Abstract
Paracetamol (acetaminophen) overdose is one of the most common causes of acute liver injury in the Western world. To improve patient care and reduce pressure on already stretched health care providers new biomarkers are needed that identify or exclude liver injury soon after an overdose of paracetamol is ingested. This review highlights the current state of paracetamol poisoning management and how novel biomarkers could improve patient care and save healthcare providers money. Based on the widely used concept of defining a target product profile, a target biomarker profile is proposed that identifies desirable and acceptable key properties for a biomarker in development to enable the improved treatment of this patient population. The current biomarker candidates, with improved hepatic specificity and based on the fundamental mechanistic basis of paracetamol-induced liver injury, are reviewed and their performance compared with our target profile.
Keywords: APAP-CYS, biomarkers, GLDH, hepatotoxicity, HMGB1, keratin-18, miR-122, miRNA, mtDNA, paracetamol
Introduction
Paracetamol (acetaminophen - APAP) is used by millions of people worldwide as a safe analgesic drug at therapeutic doses. In overdose, it is well known to be toxic to the liver. Indeed, in the Western world, paracetamol is the commonest cause of acute liver injury (ALI) and overdose is a very common reason for hospital attendance (around 100 000 UK patients each year) [1]. In terms of UK hospital admission, the number of patients per year (around 50 000) is comparable with other ‘giants’ of emergency medicine such as heart failure and hip fracture.
To improve patient care and reduce pressure on already stretched health care providers, new biomarkers are needed that identify or exclude liver toxicity soon after the drug is ingested. This review highlights the current state of paracetamol poisoning management and how novel biomarkers could improve patient care and save healthcare providers money.
Mechanism of paracetamol-induced acute liver injury
Adverse drug reactions (ADRs) have been traditionally classified into six types, labelled A through F [2]. However, recent proposals have classified ADRs as either on-target (often predictable from primary or secondary pharmacology, clear dose–response relationship) or off-target (idiosyncratic, complex dose–response relationship). Paracetamol-induced ALI is classified as type A, being predictable and dose-dependent. This has resulted in the pathophysiology being studied widely, especially in rodents, for over 40 years [3]
At therapeutic doses, the major route of paracetamol metabolism is through conjugation. Cellular injury is due to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) that is produced by the cytochrome P450 enzymes CYP2E1, CYP1A2, CYP3A4 and CYP2D6 [4]. At therapeutic paracetamol doses, only low concentrations of NAPQI are formed and this metabolite is efficiently detoxified by conjugation with glutathione. At toxic doses, the paracetamol conjugation reaction becomes saturated and more paracetamol becomes oxidized by cytochrome P450 into NAPQI. The cellular stores of glutathione become exhausted, which results in NAPQI covalently binding to sulfhydryl (SH-) groups in structural proteins, forming protein adducts leading to oxidative stress, mitochondrial injury, hepatocyte cell death by either apoptosis (minor pathway) or necrosis (major pathway), multi-organ failure and potentially patient death (Figure1) [5]. Antidote treatment with acetylcysteine (NAC) restores cellular glutathione concentrations. When administered soon after drug overdose (within about 8 h), NAC is highly effective in preventing liver injury [6,7]. However, when NAC treatment is delayed, its efficacy is substantially reduced [6].
Figure 1.
