Key Clinical Message
PTTM (Pulmonary tumor thrombotic microangiopathy) is very difficult to diagnose before death. We report a case of urothelial carcinoma of the urinary bladder associated with PTTM in which an antemortem diagnosis by PMC (pulmonary microvascular cytology). PMC may represent the only chance for diagnosis and achievement of remission in PTTM.
Keywords: Pulmonary microvascular cytology, pulmonary tumor thrombotic microangiopathy, urothelial carcinoma of the urinary bladder
Introduction
PTTM (Pulmonary tumor thrombotic microangiopathy), synonymously termed microscopic pulmonary tumor embolism, is a rare condition, and cancer-related pulmonary complications characterized by rapidly progression of dyspnea and pulmonary hypertension occasionally cause sudden death 1. PTTM is observed in 3.3% of autopsies of patients with malignant tumors and is usually associated with gastric cancer, although it was reported to affect other sites in a few cases 1,2. Rare cases of urothelial carcinoma of the urinary bladder associated with PTTM have also been reported 1,3,4. Most of the patients in these case reports were diagnosed as having PTTM following postmortem examination at autopsy. Antemortem diagnosis of PTTM is very difficult because the patient rapidly progresses to death. We report a valuable case of PTTM associated with urothelial carcinoma of the urinary bladder in which pulmonary microvascular cytology (PMC) was a useful strategy that aided in the early antemortem diagnosis, permitting the patient to undergo chemotherapy, although the patient ultimately died.
Case Report
The patient was a 77-year-old Japanese man with a history of transurethral resection of a bladder tumor at the age of 75 years for urothelial carcinoma of the urinary bladder. Pathological findings of the surgical specimens obtained indicated a urothelial carcinoma (G2) that had invaded the muscular layer (Fig.1). Afterward, he underwent intravesical Bacillus Calmette-Guerin therapy and radiation therapy that resulted in complete remission. In April 2014, he developed a relapse with abdominal metastatic lymph nodes and bone metastasis found, so he underwent chemotherapy with carboplatin and gemcitabine. After 2 cycles this chemotherapy, he developed dyspnea and was referred to our hospital in July 2014. On admission, he had tachycardia (112 beats per minute), tachypnea (31 respirations per minute), and normotensive blood pressure (118/62 mmHg). Initial peripheral arterial oxygen saturation (SpO2) was 81%, and blood gas analysis showed a PaO2 (partial pressure of oxygen in arterial blood) of 46.6 Torr and PaCO2 (partial pressure of carbon dioxide in arterial blood) of 34.2 Torr on room air, indicating hypoxemia. His white blood cell count was 5000/μL, hemoglobin 7.3 g/dL, and thrombocyte count 10.6 × 104/μL, indicating anemia and thrombocytopenia. C-reactive protein level was elevated at 6.1 mg/dL, and brain natriuretic peptide was 425 pg/mL. A transthoracic echocardiogram showed a normal left ventricular ejection fraction with an elevated estimated systolic pulmonary artery pressure of 89 mmHg. A whole-body contrast-enhanced CT (computed tomography) scan revealed diffuse small nodules, ground-glass opacities, and dilation of the pulmonary arteries in the lungs. Other findings included abdominal lymph node swelling and bone metastasis, without obvious pulmonary embolism or deep vein thrombosis (Fig.2). 99mTc-MAA scintigraphy showed multiple peripheral defects (Fig.3).
Figure 1.
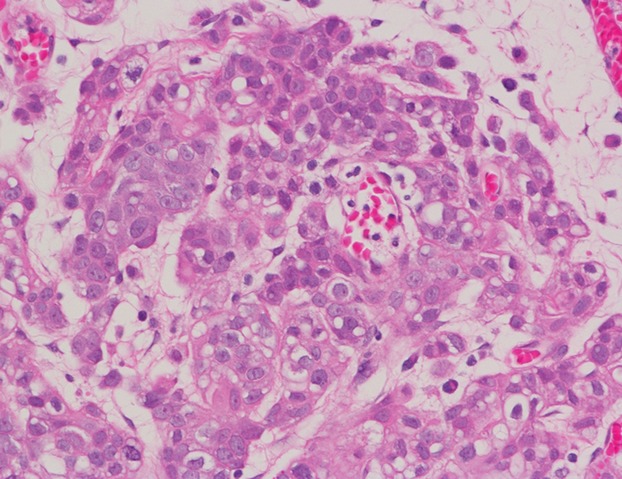
Microscopic findings of the tumor show urothelial carcinoma of the urinary bladder (Hematoxylin and eosin stain).
