Abstract
A simple clinical neurological test was developed to evaluate response to gene therapy in a preclinical canine model of X-linked myotubular myopathy (XLMTM). This devastating congenital myopathy is caused by mutation in the myotubularin (MTM1) gene. Clinical signs include muscle weakness, early respiratory failure, and ventilator dependence. A spontaneously occurring canine model has a similar clinical picture and histological abnormalities on muscle biopsy compared with patients. We developed a neuromuscular assessment score, graded on a scale from 10 (normal) to 1 (unable to maintain sternal recumbency). We hypothesize that this neurological assessment score correlates with genotype and established measures of disease severity and is reliable when performed by an independent observer. At 17 weeks of age, there was strong correlation between neurological assessment scores and established methods of severity testing. The neurological severity score correctly differentiated between XLMTM and wild-type dogs with good interobserver reliability, on the basis of strong agreement between neurological scores assigned by independent observers. Together, these data indicate that the neurological scoring system developed for this canine congenital neuromuscular disorder is reliable and valid. This scoring system may be helpful in evaluating response to therapy in preclinical testing in this disease model, such as response to gene therapy.
Introduction
Establishing a clinical scoring system for large animal preclinical gene therapy trials allows for frequent assessment without the need for anesthesia or sophisticated instrumentation. Here we report a scoring system for a canine model of myotubular myopathy. In patients, this X-linked congenital disease affects 1 in 50,000 live male human births resulting from mutations in the myotubularin gene on chromosome Xq28.1,2 The disease classically has a devastating clinical course of hypotonia, muscle weakness, and respiratory failure leading to death within the first year of life without intensive supportive care, although mild and moderate phenotypes have been described.2–5 Clinical findings in affected infants include increased head circumference, increased body length but decreased body weight, profound muscle weakness, reduced or absent tendon reflexes, external opthalmoplegia, ptosis, facial paresis, impaired suck and swallow mechanisms, and respiratory distress.2,5–7 Analogous to patients with myotubular myopathy, a series of related male Labrador retriever dogs with an early onset of weakness progressing to death by 3–6 months of age was described in Western Canada.8–10 Muscle biopsy in these dogs showed changes on histology and electron microscopy consistent with human cases of X-linked myotubular myopathy (XLMTM). Western immunoblots failed to detect myotubularin.9 A missense mutation in the MTM gene (c.465C>A; p.N155K) was discovered and a breeding colony subsequently was established.9,11 Recently, we reported response to gene therapy in canine and rodent models of XLMTM12; however, in this report a clinical evaluation strategy was not in place to assess dogs. A reliable method to monitor clinical response to therapy would strengthen further evaluation of this disease model.
Previous neurological assessment of affected Labrador retriever dogs demonstrated cervical ventroflexion with an arched back, a short-strided stilted gait in all four limbs with a tendency to collapse after several steps, normal proprioception, absent patellar reflexes, and spared withdrawal and cranial tibial reflexes until the later stages.8–10 Severely affected dogs also have dysphagia, a dropped jaw, and a hoarse bark.8,9 Affected dogs typically require humane euthanasia between 15 and 26 weeks of age because of progressive tetraparesis and laryngeal, pharyngeal, and esophageal dysfunction.9,10,12 Previous reports describe the clinical condition of affected dogs, but serial neurologic evaluations that could be used to systematically score clinical disease progression from a young age have not been reported.
To measure clinical severity in XLMTM dogs, we performed serial neurological examinations and developed a neuromuscular assessment score, graded on a scale from 10 (normal) to 1 (unable to maintain sternal recumbency) (Table 1). Clinical rating scores and neurological assessment scores of large animal research models have been reported for gene therapy in feline models of the GM1 and GM2 gangliosidoses,13,14 canine models of Duschenne muscular dystrophy,15 and in rabbit and canine stroke models.16–18 In the current project, we hypothesize that the neurological assessment score developed for the XLMTM dog model correlates with genotype and established measures of disease severity, such as hindlimb strength. We also hypothesize that scoring is reliable when performed by an independent observer.
Table 1.
