITC-IFN-γ, Pacific Blue-CD62L, PerCP-Cy5.5-CD127 (4 μg ml−1) (all obtained from BD Biosciences, San Diego, CA, USA). Cells were incubated on ice with the appropriate antibodies for 30min, washed, and data were collected using an LSR II instrument (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
In vivo CTL assay
An in vivo CTL assay was performed as previously described (19, 20). Briefly, ERAP1−/− or WT mice were immunized with Ad5-TA as described in the section describing Animal procedures. Fourteen days following the injection, syngenic splenocytes were isolated and either pulsed with a pool of the WT IDTAPs (TGYTIINGKHFYFNT, FYFNTDGIMQIGVFK, ALTSYKIINGKHFYF, STGYTIISGKHFYFN, YTSINGKHFYF NTDG, SKMVTGVFKGPNGFE) and stained with 10 μM CFSE (CFSEhigh), or pulsed with a pool of ERAP1−/− IDTAPs (AAIHLC TINNDKYYF, FEYFAPANTDANNIE, GFEYFAPANTDANNI, FAPA NTDANNIEGQA, EYFAPANTDANNIEG, ANNIEGQAIRYQNRF) and stained with 1 μM CFSE (CFSElow). Naive and immunized mice were injected with equivalent amounts of both CFSElow- and CFSEhigh-stained cells (8×106 total cells per mouse) via the retroorbital sinus. After 20h, mice were terminally sacrificed and splenocytes were recovered and analyzed on an LSRII flow cytometer. FlowJo software was used to determine percentages of CFSE-stained cells as follows: % specific killing = 1 − [(% CFSEhigh/% CFSElow)immunized/(% CFSEhigh/% CFSElow)non-immunized].
Isolation of lymphocytes from spleen and liver tissues
Splenocytes from individual mice were harvested and processed as follows: spleen tissues were physically disrupted by passage through a 40-μm sieve, followed by RBC lysis by using 2ml of ACK lysis buffer (Invitrogen, Carlsbad, CA, USA) per homogenized spleen. Splenocytes were subsequently washed two times with RPMI medium (Invitrogen) supplemented with 10% FBS, 2mM l-glutamine, 1× PSF, re-suspended and counted. Liver tissue from individual mice was minced into small pieces, incubated in collagenase/DNase media containing RPMI supplemented with 10% FBS and 1× PSF, for 1h at 37°C, with vigorous vortexing every 15min. Following incubation, tissue was passed through a 40-μm sieve, followed by RBC lysis and resuspension in complete RPMI media.
Statistical analysis
Statistically significant differences were determined using a one-way ANOVA with a Student–Newman–Keuls post hoc test or by using two-tailed homoscedastic Student’s t-tests (P value of <0.05 was deemed statistically significant). Graphs in this paper are presented as mean of the average ± SEM, unless otherwise specified. Statistical analyses were performed using GraphPad Prism (GraphPad Software).
Results
ERAP1 overrides inherent immunodominant peptide selection to completely shift which antigen-derived peptides are identified as immunodominant by the adaptive immune system
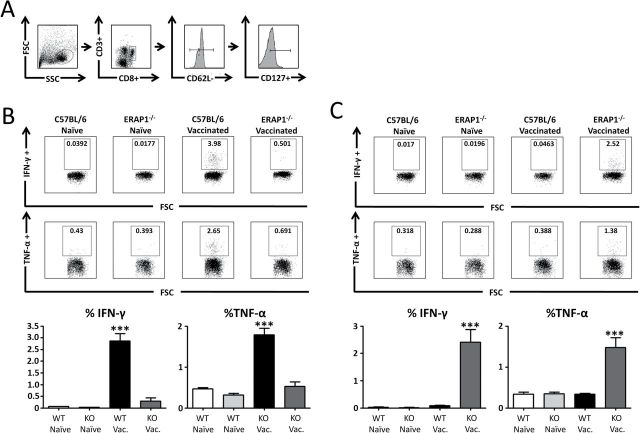
ERAP1 facilitates efficient MHC-I presentation of certain epitopes, such as SIINFEKL (3, 12) and the HF10 peptide from Toxoplasma gondii, the latter being required for T-cell-mediated immune clearance of the organism (21). However, those studies could not determine if loss of ERAP1 eradicates the ability of the adaptive immune system to generate an alternative hierarchy of immunodominant epitopes to a specific antigen. To specifically determine if and how ERAP1 affects the breadth of T-cell clonal responses to a specific antigen, we immunized mice with an adenovirus-based vaccine expressing the C. difficile-derived antigen, TA (Ad-TA) and studied the breadth of the T-cell responses to this model antigen. Specifically, WT or ERAP1−/− mice were vaccinated with Ad-TA, and TA-specific T-cell responses were evaluated at 14 days post-injection (dpi). Splenocytes from the vaccinated mice were exposed to a 15-mer overlapping peptide library (peptides overlapping by five amino acids) that spanned the entire TA protein expressed by Ad-TA. T-cell responses to these peptides were quantified by IFN-γ ELISpot assay; 15-mer peptides were selected because peptides of this length are suitable substrates for both TAP and ERAP1, yet are longer than any classic MHC-I substrates, and therefore require processing prior to MHC-I loading. Among the 84 15-mer peptides that we interrogated (Supplementary Figure 1, available at International Immunology Online), not a single peptide responded as immunodominant in both WT and ERAP1−/− mice (Fig. 1). Rather, we identified a set of ERAP1-dependent TA-derived immunodominant peptides only responsive in Ad-TA-vaccinated WT mice (the WT IDTAPs) and a set of ERAP1-independent TA-derived immunodominant peptides only responsive in Ad-TA-vaccinated ERAP1−/− mice (the ERAP1−/− IDTAPs) (Fig. 1). Together, these results demonstrate that the presence of ERAP1 completely shifts the repertoire of antigen-derived peptides ultimately identified as immunodominant by the adaptive immune system.