Figure 2.
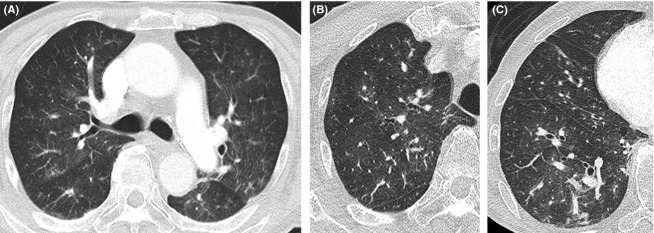
Chest CT (computed tomography) showed ground-glass opacities in both lung fields (A). High-resolution CT showed small nodules and ground-glass opacities in the upper lung field (B) and small nodules, ground-glass opacities, and dilation of the pulmonary arteries in the lower lung (C).
Figure 3.
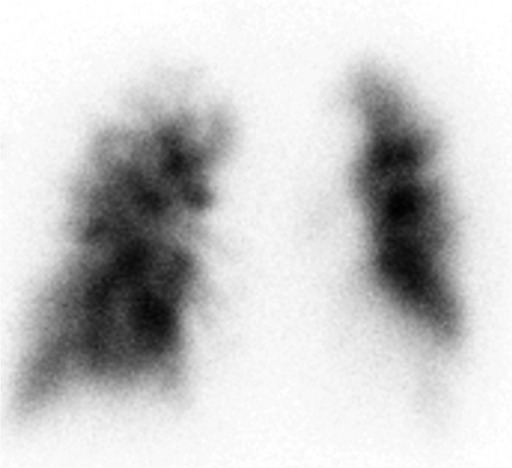
A 99mTc-MAA lung perfusion image showed multiple peripheral defects.
During the patient's hospital course, PMC was performed, for which a Swan-Ganz catheter was inserted and located in the right pulmonary artery wedge position, and blood was gently withdrawn from the wedged catheter. Hemodynamic parameters monitored during the procedure included a mean pulmonary capillary wedge pressure of 14 mmHg; mean pulmonary artery pressure of 41 mmHg; and cardiac index of 2.3 L/min/m2, which indicated pulmonary hypertension without left cardiac failure. Extracted blood samples were heparinized and centrifuged. Because malignant cells tend to accumulate in the buffy coat of centrifuged blood, slides were made from the buffy coat and were immediately fixed in 95% alcohol and stained using the Papanicolaou and Giemsa methods. The cytological specimens showed small loose clusters of large atypical cells resembling urothelial carcinoma cells (Fig.4). Upper and lower gastrointestinal tract endoscopy, small intestine endoscopy, and bone marrow biopsy provided no meaningful results: none of these tests indicated malignant disease or urothelial carcinoma of the urinary bladder. TBLB (Transbronchial lung biopsy) was performed three times in the right lower lung, but the specimens could not be reveal atypical cells in the vessels. Although specimens from TBLB also did not provide meaningful results, on the basis of the clinical, radiological, and cytological findings, we diagnosed the patient as having PTTM with urothelial carcinoma of the urinary bladder because of the existence of atypical cells resembling urothelial carcinoma cells in the pulmonary microvasculature and the complete absence of malignancy in other organs. Chemotherapy with gemcitabine and paclitaxel was started on hospital day 8. However, the patient's pulmonary hypertension progressed, his breathing rapidly deteriorated, and he died on hospital day 12.
Figure 4.