Summary of Neurological Severity Scoring with Clinical Signs and Approximate Age at Which X-linked Myotubular Myopathy Dogs Receive the Various Neurological Severity Scores
| Score | Clinical signs | Genotype/age |
|---|---|---|
| 10 | Normal neurological examination | WT/all ages; *rare XLMTM at acclimation (10 weeks) |
| 9–8 | Dropped jaw present and (8)/or (9) mild gait abnormality (weakness and bunny hop in rear) | XLMTM/10–15 weeks |
| 7–5 | Mild (7), mild–moderate (6), or moderate (5) short-strided gait, temporal & appendicular muscle atrophy, less to no running and jumping; dropped jaw present; +/− increased respiratory rate & tires after exercise | XLMTM/13–21 weeks |
| 4–3 | Marked short-strided gait & exercise intolerance (4); can only take a few steps before collapse (3); does not run or trot; profound muscle atrophy | XLMTM/15–25 weeks |
| 2–1 | Unable to ambulate (2) or maintain sternal recumbency (1); increased respiratory rate & effort at rest; dropped jaw; profound muscle atrophy | N/A—euthanasia criteria reached |
XLMTM, X-linked myotubular myopathy.
Materials and Methods
For Materials and Methods section, see Supplementary Data, available online at www.liebertpub.com/humc
Results
Validity assessments
Neurological assessment severity scoring differentiates between XLMTM and wild-type dogs
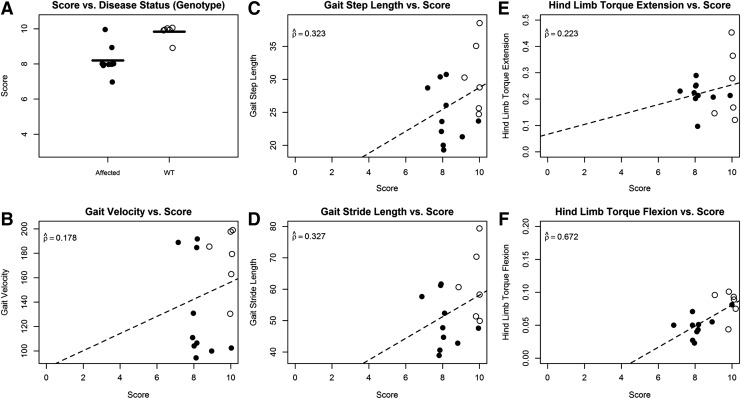
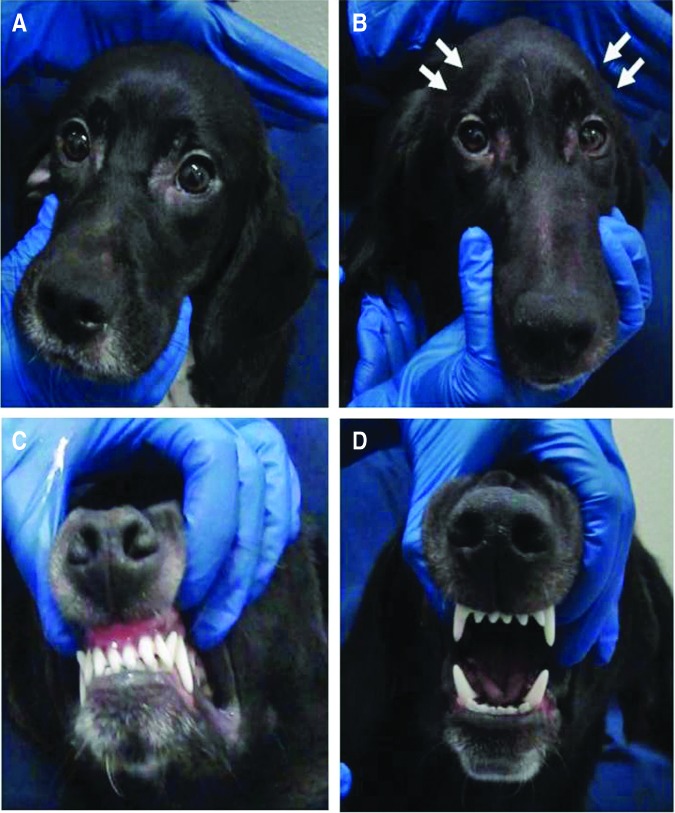
At 10 weeks of age, the mean neurological severity score in wild-type dogs was 9.8±0.41 (range 9–10) versus 8.2±0.79 (range 7–10) in XLMTM dogs (Fig. 3A). At this assessment, 9 of 10 (90%) XLMTM dogs had an abnormal neurological examination, with an inability to hold the jaw closed (dropped jaw) in 8 cases. For the two remaining dogs, one affected dog held