Fig. 1.
ERAP1 reshapes the immunodominant focus by the creation and destruction of epitopes. C56BL/6 mice (n = 5) or ERAP1−/− mice (n = 5) were immunized I.M. in the tibialis anterior with Ad5-Clostridium difficile-TA as described in Methods. At day 14, mice were sacrificed and splenocytes were harvested and stimulated with 2 μg per well of the listed 15-mer peptides. Splenocytes were then analyzed for IFN-γ production using IFN-γ ELISpot. From the 84 peptide library, the peptides that generated a strong response (100 or more spots) and showed an average of at least 100 less spots in the other strain of mouse were selected and repeated. (A) Peptides that had a very high response in WT mice compared with ERAP1−/− mice. (B) Peptides that had a very high response in ERAP1−/− mice compared with WT mice. Bars represent the mean number of spot-forming cells per 106 splenocytes ± SEM. Below are representative wells. All wells are shown in the order they appeared on the plate and all paired sets of wells are from the same plate. A P value < 0.05 was statistically significant. The figure is representative of two separate experiments.
It is also worth noting that of the ERAP1−/− IDTAPs, FAPANTDANNIEGQA and ANNIEGQAIRYQNRF were effectively immunologically silent in Ad5-TA-immunized WT mice, generating only 2.4 and 4 spot-forming cells per 106 splenocytes when the splenocytes used in these assays were derived from Ad5-TA-immunized WT mice. As previous studies relied on using peptides that were identified as potent or immunodominant peptides only in WT animals prior to their study in ERAP1−/− mice, to our knowledge the ERAP1−/− IDTAPs identified in this study are the first immunodominant antigen-derived sequences to be specifically identified as becoming immunodominant in the absence of ERAP1 activity.
ERAP1 destroys otherwise immunodominant epitopes via linear N-terminal trimming
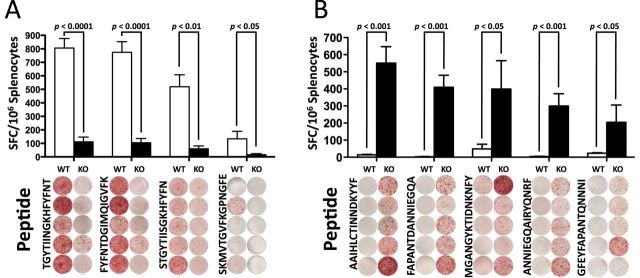
It has been observed that certain epitopes are presented at a higher frequency in cells without ERAP1 (2, 12). As it is also known that immunodominant epitopes are capable of suppressing the selection of sub-dominant epitopes (15, 22, 23), one hypothesis suggests that peptides generated by ERAP1 (WT IDTAPs) are outcompeting and suppressing immunodominant peptides generated in the absence of ERAP1 (ERAP1−/− IDTAPs), resulting in the loss of recognition of these sub-dominant epitopes by T cells in the WT animal. An alternative hypothesis is that ERAP1 is actively degrading epitopes that would otherwise be presented on the cell surface and become immunodominant (24–26). To investigate the latter hypothesis, we chose to study the peptide with the least response in WT mice, FAPANTDANNIEGQA. We constructed four 15-mer peptides with 1, 2, 3 or 4 additional N-terminal amino acids in front of the FAPANTDANNIEGQA sequence. If ERAP1 is degrading this epitope via N-terminal trimming, each N-terminal amino acid should provide incremental protection and result in increased response in WT mice. To further control for ERAP1’s strong, length-dependent substrate specificity, each peptide produced in this manner was made the same overall length by removing a C-terminal amino acid for each N-terminal amino acid added. The resulting sequences were used to stimulate splenocytes harvested from Ad-TA-immunized WT or ERAP1−/− animals. We confirmed that with each additional N-terminus extension of the FAPANTDANNIEGQA peptide, a significantly higher number of splenocytes derived from Ad-TA-vaccinated WT mice responded to the extended peptide (Fig. 2A). Furthermore, the N-terminal amino acids increased the Ad-TA-vaccinated splenocyte ELISpot responses in a linear manner, with each N-terminal amino acid resulting in an additional 13.67 (± 1.302) IFN-γ-secreting WT cells per 106 splenocytes (Fig. 2B). To further demonstrate this, a linear regression of the mean values was performed, yielding an R 2 of 0.9948 (Fig. 2B). To demonstrate that this effect was specific to ERAP1, we included ERAP1−/− cells. ERAP1−/− cells had a uniformly high response to each peptide, independent of N-terminal amino acid additions, with an R 2 of 0.19 (Fig. 2B). To our knowledge these are the first data suggesting that ERAP1 destroys potential alternative immunodominant peptide epitopes by virtue of its N-terminal trimming activity and that this destruction has functional significance on T-cell responses.