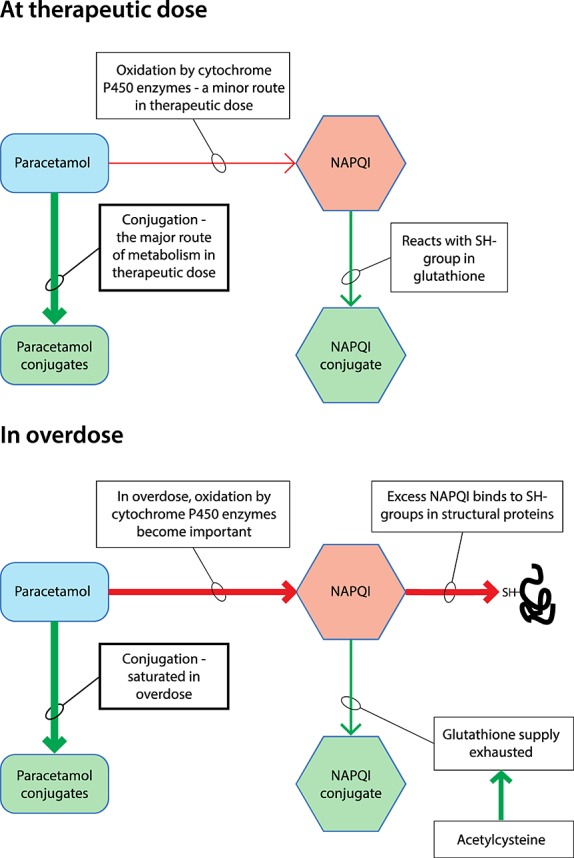
At therapeutic doses conjugation is the major route for paracetamol metabolism. Oxidation of paracetamol by cytochrome P450 is a minor route at therapeutic doses of paracetamol, forming N-acetyl-p-benzoquinone imine (NAPQI) that quickly reacts with glutathione. When an overdose of paracetamol is taken, conjugation becomes saturated and more NAPQI is formed by oxidation. When the glutathione supply is exhausted, NAPQI binds to sulfhydryl (SH-) groups in structural proteins, resulting in cell injury
Current risk assessment
Serum alanine aminotransferase (ALT) activity is the current, widely used, biomarker for hepatocyte injury after paracetamol overdose (and in many other settings). Although ALT has never been formally qualified against liver histology as a biomarker for drug-induced liver injury in humans, its utility has been qualified by decades of clinical experience [8]. However, the majority of patients present to the emergency department soon after overdose with only around 10% presenting later than 12 h post drug ingestion [9]. To exclude the development of liver injury confidently, patients require an ALT measurement at least 24 h after the overdose was ingested. This limits patient stratification in emergency care settings and potentially increases length of hospital stay. Apart from these time/kinetic issues, there are other important limitations of using ALT as a biomarker of liver injury. Changes in ALT activity do not only occur in paracetamol-induced ALI, but with a wide range of acute and chronic liver pathologies such as fatty liver disease, viral hepatitis and liver cancer which decreases the confidence in its utility for causality assessment of paracetamol-induced liver injury [10]. Increases in serum ALT activity can also be a result of myocardial damage or extreme exercise [11], which may generate false positive results. Because paracetamol-induced ALI cannot be confidently confirmed or excluded by using serum ALT activity as a biomarker at the hospital ‘front door’, the decision to treat with NAC following an overdose is primarily based on the blood paracetamol concentration [12]. To stratify patients as being ‘at risk’ for hepatotoxicity after a single overdose (total ingestion of paracetamol taking less than around 1–2 h) a nomogram is used that plots blood paracetamol concentration against time after overdose. This nomogram can only be confidently applied 4 h after overdose ingestion, when absorption is believed to be complete [13]. The utilization of the blood paracetamol nomogram after overdose depends on the correct reporting of the time of overdose. Small errors in timing can result in an incorrect treatment [5]. When the paracetamol overdose is ingested over a longer time period (staggered overdose) or if the patient was exposed to a modified release or intravenous formulation [14] the nomogram cannot be used and the treatment decision is based on the reported dose of paracetamol and the serum ALT activity. There is an unmet clinical need for new biomarkers that can guide treatment to patients at high risk of paracetamol-induced ALI and identify patients with low risk of liver injury who may require shorter, lower doses of NAC or even no treatment at all. To decrease the risk of developing ALI, the MHRA altered the guidelines associated with NAC and lowered its utility threshold. Recent health economic calculations have estimated that this change resulted in an increase of around £8M in the annual spend by UK healthcare providers [1], which further strengthens the case for new biomarkers that improve stratification of patients with paracetamol overdose.