the jaw closed and the other could not be assessed. Eight of the 10 (80%) XLMTM dogs had abnormal gaits, with less running, a pronounced tendency to bunny hop in 6 dogs, and a short stride in 3 dogs. At the same age, 5 of 6 (83%) of wild-type dogs had a normal neurological examination (Fig. 1). No wild-type dog had a dropped jaw. One wild-type dog scored a 9 at 10 weeks of age as a result of a mild hind limb gait abnormality. At 10 weeks of age, the mean patellar reflex score for XLMTM dogs was 0.26±0.34 (range 0–0.875) and the mean cranial tibial reflex score was 0.97±0.45 (range 0.25–2). The mean patellar (1.67±0.75, range 0.5–2.5) and cranial tibial (1.67±0.60, range 0.5–2) reflex scores were higher for wild-type dogs at 10 weeks of age. At the acclimation assessment, most XLMTM dogs had mild temporal and generalized limb atrophy, which worsened over time (Fig. 2).
FIG. 3.
Validity results at 10 weeks of age in untreated XLMTM and wild-type (WT) littermate dogs. Open circles denote WT dogs, while closed circles denote affected dogs. (A) Box plot of scores for WT and affected dogs. Black horizontal lines indicate mean neurological assessment score. (B–F) Comparison of neurological assessment scores to traditional severity data available at the time of analysis (B, gait velocity; C, gait step length; D, gait stride length; E, hind limb torque extension; F, hind limb torque flexion). Dashed line indicates the estimated linear trend. Estimated correlation coefficients are indicated on all plots. All points are jittered to avoid overplotting.
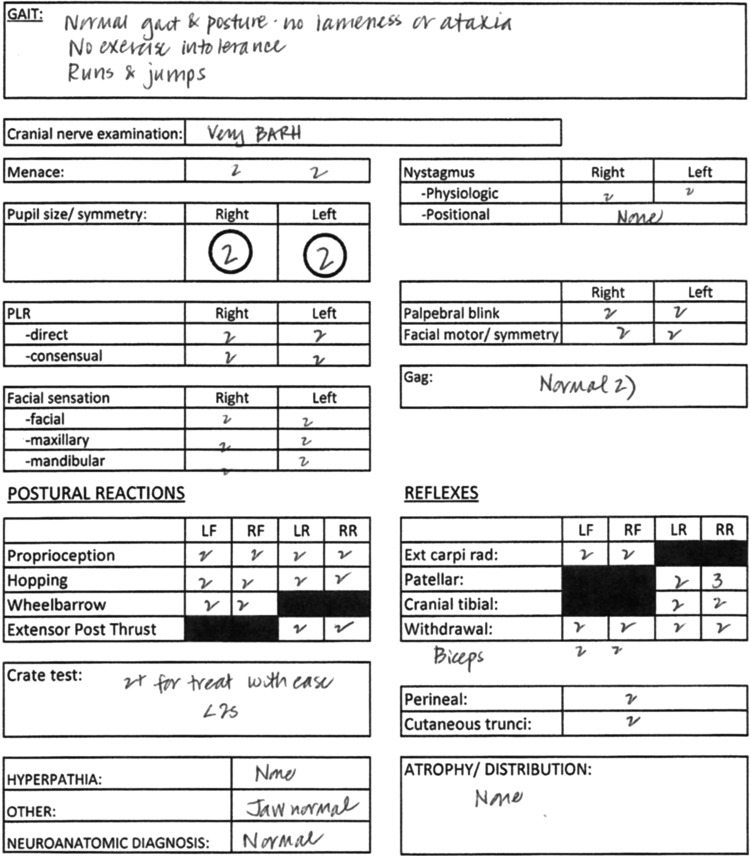
FIG. 1.
Example of the neurological assessment form completed for all dogs at each evaluation. This form reflects a normal neurological examination.
FIG. 2.