Fig. 2.
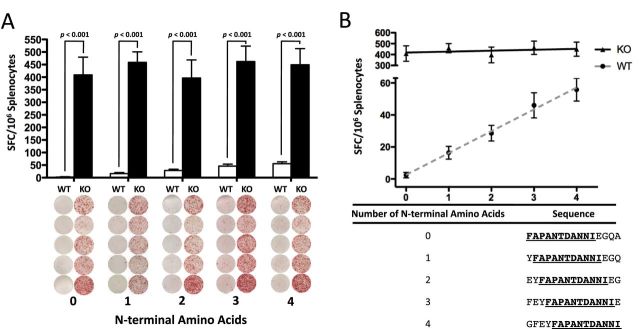
ERAP1 ablates response to ERAP1−/− IDTAP linearly with the number of N-terminal residues prior to a consensus sequence. C56BL/6 mice (n = 5) or ERAP1−/− mice (n = 5) were immunized I.M. in the tibialis anterior with Ad5-C. difficile-TA as described in Methods. At day 14, mice were sacrificed and splenocytes were harvested and stimulated with 2 μg per well of the listed 15-mer peptides. Five peptides were generated that contained the consensus sequence FAPANTDANNI and 0, 1, 2, 3 or 4 additional N-terminal residues. These peptides were used to stimulate splenocytes using an ELISpot technique. As expected each peptide generated a strong response in ERAP1−/− mice, and very low responses in corresponding WT animals (A). A linear regression was performed (dotted gray line) to examine if N-terminal extension of the consensus sequence would provide incremental protection from ERAP1-mediated destruction (B). The number of specific spots per 106 splenocytes stimulated were graphed on the y-axis and the number of N-terminal amino acids before the consensus sequence were plotted on the x-axis. In WT cells, for each additional N-terminal amino acid in front of the consensus sequence, 13.67 (± 1.892) additional spots per 106 were observed to respond. There was no strong relationship observed in ERAP1−/− cells. R 2 of 0.9948 for WT and 0.1903 for ERAP1−/−. Error is shown above and below each point. The figure is representative of two separate experiments.
Different cytokine secretion profiles of CD8+ T lymphocytes derived from Ad5-TA-vaccinated WT or ERAP1-deficient mice
To further characterize the ERAP1-dependent aspects of induction of peptide-specific T-cell responses to TA-derived epitopes, WT or ERAP1−/− mice were vaccinated with Ad5-TA, and at 14 dpi the splenocytes derived from these animals were stimulated with either a pool of the WT IDTAPs or a pool of the ERAP1−/− IDTAPs and then subjected to FACS analysis. We observed a significantly (P < 0.001) increased frequency of IFN-γ- and TNF-α-secreting CD3+ CD8+ T cells in splenocytes derived from WT mice vaccinated with Ad5-TA and exposed to the WT IDTAPs (Fig. 3A), as compared with similar exposures of T cells derived from ERAP1−/− animals vaccinated with Ad5-TA (Fig. 3B). In contrast, ERAP1−/− IDTAPs induced a significant (P < 0.01) increase in the frequency of IFN-γ- and TNF-α-expressing CD8+ T cells in splenocytes derived from Ad5-TA-vaccinated ERAP1−/− mice, as compared with splenocytes from Ad5-TA-vaccinated WT mice similarly exposed to the ERAP1−/− IDTAPs (Fig. 3C and D). CD3+, CD4+, CD8− T cells were also examined and found to not respond significantly (Supplementary Figure 2, available at International Immunology Online).
Fig. 3.
Cytokine secretion profile of CD8+ T cells derived from WT C57BL/6 or ERAP1-deficient mice. C56BL/6 mice (n = 8) or ERAP1−/− mice (n = 8) were immunized I.M. in the tibialis anterior with viral particles of Ad5-C. difficile-TA (1×1010). At day 21, mice were sacrificed and splenocytes were harvested and stimulated for 6h at 37°C with a pool of peptides that responded in the WT mice only, the WT IDTAPs as described in Methods, (A and B), with a pool of peptides that responded in the ERAP1−/− mice only, the ERAP1−/− IDTAPs as described in Methods (C and D). (A) Representative example of IFN-γ-, TNF-α- or IFN-γ/TNF-α-producing splenic CD8+ T cells stimulated with the WT IDTAPs. (C) Representative example of IFN-γ-, TNF-α- or IFN-γ/TNF-α-producing splenic CD8+ T cells stimulated with the ERAP1−/− IDTAPs. Gates were set based on negative control (naIve) and placed consistently across samples. (B) The total frequency of splenic CD8+ T cells expressing IFN-γ, TNF-α or IFN-γ/TNF-α stimulated with the WT IDTAPs. (D) The total frequency of splenic CD8+ T cells expressing IFN-γ, TNF-α or IFN-γ/TNF-α stimulated with the ERAP1−/− IDTAPs. The bars represent means ± SEM. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A P value < 0.05 was statistically significant. ***P < 0.001, statistically significant from the other three groups of animals. **P < 0.01, statistically significant from the other groups of animals.