Target biomarker profile
In order to identify new biomarkers that could add real value to the management of paracetamol poisoning we propose desirable and acceptable properties, our target biomarker profile (TBP, Table 1). This approach is widely used in biomarker development to set criteria that will be used to define future success. For further background the US Food and Drug Administration have produced guidance [15]. Our suggested biomarker properties specifically relate to the clinical management of paracetamol overdose. What is desirable or acceptable in other settings may be different. For all biomarkers their diagnostic performance may change when they are measured on different validated assay platforms (for example, a new point-of-care platform in contrast with the laboratory gold standard), so our TBP would need to be re-assessed when new clinical assays become available and validated. It is also important to note that defined desirable and acceptable properties can assist with the development of prospective qualification studies in man to define the context of use for a putative biomarker [16].
Table 1.
Desired and acceptable biomarker attributes
| Attribute | Desired | Acceptable |
|---|---|---|
| Specific for paracetamol overdose | Exclusively elevated by paracetamol-induced injury | Liver injury |
| Sensitivity for ruling out injury | ROC-AUC 1 | AUC ≥ 0.90 |
| Rapidly assayed | At point of care | <60 min turn around time |
| Feasibility of assay | Feasible in settings where resources are sparse (developing countries) | Feasible in standard clinical laboratories (developed countries) |
| Invasiveness / sample preparation time | Whole blood | Plasma/serum |
| Conserved (translational) across in vitro models, in vivo models and humans | Fully conserved between in vitro models, in vivo models and humans | Conserved between rodent models and humans |
| Time after overdose at which it is able to predict the onset of liver injury | 4 h | 8 h |
| Signal to noise | Single measure required to differentiate between healthy reference value and disease | Requires measurement at two time points |
| Quantitative relationship with disease severity | Quantitative | Qualitative |
| Distinguish benign and clinical relevant increase in ALT | Predicts liver failure | Predicts ALT rise |
| Mediator of liver injury | Has existing therapeutic intervention | Potential drug target |
A biomarker specific for paracetamol toxicity is desired, as this test could not be misinterpreted due a signal produced by other causes of liver injury. A biomarker diagnostic for paracetamol toxicity would be valuable when the aetiology of liver injury cannot be identified, reported to be the case in 17% of patients with ALI [17]. However, a marker that reports liver injury due to any cause is acceptable, since in most cases it is known that the patient has ingested an overdose (proven by blood paracetamol measurement).
Ideally, a new biomarker would differentiate disease from non-disease with 100% sensitivity and specificity (area under the receiver operator curve (ROC-AUC) of 1), but we propose an acceptable performance as a ROC-AUC of 0.90. This is a comparable accuracy to troponin T assays when they were first introduced into clinical medicine for acute myocardial infarction stratification [18]. In real clinical practice the biomarker's context of use, derived from prognostic qualification studies in man, may prioritize sensitivity or specificity and our acceptable criterion is a starting point for development.
If the biomarker could be assayed rapidly at point-of-care it could be used outside of standard hospital laboratories, for example, in an ambulance, phase 1 clinical trial unit or in the developing world. An acceptable level of performance would be a turnaround time of 60 min in a standard hospital laboratory, as per the guidance of the clinical biochemists/chemical pathologists and clinical biochemistry services regarding the measurement of commonly requested routine clinical biochemistry and haematology tests in emergency departments [19]. Ideally, the assay would be performed on a drop of whole blood obtained from the fingertip, resulting in a minimally invasive test with short sample preparation time. Acceptable would be measuring the biomarker in plasma or serum. We would desire the biomarker assay to be measurable in settings where resources are sparse, such as in developing countries, but acceptable would be the ability to perform the assay in standard hospital laboratories. Recently, data suggest that ALT can be measured using a robust point-of-care, finger stick test with a rapid turn around time [20]. Despite the drawbacks of ALT, this technology might provide the clinician with a signal that triggers an improved sensitive and specific liver safety assessment. Furthermore, advances in these technologies also point to a pathway of development and validation of such methodologies to assess potential biomarkers with improved characteristics at the point-of-care.
If the biomarker can report liver injury as early as 4 h after paracetamol ingestion, it would complement the paracetamol blood concentration nomogram for making treatment decisions in patients presenting early after overdose. Acceptable would be the ability to predict liver injury at 8 h after paracetamol ingestion, the time point after which NAC treatment loses efficacy [6,21].