Clinical signs observed in wild type as compared with XLMTM dogs. (A) Wild-type dog with normal masticatory muscles. (B) XLMTM dog with pronounced temporal muscle atrophy (arrows). (C) Wild-type dog able to hold mouth in a normal, closed position. (D) XLMTM dog unable to maintain mouth in closed position (dropped jaw). XLMTM, X-linked myotubular myopathy. Color images available online at www.liebertpub.com/humc
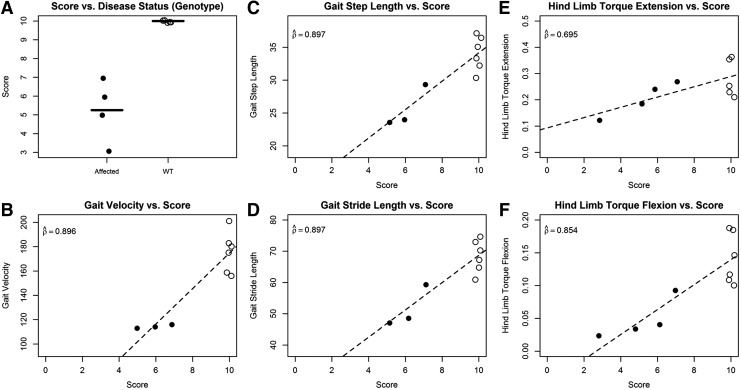
At 13, 15, and 17 weeks of age, all XLMTM dogs had abnormal neurological examinations and the mean neurological severity score decreased at each subsequent assessment. Wild-type dogs maintained a normal neurological examination throughout the study. Reflex scores gradually decreased at each assessment, although withdrawal reflexes were maintained in affected dogs until late stages of disease. Pelvic and thoracic limb withdrawal reflexes were decreased in 2/4 (50%) of XLMTM dogs at 17 weeks of age, and at this assessment there was a dramatic difference in neurological severity score between XLMTM and wild-type dogs (mean score in wild-type dogs 10 [all dogs] versus 5.2±1.7 [range 3–7] in affected dogs; Fig. 4A).
FIG. 4.
Validity results at 17 weeks of age in untreated XLMTM and wild-type (WT) littermate dogs. Open circles denote WT dogs, while closed circles denote affected dogs. (A) Box plot of scores for WT and affected dogs. Black horizontal lines indicate mean neurological assessment score. (B–F) Comparison of neurological assessment scores to traditional severity data available at the time of analysis (B, gait velocity; C, gait step length; D, gait stride length; E, hind limb torque extension; F, hind limb torque flexion). Dashed line indicates the estimated linear trend. Estimated correlation coefficients are indicated on all plots. All points are jittered to avoid overplotting.
Correlation between gait tests and neurological score
At 10 weeks of age, there was poor correlation between measures of gait testing (gait step length, gait velocity, and gait stride length) and neurological severity score, with estimated correlation coefficients ranging from 0.178 to 0.327 (Fig. 3B–D). In contrast, at 17 weeks of age there was strong correlation between measures of gait testing and neurological severity score, with estimated correlation coefficients (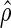 ) of 0.897 (gait step length), 0.896 (gait velocity), and 0.897 (gait stride length) (Fig. 4). At 17 weeks of age, 1 of 4 XLMTM dogs could not complete gait testing given severe weakness, but a neurological severity score was assigned to this dog.
) of 0.897 (gait step length), 0.896 (gait velocity), and 0.897 (gait stride length) (Fig. 4). At 17 weeks of age, 1 of 4 XLMTM dogs could not complete gait testing given severe weakness, but a neurological severity score was assigned to this dog.
The higher correlations observed at 17 weeks may have been because of differences between the XLMTM dogs included in the analysis at 10 (n=10) and 17 weeks (n=3). However, when we restricted the analysis at 10 weeks to only those dogs measured at both 10 and 17 weeks, the results were very similar (correlations 0.153–0.279).
Correlation between force testing and neurological score
At 10 weeks of age, there was poor correlation between hind limb torque extension and neurological severity score (0.223) and moderate correlation between hind limb torque flexion and neurological severity score (0.672) (Fig. 3). When only those XLMTM dogs that were also measured at 17 weeks of age were included in the analysis of the measurements made at 10 weeks (n=4), the estimated correlation coefficients for hind limb torque extension versus neurological score (0.206) and hind limb torque flexion versus neurological score (0.515) were similar to those obtained when all XLMTM dogs were included at 10 weeks of age.