In vivo cytolytic activity of T lymphocytes against specific antigenic epitopes is dependent upon ERAP1 functions
As we have recently demonstrated, ERAP1 functions modulate the secretion of certain cytokines during innate immune responses, independent of its canonical role as an antigen-processing molecule (16). To avoid these potentially confounding effects of ERAP1 and directly investigate if the T-cell activation observed in this study translates to a functional difference in vivo, we tested the role that ERAP1 may have in directing the in vivo cytolytic activity of T cells, using in vivo functional cytotoxic lysis assays (20). Normal splenocytes were harvested, pulsed with the WT IDTAPs or the ERAP1−/− IDTAPs and differentially labeled with high or low concentration of the CFSE dye so as to allow us to track the survival of the two groups of differentially labeled splenocytes in vivo after administrations into syngenic Ad-TA-vaccinated WT or ERAP1−/− mice. In Ad5-TA-vaccinated WT mice splenocytes pulsed with WT IDTAPs were specifically killed compared with identical splenocytes pulsed with the ERAP1−/− IDTAPs (Fig. 4A). In contrast, adoptive transfer of ERAP1−/− splenocytes pulsed with either WT IDTAPs or ERAP1−/− IDTAPs into Ad-TA-vaccinated ERAP1−/− mice resulted in preferential killing of only splenocytes pulsed with ERAP1−/− IDTAPs (Fig. 4B). We also noted that although ERAP1−/− T cells were able to generate potent cytokine secretion profiles, (Fig. 3C) the cytolytic immune system of Ad5-TA-vaccinated ERAP1−/− animals did not kill cells loaded with ERAP1−/− IDTAPs as quickly or potently as the cytolytic immune system of Ad5-TA-vaccinated WT animals killed WT IDTAP-loaded cells (Fig. 4B).
Fig. 4.
ERAP1 directs in vivo cytolytic activity of T lymphocytes against a specific set of antigens, and absence of ERAP1 directs in vivo cytolytic activity of T lymphocytes against a different, distinct set of antigens. C56BL/6 mice (n = 8) or ERAP1−/− mice (n = 7) were immunized I.M. in the tibialis anterior with viral particles of Ad5-C. difficile-TA (1×1010). At day 14, syngenic splenocytes were pulsed with either the pool of WT IDTAPs and stained with 10 μM (CFSEhigh) or with the pool of ERAP1−/− IDTAPs and stained with 1 μM (CFSElow), then transplanted into the immunized mice, or corresponding naive animals (n = 5). Twenty-four hours after adoptive transfer, splenocytes were harvested and analyzed using an LSRII flow cytometer. (A) Representative examples of CFSE+ splenocytes from C57BL/6 mice and corresponding statistical analysis. (B) Representative examples of CFSE+ splenocytes from ERAP1−/− mice and corresponding statistical analysis. The percentage of CFSE+ cells was quantified using FlowJo software. % specific killing = 1 − [(% CFSEhigh/% CFSElow)immunized/(% CFSEhigh/% CFSElow)non-immunized].
ERAP1 functions are necessary to regulate the antigen-specific effector memory T-cell response in vivo
After an acute infection, antigen-specific memory T cells persist for long periods of time, via a cytokine-directed, antigen-independent mechanism (27). To study if ERAP1 impacted long-term antigen-specific memory responses, we examined induction of memory CD8+ T cells after Ad-TA vaccinations of WT and ERAP1−/− mice. In addition, because of the important functional differences between the population of central and effector memory CD8+ T-cell development, we investigated the effects that ERAP1 had on T central memory (TCM) and T effector memory (TEM) cells (28–30). Following immunization of WT or ERAP1−/− mice with Ad-TA, splenocytes were harvested and stimulated with TA peptides. Subsequently, the response of effector memory CD8+ T cells (CD3+, CD8+, CD62L−, CD127+ cells) was quantified by flow cytometry (Fig. 5A). We observed that, TEM cells from Ad-TA-vaccinated WT mice had a potent and specific response to WT IDTAPs (Fig. 5B). TEM from the vaccinated WT mice secreted significantly (P < 0.001) more IFN-γ and TNF-α in response to WT IDTAPs than TEM derived from Ad5-TA-vaccinated ERAP1−/− mice. The Ad5-TA WT derived TEM cells responded at a significantly greater level to the WT IDTAPs than to the ERAP1−/− IDTAPs (P < 0.001), demonstrating that ERAP1-mediated alterations in antigen-derived immunodominance (observed in effector T cells) also alters the activity of antigen-specific TEM cells.
Fig. 5.