A biomarker that is fully translational between in vitro models, animals and humans would aid the detection of hepatotoxic compounds in drug development. However, a biomarker being translational between rodents and humans would be acceptable.
A biomarker with a high signal-to-noise ratio that only requires a single measurement is desirable. Serial measurement would be acceptable, as is the case with troponin for acute myocardial infarction [22].
Ideally, the biomarker could distinguish between a benign ALT rise, the development of serious liver injury or potential Hy's Law cases and imminent liver failure. Acceptable would be the prediction of an ALT rise. The ability of a biomarker to have a quantitative relationship with the severity of the paracetamol toxicity would allow for different treatment strategies in patients with different severities of liver injury. Acceptable would be a biomarker that qualitatively discriminates injury from non-injury.
Should a biomarker also be a mediator of liver injury then the marker could represent a companion diagnostic that identifies patients for a novel therapeutic. A desirable criterion would be a marker that has a drug already approved for human use, acceptable would be a marker that mediates the disease process and has potential therapeutics in development.
New biomarker candidates for paracetamol-induced liver injury and their performance compared with the target biomarker profile
Currently there is huge investment into the development and qualification of novel biomarkers to improve the prediction and monitoring of drug-induced liver injury in preclinical species and in man (Figure2). A number of public-private consortia exist such as the IMI funded SAFE-T (Safer And Faster Evidence-based Translation) project and the Critical Path institute supported PSTC (Predicative Safety Testing Consortium) with these objectives as specific goals. Although the efforts and achievements of these consortia have been previously reported, here we present the case for our TBP to improve treatment and to aid the understanding of the mechanistic basis of paracetamol overdose.
Figure 2.
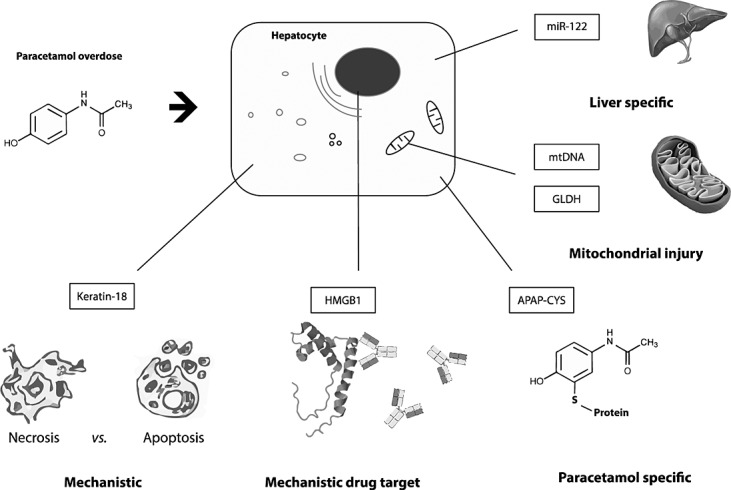
Key properties of novel biomarkers for paracetamol-induced liver injury
Paracetamol protein adducts
Key properties:
Conserved between rodent models and humans.
Exclusively selective for paracetamol overdose
Reflective of the initial molecular initiating event (MIE)
When NAPQI is formed during paracetamol metabolism it covalently binds with proteins forming paracetamol-protein adducts, of which cysteine adducts are the most common [23]. After the binding of NAPQI with cysteine, the structure of NAPQI reverts to that of paracetamol resulting in paracetamol-cysteine (APAP-CYS). In mouse models of paracetamol-induced ALI, immunohistochemical methods report that APAP-CYS is formed in the liver in a temporally progressive, central to peripheral pattern [24]. Experiments in mice also report APAP-CYS in serum, suggesting that injured liver cells release APAP-CYS into the circulation. Notably, serum APAP-CYS was only detectable after toxic doses of paracetamol [25].