At 17 weeks of age, the correlation between the measures of force tests (hind limb torque extension and hind limb torque flexion) and neurological severity score increased, with estimated correlation coefficients of 0.695 and 0.854, respectively. Hind limb torque flexion had the highest correlation with neurological severity score of all the measures of gait and force tests at 10 weeks. The correlation between hind limb flexion and neurological severity score at 10 weeks was not much lower than for measures of gait analysis at 17 weeks (Fig. 4).
Reliability assessments
Interobserver agreement in neurological severity scoring
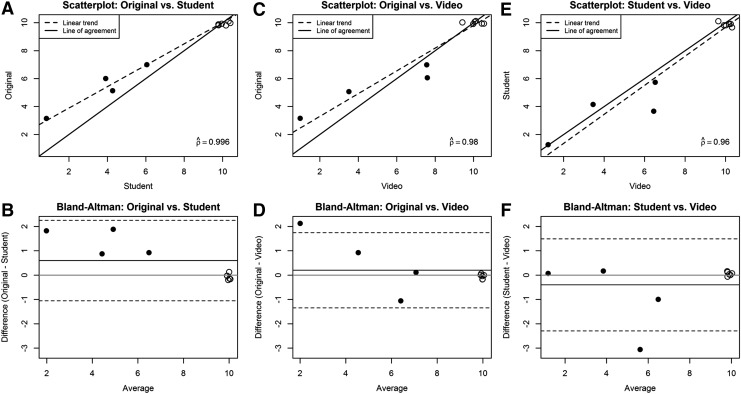
Interobserver reliability was assessed by independent scoring of completed neurological examination forms (Fig. 1) and videotaped neurological examinations by a second trained observer. The neurological severity scores of the neurologist and the second observer agreed in 35/46 cases (76%), with scores within 1 point in 43/46 cases (93%), when based on evaluation of the written neurological examination. When based on evaluation of the videotaped neurological examination, the neurological severity scores of the observer and the neurologist agreed in 20/29 cases (69%), with scores within 1 point in 28/29 cases (96%). In general, the associations between neurological severity scores assigned by the neurologist and the second observer were quite strong, with high correlation coefficients (0.91 and higher). Nearly all pairs of scores were within one unit of each other. The estimated correlation coefficients for the original scoring by the neurologist and the second observer scoring written neurological examination forms were 0.914 (10 weeks), 0.982 (13 weeks), 0.988 (15 weeks), and 0.996 (17 weeks; Fig. 5). The estimated correlation coefficients for the original scoring by the neurologist and the independent observer scoring videotaped neurological examinations were 0.978 (15 weeks) and 0.98 (17 weeks; Fig. 5). The correlation between the score assigned by the second observer based on review of the completed neurological examination form and of the videotaped examination was also high, with estimated correlation coefficients of 0.983 (15 weeks) and 0.96 (17 weeks). There were a few instances of scores two or three units apart, but these were uncommon. There was some indication of bias between the scoring methods (i.e., places where the mean difference in scores was nonzero), although in all cases the absolute mean difference was less than one.
FIG. 5.
Reliability results at 17 weeks. Open circles denote wild-type (WT, n=6) dogs, while closed circles denote XLMTM dogs. Graphs A, C, and E present scatterplots with estimated correlation coefficients. The dashed line indicates the estimated linear trend, and the solid line shows the line of agreement. Graphs B, D, and F present Bland–Altman plots. The gray line shows the zero line, the solid black line shows the average of the differences, and the dotted black line shows the limits of agreement. All points were jittered to avoid overplotting. Original, original score provided by neurologist; Student, score provided by second independent observer based on written neurological examination record; Video, score provided by second independent observer based on videotaped neurological examination.