T effector memory cells directed at distinct sets of epitopes. C56BL/6 mice (n = 8) or ERAP1−/− mice (n = 8) were immunized I.M. in the tibialis anterior with viral particles of Ad5-C. difficile-TA (1×1010). At day 21, mice were sacrificed and splenocytes were harvested and stimulated for 6h at 37°C with the labeled peptides. (A) Representative sample demonstrating our gating strategy. (B) Representative example of IFN-γ-, TNF-α-producing splenic CD8+ CD62L− CD127+ T cells stimulated with the WT IDTAP and percentage of IFN-γ-+, TNF-α+ TEM cells. (C) Representative example of IFN-γ-, TNF-α-producing splenic CD8+ CD62L− CD127+ T cells stimulated with the ERAP1−/− IDTAP and percentage of IFN-γ+, TNF-α+ TEM cells. The bars represent means ± SEM. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A P value < 0.05 was statistically significant. ***P < 0.001, statistically significant from the other three groups of animals.
In contrast, Ad5-TA-vaccinated ERAP1−/− mice generated a population of TEM cells that generated a significantly more potent response to the ERAP1−/− IDTAPs, than did TEM cells from WT mice exposed to these same IDTAPs (P < 0.001) (Fig. 5C). Specifically, the TEM from ERAP1−/− mice responded to the ERAP1−/− IDTAPs by secreting significantly more IFN-γ and TNF-α than they secreted in response to the WT IDTAPs.
Because of our recent identification of ERAP1’s effect on the cytokine response to innate immune stimulation, including changes in many important regulatory cytokines such as TNF-α and IL-12 (16), and because of the critical role of IL-12 in regulating the transition of memory T cells toward a TEM phenotype, we also investigated if ERAP1 deficiency impacted on the phenotype of antigen-specific memory T cells induced following Ad-TA vaccination of WT or ERAP1−/− mice. Expression of IFN-γ, TNF-α or both cytokines in CD8+ T cells was quantified, and from that population, the frequency of TCM (CD62L+, CD127+) and TEM (CD127+, CD62L−) CD8+ T cells were analyzed using flow cytometry as previously described (19). Interestingly, following ERAP1−/− IDTAPs stimulation, we observed a significantly (P < 0.001) higher frequency of antigen-specific TEM cell populations in Ad-TA-vaccinated ERAP1−/− mice, as compared with the number of similar cells generated in Ad-TA-vaccinated WT mice exposed to the WT IDTAPs (Fig. 6). Specifically, of the responsive antigen-specific CD8+ T cells secreting TNF-α, a significantly (P < 0.001) higher frequency of TEM CD8+ T cells were observed in Ad-TA-vaccinated ERAP1−/− mice compared with identically vaccinated WT mice (Fig. 6A). The same trend was observed in TA antigen-specific CD8+ T splenocytes in that there were greater numbers of cells secreting IFN-γ (Fig. 6B), and cells secreting both TNF-α and IFN-γ derived from Ad-TA-vaccinated ERAP1−/− mice as compared with identically vaccinated WT mice (Fig. 6C). The antigen-specific CD8+ T cells that were positive for both TNF-α and IFN-γ also showed a significant (P < 0.05) increase in the percentage of TCM (CD62L+, CD127+) cells in Ad-TA-vaccinated ERAP1−/− mice, as compared with identically vaccinated WT animals. Together, these results demonstrate that ERAP1 alters the quality and, interestingly, suppresses the quantity of the CD8+ memory T cells responding to specific, antigen-derived peptides.
Fig. 6.
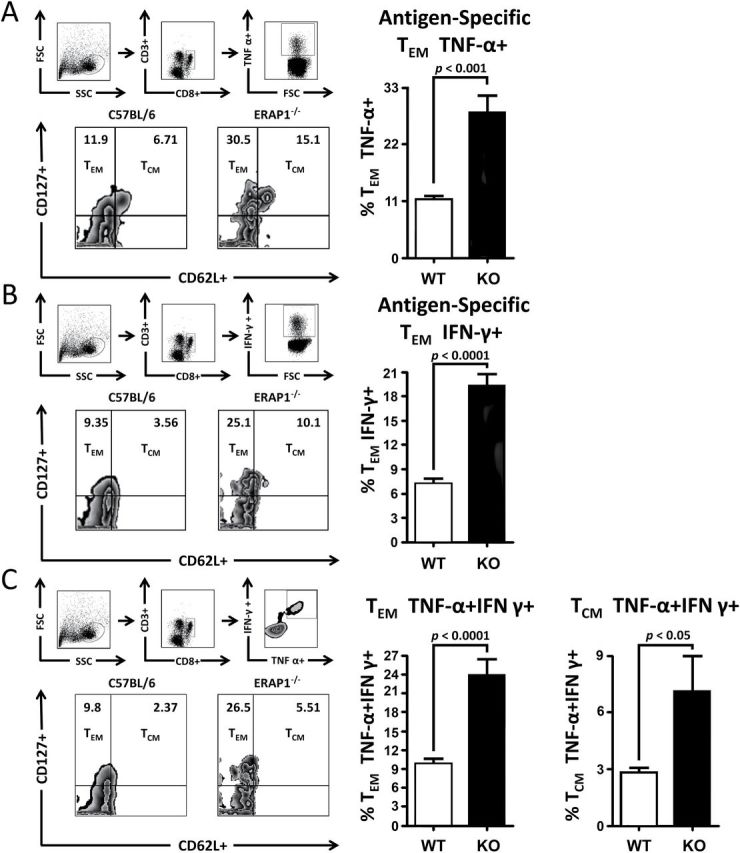
Deficiency of ERAP1 reprograms antigen-specific memory T cell toward an effector memory T-cell phenotype. C56BL/6 mice (n = 8) or ERAP1−/− mice (n = 8) were immunized I.M. in the tibialis anterior with viral particles of Ad5-C. difficile-TA (1×1010). At day 21, mice were sacrificed and splenocytes were harvested and stimulated for 6h at 37°C with antigens. (A) Percentages of antigen-specific, TNF-α+ TEM and TCM shown. (B) Percentages of antigen-specific, IFN-γ+ TEM and TCM shown. (C) Percentages of antigen-specific, IFN-γ+ TNF-α+ double-positive TEM and TCM shown. The bars represent means ± SEM. Statistical analysis was completed using a two-tailed Student’s t-test.