More recently, a high performance liquid chromatography with electrochemical detection (HPLC-ECD) assay has been developed. This assay has been used to detect APAP-CYS in liver and serum after hepatotoxic dosing of paracetamol to mice [26] and in both adult and paediatric human serum samples with paracetamol-induced ALI [27–29]. Due to the relatively long plasma half time of 1.7 ± 0.3 days in adults [30] and 1.5 ± 0.3 days in children and adolescents [31], APAP-CYS can be detected up to 7 days after a large overdose [27]. By contrast, the plasma half-life for paracetamol is 1.5–2.5h [32]. The longer half-life of APAP-CYS could potentially allow risk assessment/diagnosis in patients who present when paracetamol has been cleared from the circulation. When the aetiology of ALI is known, APAP-CYS has no defined advantage over ALT because kinetic changes in APAP-CYS track ALT activity [27]. A point-of-care test is currently in development [33].
microRNA-122 (miR-122)
Key properties:
Specific for liver injury
Fully conserved (translational) across in vitro models, in vivo models and humans
Early marker for ALI with a ROC-AUC of >0.90
Predicts ALT rise
MicroRNAs (miRNAs) are small (∼22 nucleotides long) non-protein coding RNA species involved in post-transcriptional gene product regulation. miRNAs are involved in a wide range of biological processes and the biogenesis and function of miRNAs have been comprehensively reviewed elsewhere [34,35]. Outside the cell, miRNAs are very stable due to protection from degradation by RNAses by microvesicles [36] and RNA binding protein complexes [37]. Although a number of miRNAs are widely expressed, certain miRNAs appear to be highly organ specific [38]. Liver tissue expresses a number of distinct miRNAs, especially miR-122, the most abundant hepatic miRNA that has very low to no expression in other healthy tissues, which makes this marker highly liver specific [38]. miR-122 is a multifunctional RNA species that modulates multiple pathways involved in stress response [39], fatty acid metabolism [40], cholesterol synthesis [41] and hepatocellular carcinoma [42].
The potential of circulating miRNAs to serve as biomarkers for ALI was first reported in mice treated with a toxic dose of paracetamol [43]. miR-122 was the miRNA species that had the largest fold change between control and paracetamol treated mice [43]. Subsequently circulating miR-122 has been reported as a marker for ALI in rats [44], dogs [45], zebrafish [46] and pigs [47].
In patients with established ALI circulating miR-122 is around 100-fold higher compared with healthy controls and overdose patients without ALI [48]. Furthermore, miR-122 has also been shown to provide utility at reporting liver injury in paediatric populations of paracetamol overdose. At first presentation to hospital, in a UK cohort of 129 patients from Edinburgh and Newcastle-upon-Tyne, miR-122 was measured at a median of 8 h post-overdose in patients requiring subsequent NAC therapy (Edinburgh and Newcastle study) [49]. In this first sample miR-122 correlated significantly with peak hospital stay ALT activity and INR. miR-122 was significantly higher in those patients who developed subsequent ALI. ROC analysis revealed that miR-122 had an AUC value (sensitivity at 90% specificity) of 0.93 (0.83, 95% CI 0.86, 1.0, P < 0.0001) suggesting that miR-122 could accurately separate patients with and without ALI at an early time when ALT activity was still normal.
Keratin-18
Key properties:
Conserved between rodent models and humans.
Mechanism-based (apoptosis vs. necrosis)
Early marker for ALI with a ROC-AUC of >0.90
Predicts ALT rise
Prognostic marker
Keratins are intermediate filament proteins, expressed by epithelial cells, that are responsible for cell structure, differentiation, mitosis and apoptosis [50,51]. Keratin 18 (K18) is abundantly expressed in the liver and other digestive epithelia cells [52]. During apoptosis, phosphorylation and cleavage of K18 results in cellular rearrangement. Full length K18 is passively released from necrotic cells whereas cleaved K18 fragments are released from apoptotic cells once membrane integrity is lost [53]. Circulating cleaved K18 (apoptosis) and full length K18 (necrosis) can be measured with ELISA to report apoptosis and necrosis [54,55].