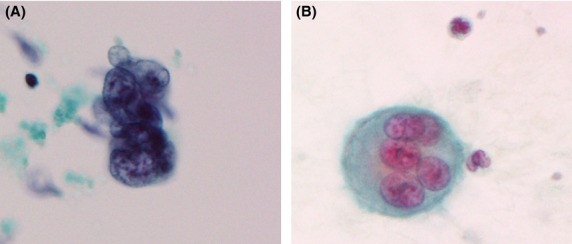
Pulmonary microvascular cytology showed large atypical cells resembling urothelial carcinoma cells (A: Papanicolaou stain, B: Giemsa stain).
Discussion
We describe a case of PTTM in which PMC detected tumor cells strongly suspicious of urothelial carcinoma of the urinary bladder. PTTM should be considered in the differential diagnosis of a patient with cancer who presents with subacute dyspnea. This rare and poorly understood entity is underrecognized before death. PTTM is characterized by the following findings: (1) the presence of tumor emboli in the small pulmonary arteries; (2) fibrocellular and fibromuscular intimal proliferation in the small pulmonary arteries; and (3) organization and recanalization of thrombi. The second finding is particularly serious and is very difficult to differentiate from a simple pulmonary arterial tumor emboli. The presence of minute tumor emboli in the peripheral pulmonary arteries can damage the vascular endothelium, leading to thrombus formation and accelerated coagulation 5. PTTM is usually associated with adenocarcinoma, particularly gastric adenocarcinoma and adenocarcinomas of the pancreas, breast, lung, and liver 1,2. However, there are a few cases reports of PTTM or microscopic pulmonary tumor embolism associated with urothelial carcinoma of the urinary bladder 1,3,4,6–9. To the best of our knowledge, including the present case, only two cases have been reported in which PMC detected tumor cells that led to a diagnosis of PTTM associated with urothelial carcinoma of the urinary bladder 9.
The clinical diagnosis of PTTM is extremely difficult 10. The development of clinical signs of pulmonary hypertension result in acute or subacute cor pulmonale and subacute respiratory failure. Moreover, stenosis and/or occlusion of the pulmonary arteries occur along with an increase in pulmonary vascular resistance that results in pulmonary hypertension, hemolytic anemia, and disseminated intravascular coagulation 1. The CT findings of PTTM include consolidation, ground-glass opacities, small nodules, and tree-in-bud appearance 2. In the present patient, a chest CT scan revealed nodules and ground-glass opacities. In addition, multiple small and wedge-shaped perfusion detects in a lung perfusion scan are characteristic of PTTM 11. On the basis of the clinical and radiological findings, we considered a diagnosis of PTTM induced by urothelial carcinoma of the urinary bladder, and PMC, bone marrow biopsy, and TBLB were performed.
Pulmonary microvascular cytology, the cytological study of blood drawn from a wedged pulmonary artery catheter to detect material in the pulmonary microvasculature, was first reported in 1979 in patients with amniotic fluid embolism 12. Dexter et al. 13 showed the oxygen saturation of blood withdrawn from pulmonary artery catheters in the wedge position has characteristics of pulmonary capillary blood. In reality, since the catheter lodges in an artery of substantial size, a “wedge” sample most likely represents a mixture of blood from the terminal pulmonary arteries, arterioles, and capillaries in the subsegmental distribution of the occluded vessel. We also thought that PMC was useful diagnosis method of PTTM, which was characterized by the occlusion of arterioles, capillaries, and venules throughout the body by malignant tumor cells. PMC has been extended to the diagnosis of fat embolism, pulmonary microvascular metastasis, and intravascular lymphoma 14–17. Similar to these disorders, a few cases of PTTM were reported in which tumor cells were detected by PMC 14,18. Other antemortem diagnostic methods have been suggested, including video-assisted thoracic surgery and TBLB 19,20. Our patient could not be diagnosed as having PTTM from the TBLB findings, and we could not perform further examinations because of the patient's poor condition. However, PMC was successful in detecting tumor cells in the pulmonary microvasculature of our patient. The point where we should be careful about the cytological study is that the presence of megakaryocytes is rarely seen in the peripheral blood. Therefore, we should avoid of making mistake such that the megakaryocyte was recognized as atypical cell. Although an invasive procedure, pulmonary artery catheterization is generally well tolerated in comparison with video-assisted thoracic surgery and TBLB. Due to the extremely rapid progression of PTTM, few cases have been reported in which systemic chemotherapy has improved the prognosis 19, and no previous cases of chemotherapy administered after the diagnosis of urothelial carcinoma of the urinary bladder associated with PTTM have been reported. Although the condition of our patient did not improve after chemotherapy and progressed to death, we believe that early diagnosis by PMC may be valuable in improving the prognosis of patients with PTTM. On the other hand, anticoagulation therapies and new drugs for the treatment of pulmonary arterial hypertension, such as endothelin antagonists, prostacyclin analogs, and phosphodiesterase type 5 inhibitors, are controversial for the treatment of PTTM 21. Moreover, Ogawa et al. 22 reported that imatinib may be effective for ameliorating pulmonary hypertension that is caused by PTTM. Therefore, further studies are warranted focusing on these treatments of PTTM.