Discussion
Many genome-wide association studies have identified ERAP1 variants, that when present, increase the risk of several human autoimmune diseases (24, 31–35). It is also well documented that the aminopeptidase activity of ERAP1 has an important role in the antigen presentation component of adaptive immunity (13). Because of ERAP1’s role in antigen presentation it has been proposed that significant alterations in antigen presentation may be the mechanism underlying how single nucleotide polymorphisms (SNPs) in ERAP1 contribute as a risk factor for many autoimmune diseases (32, 36). Here, we have demonstrated that ERAP1 functions are not only important during the presentation of antigen-derived peptides, but these functions can also completely change which antigen-derived peptides ultimately become selected as immunodominant T-cell epitopes. Our results suggest that ERAP1 may do this by destroying epitopes that would otherwise become immunodominant in the absence of adequate ERAP1 functionality. By virtue of our use of classical peptide-pulsing experiments to stimulate splenocytes, we cannot discern if loss of ERAP1 functions may unmask effects of cross-presentation and/or the impact that cytosolic aminopeptidases may be having on the experimental results, a caveat that will require future studies to fully understand.
We further establish that ERAP1-mediated influences on T-cell functions are both qualitative and quantitative, by demonstrating that loss of ERAP1 function redirects CTL killing toward a different set of epitopes and increases the percent of antigen-specific memory T cells elicited by antigen exposure. In fact, our studies suggest that normal ERAP1 activity can act to suppress the numbers of TEM cells that respond to a given antigen, as lack of normal ERAP1 activity allows for increased numbers of antigen-specific TEM cells. Because TEM cells have been implicated in autoimmunity (37, 38), this unique finding may shed light on why ERAP1 SNPs are associated with several autoimmune diseases, for example, by significantly altering the robustness and quality of CD8+ T-cell memory responses to antigenic proteins.
While it had been hypothesized that epitopes that are immunologically silent in WT adaptive immune responses may yet be important in generating CD8+ T-cell responses in ERAP1−/− backgrounds, it had not been directly demonstrated until our study (12). We have now identified antigen-derived peptides that become immunodominant T-cell targets only in the absence of ERAP1 functions and further demonstrated that these epitopes are fully functional and capable of directing T-cell killing in vivo. These results confirm that ERAP1 is not essential to the process of immunodominant peptide selection and suggest that other factors such as dominant peptide suppression, the proteasome, TAP and MHC-I are sufficient to generate an immunodominant T-cell response. Despite the production of intact immunodominant peptide responses without ERAP1, the evolutionary conservation of ERAP1 suggests that ERAP1 must somehow enhance this process, providing for optimal induction of antigen-specific T-cell clones to a respective peptide target.
One reason for the evolutionary conservation of ERAP1 may be that for some antigens the absence of ERAP1 results in detrimental outcomes, such as diminished capability of the cellular arm of the adaptive immune response to kill pathogen-infected cells. Consistent with these notions, it has been demonstrated that ERAP1 is required to mount an effective immune response against T. gondii (21). Alternatively, the immunodominant epitopes generated in the absence of ERAP1 may be less potent, as our study demonstrates that although ERAP1−/− T cells were able to generate potent cytokine secretion profiles, ERAP1−/− animals did not kill cells loaded with ERAP1−/− IDTAPs as quickly or potently as WT animals killed WT IDTAP-loaded cells.
Evolutionary benefits may also explain why the presence of ERAP1 may eliminate certain peptides from the repertoire of presented epitopes, for example, to decrease the likelihood of generating immune responses to self-antigens. It has been demonstrated that certain pathogen-derived peptides with high homology to host peptides can break tolerance and result in CD8+ T-cell-mediated autoimmunity due to molecular mimicry (39–41). Our data suggest that the presence of ERAP1 has the potential to ‘over ride’ immunodominant peptide selection so as to limit the number of homologous peptides presented to host T cells, by destroying immunodominant peptides that overlap between pathogen and host. ERAP1 may have evolved to eliminate certain problem epitopes or it may merely add a layer of complexity that must be overcome to generate self-mimicking epitopes. ERAP1’s ability to specifically eliminate certain peptides could ultimately be functionally responsible for why the presence of specific ERAP1 alleles has been repeatedly associated with several autoimmune diseases, and specifically why ERAP1 has been shown to contribute risk to these diseases in an HLA-dependent manner.