Discussion
The objective of this study was to determine validity and reliability of a neurological severity scoring system for a spontaneous canine model of XLMTM. This test represents a relatively simple test that does not require advanced instrumentation or general anesthesia and could be used to evaluate response to therapy in preclinical testing in this disease model, such as response to gene therapy. There is evidence that the scoring system is valid at the late stage of the disease (17 weeks of age) based on correlations between neurological score and established methods of severity scoring, such as hind limb torque measures and gait analysis. Results also indicate that in this small group of animals, the neurological severity score correctly differentiates between XLMTM and wild-type dogs with strong interobserver reliability, on the basis of strong agreement between neurological score assigned by independent observers.
At 17 weeks, estimated correlation coefficients between neurological score and the measures of gait analysis and hind limb extension and flexion torque ranged from 0.695 to 0.897. The only measure of disease severity that correlated moderately well with the neurological severity score at 10 weeks of age (i.e., baseline) was hind limb torque flexion, an established technique in this animal model and in other models of neuromuscular disease.12,20–22 There was a difference between XLMTM and wild-type dogs in neurological severity scores at the earliest age tested (10 weeks), although dogs were relatively mildly affected at this age. Clinical signs at this age included an inability to hold the jaw in a closed position and an abnormal gait characterized by a tendency to push off with both hind limbs (“hopping”) when trotting and running and occasionally a short stride or stilted gait. Patellar reflexes and cranial tibial reflexes were decreased in XLMTM dogs compared with wild-type dogs at 10 weeks of age, although withdrawal reflexes generally were spared until later stages of the disease.
For gait analysis, estimated correlation between gait measures and the neurological score was relatively poor at 10 weeks of age. Correlations at 17 weeks were much stronger (∼0.90 for all three measures of gait analysis). Further, one dog was too weak to perform gait testing at 17 weeks and could not be included in the analysis, although it had a very low neurological severity score at 17 weeks (score of 3). If this dog could have been included, it likely would have had poor gait measurements, which would strengthen the observed correlation further. This dog was not able to be evaluated by gait analysis, but could be assessed for neurological severity scoring, which represents another advantage of this test. The correlation between hind limb torque extension and flexion and neurological score was also higher at 17 weeks of age than at 10 weeks of age. At 10 weeks of age, dogs are less severely affected and there is less spread in neurological severity scores, which may explain the low correlation coefficients. These differences could also be related to differences between the dogs included at 10 weeks and the dogs included at 17 weeks, although this is less likely because when we restricted the analysis at 10 weeks to those XLMTM dogs measured at both 10 and 17 weeks, the patterns seen were similar. The small number of XLMTM dogs and wild-type dogs, especially at the later time points, is a limitation of the study. However, the dramatic difference between XLMTM and wild-type dogs, especially at the later ages, supports the rationale for such a small sample size.
Reliability assessment indicated that agreement between scores assigned by the neurologist and the second observer was good, with scores within 1 point for 93% of cases based on review of the neurological examination form and for 96% of cases based on review of the videotaped neurological examination. As expected, agreement was better for wild-type dogs since healthy dogs are easier to score once they are suspected to be normal. For XLMTM dogs, there may be observer variation in what is considered “mild” versus “moderate,” which may lead to minor scoring differences (a score of 7 vs. 6, for example). The agreement based on evaluation of the neurological examination record indicates that the scoring criteria generally were clear and reproducible based on the presence or absence of certain findings such as dropped jaw, gait abnormality, weakness, exercise intolerance, and muscle atrophy. The agreement based on evaluation of the videotaped neurological examination indicates that an observer can be trained to recognize key abnormalities on the neurological examination and assign a consistent score. The agreement between scoring of the written examination by the second independent observer and scoring of the videotaped examination by the same observer was also satisfactory. In this study, we did not have a second observer perform the neurological examination because of time and animal protocol constraints, although the videotaped examination and written examination data suggest that with additional time a second observer could be trained to perform a neurological examination and successfully assign a neurological severity score.
In summary, the neurological assessment we describe is a valid and reliable clinical measure of disease severity. The developed scoring system differentiates between wild-type and XLMTM dogs at an early age and correlates well with other established measures of gait and strength analysis at later ages. This instrument could be used to assess treatment effects in XLMTM dogs. For example, we recently reported that gene therapy markedly improved muscle weakness and respiratory impairment and prolonged life span to more than 1 year in treated XLMTM dogs.12 Further analysis would be required to evaluate the utility of the described neurological severity scoring system in predicting response to treatment in this spontaneous animal model of XLMTM.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health (1R01HL115001 [NHLBI], 1R21AR064503 [NIAMS]); Muscular Dystrophy Association (Academic Translational Grant); Joshua Frase Foundation; Khuri Myopathy Research fund; and Where There's a Will There's a Way.