We report a rare case of urothelial carcinoma of the urinary bladder associated with PTTM in which an antemortem diagnosis was made with the aid of PMC, permitting the patient to undergo chemotherapy. If the possibility of PTTM is considered in cancer patients with a rapidly worsening respiratory condition, we believe that PMC may represent the only chance for diagnosis and achievement of remission in this aggressive disease, particularly for critically ill patients.
Conflict of Interest
The authors state that they have no conflicts of interest.
References
- von Herbay A, Illes A, Waldherr R. Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587–592. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Uruga H, Fujii T, Kurosaki A, Hanada S, Takaya H, Miyamoto A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern. Med. 2013;52:1317–1323. doi: 10.2169/internalmedicine.52.9472. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Oharaseki T, Wakayama M, Yamamoto M, Sugi K. Takahashi K. An autopsy case of pulmonary tumor thrombotic microangiopathy of bladder cancer. Diag. Pathol. 2004;21:336–339. , and (in Japanese) [Google Scholar]
- Hirano H, Ichibori H, Kizaki T, Matsumoto T, Ohka Z, Mori T, et al. Pulmonary tumor thrombotic microangiopathy showing aggressive course after transurethral resection of urinary bladder: an autopsy case report. Med. Mol. Morphol. 2012;45:238–242. doi: 10.1007/s00795-012-0586-3. [DOI] [PubMed] [Google Scholar]
- Miyano S, Izumi S, Takeda Y, Tokuhara M, Mochizuki M, Matsubara O, et al. Pulmonary tumor thrombotic microangiopathy. J. Clin. Oncol. 2007;25:597–599. doi: 10.1200/JCO.2006.09.0670. [DOI] [PubMed] [Google Scholar]
- Suyama N, Mashimoto H, Araki J, Asai S, Ikeno Y. Ikeda T. An autopsy case of urinary bladder carcinoma with pulmonary infarction and subacute cor pulmonale caused by tumor embolization. Nihon Kyobu Shikkan Gakkai Zasshi. 1994;32:491–496. , and (in Japanese) [PubMed] [Google Scholar]
- Rüchardt A, Gruber B. Trenkwalder P. Intrapulmonary tumor cell embolism from cancer of the bladder as the cause of a subacute cor pulmonale. Dtsch. Med. Wochenschr. 2001;126:847–850. doi: 10.1055/s-2001-16015. , and (in German) [DOI] [PubMed] [Google Scholar]
- Dhillon SS, Singh DJ, Dass B. Schaub CR. Transitional cell carcinoma manifesting as acute cor pulmonale: cause of microscopic tumor embolism. South. Med. J. 2001;94:1030–1032. [PubMed] [Google Scholar]
- Roberts KE, Hamele-Bena D, Saqi A, Stein CA. Cole RP. Pulmonary tumor embolism: a review of the literature. Am. J. Med. 2003;115:228–232. doi: 10.1016/s0002-9343(03)00305-x. [DOI] [PubMed] [Google Scholar]
- Gavin MC, Morse D, Partridge AH, Levy BD. Loscalzo J. Clinical problem-solving. Breathless. N. Engl. J. Med. 2012;366:75–81. doi: 10.1056/NEJMcps1011918. [DOI] [PubMed] [Google Scholar]
- Crane R, Rudd TG. Dail D. Tumor microembolism: pulmonary perfusion pattern. J. Nucl. Med. 1984;25:877–880. [PubMed] [Google Scholar]