In addition to demonstrating that ERAP1 alters the cytolytic activity of antigen-specific CD8+ T cells, we show an increased percentage of antigen-specific CD8+ T cells that express an effector memory (CD62L−, CD127+) phenotype in Ad-TA-vaccinated ERAP1−/− mice.TEM cells have also been implicated in autoimmune diseases (37, 38, 42, 43). The increased frequency of CD8+ TEM cells within the antigen-specific population of memory T cells in ERAP1−/− mice suggest that ERAP1 may be playing a role in autoimmunity by increasing the number of, or modifying the phenotype of memory T cells generated during an immune response to a degree that may contribute to increased autoimmune disease risk.
Our data demonstrating that ERAP1 suppresses TEM responses appear counterintuitive relative to the known important role that ERAP1 plays during antigen generation and raises interesting questions about the evolutionary retention of ERAP1’s functions with regard to TEM. Our findings suggest that in the absence of ERAP1, greater percentages of memory T cells expressing the effector memory phenotype are generated, contrary to what might be expected of an important enzyme that facilitates CD8+ T-cell responses. However, memory T-cell expansion and contractions are tightly regulated by a network of positive and negative feedback loops (44–46). Our data allow for the possibility that ERAP1 is involved in one of these regulatory mechanisms, affecting the number of TEM cells elicited during an immune response. In this manner, the level of ERAP1 expression may act as a modulator, enhancing the effector function of T cells and slowing their progression into memory cells during an acute infection. Alternatively, ERAP1 may have important roles in other immune pathways, including the innate immune response, which may also significantly impact on the potency of downstream adaptive immune responses. For example, we recently demonstrated that there are major differences in cytokine responses to TLR stimulation in ERAP1−/− mice (16). Therefore, future studies will have to discern if one of these mechanisms, a combination of these mechanisms, or none of these mechanisms are mediating this interesting effect on T-cell memory.
This study clarifies ERAP1’s role in shaping immunodominance through creation and destruction of peptides in vivo and demonstrates the functional significance of ERAP1 in modulating T-cell killing based upon this role. However, we also confirm that ERAP1 is not necessary to establish an antigen-derived peptide as immunodominant. The work also expands upon ERAP1’s roles in regulating some aspects of the adaptive immune system, beyond its known role in peptide processing and trimming for MHC-I display, by demonstrating that ERAP1 regulates the quantity of memory T cells with TEM phenotype generated in response to an infection. These multiple functions of ERAP1 may play critical roles in the regulation of T-cell memory and may further explain the genetic link between ERAP1 and the seronegative spondyloarthropathies.
Supplementary data
Supplementary data are available at International Immunology Online.
Acknowledgements
We wish to thank Michigan State University Laboratory Animal support facility for their assistance in the humane care and maintenance of the animals utilized in this work. A.A. was supported by the National Institutes of Health grant 5R01AR056981, the MSU Foundation, as well the Osteopathic Heritage Foundation.
Conflict of interest statement: The authors declared no conflicts of interest.
References
- 1. Saric T., Chang S. C., Hattori A., et al. 2002. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3:1169. [DOI] [PubMed] [Google Scholar]
- 2. Serwold T., Gonzalez F., Kim J., Jacob R., Shastri N. 2002. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419:480. [DOI] [PubMed] [Google Scholar]
- 3. York I. A., Chang S. C., Saric T., et al. 2002. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat. Immunol. 3:1177. [DOI] [PubMed] [Google Scholar]
- 4. Kochan G., Krojer T., Harvey D., et al. 2011. Crystal structures of the endoplasmic reticulum aminopeptidase-1 (ERAP1) reveal the molecular basis for N-terminal peptide trimming. Proc. Natl Acad. Sci. USA 108:7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rock K. L., Farfán-Arribas D. J., Shen L. 2010. Proteases in MHC class I presentation and cross-presentation. J. Immunol. 184:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang S. C., Momburg F., Bhutani N., Goldberg A. L. 2005. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl Acad. Sci. USA 102:17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Endert P. 2011. Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell. Mol. Life Sci. 68:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandhi A., Lakshminarasimhan D., Sun Y., Guo H. C. 2011. Structural insights into the molecular ruler mechanism of the endoplasmic reticulum aminopeptidase ERAP1. Sci. Rep. 1:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stratikos E., Stern L. J. 2013. Antigenic peptide trimming by ER aminopeptidases–insights from structural studies. Mol. Immunol. 55:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen T. T., Chang S. C., Evnouchidou I., et al. 2011. Structural basis for antigenic peptide precursor processing by the endoplasmic reticulum aminopeptidase ERAP1. Nat. Struct. Mol. Biol. 18:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blanchard N., Kanaseki T., Escobar H., et al. 2010. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. J. Immunol. 184:3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. York I. A., Brehm M. A., Zendzian S., Towne C. F., Rock K. L. 2006. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc. Natl Acad. Sci. USA 103:9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammer G. E., Gonzalez F., James E., Nolla H., Shastri N. 2007. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8:101. [DOI] [PubMed] [Google Scholar]