Author Disclosure
No competing financial interests exist.
References
- 1.Laporte J, Hu LJ, Kretz C, et al. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 1996;13:175–182 [DOI] [PubMed] [Google Scholar]
- 2.Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis 2008;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taratuto AL. Congenital myopathies and related disorders. Curr Opin Neurol 2002;15:553–561 [DOI] [PubMed] [Google Scholar]
- 4.Biancalana V, Caron O, Gallati S, et al. Characterisation of mutations in 77 patients with X-linked myotubular myopathy, including a family with a very mild phenotype. Hum Genet 2003;112:135–142 [DOI] [PubMed] [Google Scholar]
- 5.Das S, Dowling J, Pierson CR. X-linked centronuclear myopathy. In: GeneReviews® (Internet). Pagon RA, Adam MP, Bird TD, et al., eds. (Seattle, WA: ) University of Washington, Seattle;1993–2015 [Google Scholar]
- 6.Pierson CR, Tomczak K, Agrawal P, et al. X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 2005;64:555–564 [DOI] [PubMed] [Google Scholar]
- 7.Joseph M, Pai GS, Holden KR, et al. X-linked myotubular myopathy: clinical observations in ten additional cases. Am J Med Genet 1995;59:168–173 [DOI] [PubMed] [Google Scholar]
- 8.Cosford KL, Taylor SM, Thompson L, et al. A possible new inherited myopathy in a young Labrador retriever. Can Vet J 2008;49:393–397 [PMC free article] [PubMed] [Google Scholar]
- 9.Beggs AH, Bohm J, Snead E, et al. MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci USA 2010;107:14697–14702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snead EC, Taylor SM, van der Kooij M, et al. Clinical phenotype of X-linked myotubular myopathy in labrador retriever puppies. J Vet Intern Med 2015;29:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goddard MA, Mitchell EL, Smith BK, et al. Establishing clinical end points of respiratory function in large animals for clinical translation. Phys Med Rehabil Clin N Am 2012;23:75–94, xi. [DOI] [PubMed] [Google Scholar]
- 12.Childers MK, Joubert R, Poulard K, et al. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med 2014;6:220ra210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCurdy VJ, Rockwell HE, Arthur JR, et al. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease. Gene Ther 2015;22:181–189 [DOI] [PubMed] [Google Scholar]
- 14.McCurdy VJ, Johnson AK, Gray-Edwards HL, et al. Sustained normalization of neurological disease after intracranial gene therapy in a feline model. Sci Transl Med 2014;6:231ra248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampaolesi M, Blot S, D'Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 2006;444:574–579 [DOI] [PubMed] [Google Scholar]
- 16.Brown A, Woods S, Skinner R, et al. Neurological assessment scores in rabbit embolic stroke models. Open Neurol J 2013;7:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zu QQ, Liu S, Xu XQ, et al. An endovascular canine stroke model: middle cerebral artery occlusion with autologous clots followed by ipsilateral internal carotid artery blockade. Lab Invest 2013;93:760–767 [DOI] [PubMed] [Google Scholar]
- 18.Purdy PD, Devous MD Sr, Batjer HH, et al. Microfibrillar collagen model of canine cerebral infarction. Stroke 1989;20:1361–1367 [DOI] [PubMed] [Google Scholar]
- 19.Goddard MA, Burlingame E, Beggs AH, et al. Gait characteristics in a canine model of X-linked myotubular myopathy. J Neurol Sci 2014;346:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tegeler CJ, Grange RW, Bogan DJ, et al. Eccentric contractions induce rapid isometric torque drop in dystrophin-deficient dogs. Muscle Nerve 2010;42:130–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange RW, Doering J, Mitchell E, et al. Muscle function in a canine model of X-linked myotubular myopathy. Muscle Nerve 2012;46:588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childers MK, Grange RW, Kornegay JN. In vivo canine muscle function assay. J Vis Exp 2011; (50). pii: ; 10.3791/2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. A note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Comput Biol Med 1990;20:337–340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.