- Masson RG, Ruggieri J. Siddiqui MM. Amniotic fluid embolism: definitive diagnosis in a survivor. Am. Rev. Respir. Dis. 1979;120:187–192. doi: 10.1164/arrd.1979.120.1.187. [DOI] [PubMed] [Google Scholar]
- Dexter L, Haynes FW, Burwell CS, Eppinger EC, Sagerson RP. Evans JM. Studies of congenital heart disease: 2. the pressure and oxygen content of blood in the right auricle, right ventricle, and pulmonary artery in control patients, with observations on the oxygen saturation and source of pulmonary “capillary” blood. J. Clin. Investig. 1947;26:554–560. doi: 10.1172/JCI101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson RG. Ruggieri J. Pulmonary microvascular cytology. A new diagnostic application of the pulmonary artery catheter. Chest. 1985;88:908–914. doi: 10.1378/chest.88.6.908. [DOI] [PubMed] [Google Scholar]
- Masson RG, Krikorian J, Lukl P, Evans GL. McGrath J. Pulmonary microvascular cytology in the diagnosis of lymphangitic carcinomatosis. N. Engl. J. Med. 1989;321:71–76. doi: 10.1056/NEJM198907133210202. [DOI] [PubMed] [Google Scholar]
- Abati A, Landucci D, Danner RL. Solomon D. Diagnosis of pulmonary microvascular metastases by cytologic evaluation of pulmonary artery catheter-derived blood specimens. Hum. Pathol. 1994;25:257–262. doi: 10.1016/0046-8177(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Demirer T, Dail DH. Aboulafia DM. Four varied cases of intravascular lymphomatosis and a literature review. Cancer. 1994;73:1738–1745. doi: 10.1002/1097-0142(19940315)73:6<1738::aid-cncr2820730631>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ota K, Matsuyama M, Kokuho N, Masuko H, Hayashi H, Iizuka T. An autopsy case of pulmonary tumor thrombotic microangiopathy complicated with interstitial pneumonia and lipoid pneumonia. Nihon Kokyuki Gakkai Zasshi. 2009;47:518–523. , et al. (in Japanese) [PubMed] [Google Scholar]
- Kayatani H, Matsuo K, Ueda Y, Matsushita M, Fujiwara K, Yonei T, et al. Pulmonary tumor thrombotic microangiopathy diagnosed antemortem and treated with combination chemotherapy. Intern. Med. 2012;51:2767–2770. doi: 10.2169/internalmedicine.51.7682. [DOI] [PubMed] [Google Scholar]
- Ueda A, Fuse N, Fujii S, Sasaki T, Sugiyama J, Kojima T, et al. Pulmonary tumor thrombotic microangiopathy associated with esophageal squamous cell carcinoma. Intern. Med. 2011;50:2807–2810. doi: 10.2169/internalmedicine.50.6113. [DOI] [PubMed] [Google Scholar]
- Kitamura A, Nishimura N, Jinta T, et al. A case of pulmonary tumor thrombotic microangiopathy diagnosed by transbronchial lung biopsy and treated with chemotherapy and long-term oxygen and anticoagulation therapies. Case Rep. Pulmonol. 2013:1–13. doi: 10.1155/2013/259080. article 259080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Yamadori I, Matsubara O. Matsubara H. Pulmonary tumor thrombotic microangiopathy with circulatory failure treated with imatinib. Intern. Med. 2013;52:1927–1930. doi: 10.2169/internalmedicine.52.0718. [DOI] [PubMed] [Google Scholar]