- 14. Yan J., Parekh V. V., Mendez-Fernandez Y., et al. 2006. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 203:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yewdell J. W. 2006. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity 25:533. [DOI] [PubMed] [Google Scholar]
- 16. Aldhamen Y. A., Seregin S. S., Rastall D. P., et al. 2013. Endoplasmic reticulum aminopeptidase-1 functions regulate key aspects of the innate immune response PLoS One 8:e69539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanaseki T., Blanchard N., Hammer G. E., Gonzalez F., Shastri N. 2006. ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulum. Immunity 25:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seregin S. S., Aldhamen Y. A., Rastall D. P., Godbehere S., Amalfitano A. 2012. Adenovirus-based vaccination against Clostridium difficile toxin A allows for rapid humoral immunity and complete protection from toxin A lethal challenge in mice. Vaccine 30:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aldhamen Y. A., Seregin S. S., Schuldt N. J., et al. 2012. Vaccines expressing the innate immune modulator EAT-2 elicit potent effector memory T lymphocyte responses despite pre-existing vaccine immunity. J. Immunol. 189:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aldhamen Y. A., Appledorn D. M., Seregin S. S., et al. 2011. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J. Immunol. 186:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanchard N., Gonzalez F., Schaeffer M., et al. 2008. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 9:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas P. G., Brown S. A., Keating R., et al. 2007. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J. Immunol. 178:3091. [DOI] [PubMed] [Google Scholar]
- 23. Jenkins M. R., Webby R., Doherty P. C., Turner S. J. 2006. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J. Immunol. 177:2917. [DOI] [PubMed] [Google Scholar]
- 24. Guerini F. R., Cagliani R., Forni D., et al. 2012. A functional variant in ERAP1 predisposes to multiple sclerosis. PLoS One 7:e29931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hearn A., York I. A., Rock K. L. 2009. The specificity of trimming of MHC class I-presented peptides in the endoplasmic reticulum. J. Immunol. 183:5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reeves E., Edwards C. J., Elliott T., James E. 2013. Naturally occurring ERAP1 haplotypes encode functionally distinct alleles with fine substrate specificity. J. Immunol. 191:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaech S. M., Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hansen S. G., Vieville C., Whizin N., et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen S. G., Ford J. C., Lewis M. S., et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708. [DOI] [PubMed] [Google Scholar]
- 31. Davidson S. I., Wu X., Liu Y., et al. 2009. Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 60:3263. [DOI] [PubMed] [Google Scholar]
- 32. Evans D. M., Spencer C. C., Pointon J. J., et al. ; Spondyloarthritis Research Consortium of Canada (SPARCC); Australo-Anglo-American Spondyloarthritis Consortium (TASC); Wellcome Trust Case Control Consortium 2 (WTCCC2). 2011. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirino Y., Bertsias G., Ishigatsubo Y., et al. 2013. Genome-wide association analysis identifies new susceptibility loci for Behçet’s disease and epistasis between HLA-B*51 and ERAP1. Nat. Genet. 45:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strange A., Capon F., Spencer C. C. A., et al. 2010. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsai F. J., Lee Y. C., Chang J. S., et al. 2011. Identification of novel susceptibility Loci for kawasaki disease in a Han chinese population by a genome-wide association study. PLoS One 6:e16853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fierabracci A., Milillo A., Locatelli F., Fruci D. 2012. The putative role of endoplasmic reticulum aminopeptidases in autoimmunity: insights from genomic-wide association studies. Autoimmun. Rev. 12:281. [DOI] [PubMed] [Google Scholar]
- 37. Lünemann J. D., Frey O., Eidner T., et al. 2008. Increased frequency of EBV-specific effector memory CD8+ T cells correlates with higher viral load in rheumatoid arthritis. J. Immunol. 181:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho B. A., Sim J. H., Park J. A., et al. 2012. Characterization of effector memory CD8+ T cells in the synovial fluid of rheumatoid arthritis. J. Clin. Immunol. 32:709. [DOI] [PubMed] [Google Scholar]
- 39. Albert L. J., Inman R. D. 1999. Molecular mimicry and autoimmunity. N. Engl. J. Med. 341:2068. [DOI] [PubMed] [Google Scholar]
- 40. Wucherpfennig K. W., Strominger J. L. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guilherme L., Kalil J., Cunningham M. 2006. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity 39:31. [DOI] [PubMed] [Google Scholar]
- 42. Beeton C., Pennington M. W., Wulff H., et al. 2005. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 67:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diz R., Garland A., Vincent B. G., et al. 2012. Autoreactive effector/memory CD4+ and CD8+ T cells infiltrating grafted and endogenous islets in diabetic NOD mice exhibit similar T cell receptor usage. PLoS One 7:e52054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belz G. T., Zhang L., Lay M. D., Kupresanin F., Davenport M. P. 2007. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cell. Proc. Natl Acad. Sci. USA 104:6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Helft J., Jacquet A., Joncker N. T., et al. 2008. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood 112:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong P., Pamer E. G. 2003. Feedback regulation of pathogen-specific T cell priming. Immunity 18:499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.