Cleaved K18 and full length K18 have been reported to be circulating mechanistic biomarkers for apoptosis and necrosis in mouse models of paracetamol-induced ALI [56,57]. Full length and cleaved K18 were measured in a mixed UK and US cohort of paracetamol-induced ALI patients. They were increased in the circulation of paracetamol-induced ALI patients compared with paracetamol overdose patients without ALI and healthy controls.
In patients with established ALI full length and cleaved K18 were significantly higher in patients who subsequently reached the King's College Criteria (KCC) for liver transplantation. Further analysis revealed that the percentage of total circulating K18 derived from cleaved K18 (from apoptotic cells) was relatively lower in patients who reached the KCC compared with those who did not [57]. ROC analysis confirmed that full length K18 had a higher AUC than ALT for predicting patients who met KCC and for the outcome of liver transplant/death [57]. Another research group has recently confirmed the ability of circulating cleaved and full length K18 to report liver injury after paracetamol overdose [58]. In this study both cleaved and full length K18 correlated with poor outcome (death or liver transplant).
At first presentation to hospital, in the Edinburgh and Newcastle first presentation study, cleaved and full length K18, measured in the first sample, correlated with peak ALT activity and INR during hospital stay. ROC analysis revealed that full length K18 had an AUC value (sensitivity at 90% specificity) of 0.94 (0.9, 95% CI 0.87, 1.0, P < 0.0001) suggesting that this form of K18 could accurately separate patients with and without ALI at an early time when ALT activity was still normal. The performance of the cleaved form of K18 was less accurate with regard to reporting ALI at first presentation to hospital (AUC 0.77; sensitivity at 90% specificity 0.21) [49]. This is consistent with necrosis being more prominent than apoptosis in the pathophysiology of paracetamol-induced acute liver injury.
High-mobility group box-1
Key properties:
Prognostic marker
Mechanism-based (DAMP – inflammatory mediator)
Early marker for ALI with a ROC-AUC of >0.90
Predicts ALT rise
Conserved between rodent models and humans
Potential drug target
High-mobility group box-1 (HMGB1) is an evolutionary conserved chromatin-binding protein expressed in the nucleus of virtually all cells. HMGB1 is passively released into the extracellular space by cells that are undergoing necrosis and plays a key role in alerting the immune system to dying cells and thus works as a damage-associated molecular pattern (DAMP) molecule [59–61]. HMGB1 stimulates an immune response by activating toll-like receptors (TLR) and the receptor for advanced glycation end products (RAGE) [62–64]. Besides being passively released, HMGB1 is actively released as a cytokine in a hyper-acetylated form by various immune cells such as monocytes and macrophages after activation by inflammatory stimuli [65]. Whether the extracellular cytokine activity of HMGB1 functions as a chemo attractant or pro-inflammatory mediator depends on the redox state of three key cysteine residues [64,66]. HMGB1 can be measured in the circulation and increased levels are related to increased disease activity in sepsis [67], pancreatitis [68] and rheumatoid arthritis [69]. In a mouse model of paracetamol toxicity circulating total and acetylated forms of HMGB1 displayed temporal kinetics that correlated with the onset of necrosis and inflammation respectively, confirming the potential of HMGB1 as an indicator of cell death processes [70]. HMGB1 is a potential mediator of paracetamol-induced hepatotoxicity. Anti-HMGB1 antibodies and knocking out HMGB1 in the liver reduced hepatic inflammation and liver injury in mouse models of paracetamol poisoning [59,71,72]. Anti-HMGB1 antibodies are in development for the treatment of human disease.
In patients with established ALI total and acetylated HMGB1 was increased in the circulation compared with paracetamol overdose patients without ALI and healthy controls [57]. Acetylated HMGB1 was significantly increased in patients who reached KCC compared with patients who did not. ROC analysis demonstrated that HMGB1 (both total and acetylated) had a higher AUC than ALT for predicting patients who will reach KCC and predicting liver transplant/death [57].
At first presentation to hospital HMGB1 had an AUC value (sensitivity at 90% specificity) of 0.97 (0.91, 95% CI 0.91, 1.0, P < 0.0001), suggesting that HMGB1 could accurately identify patients with ALI at the time when ALT activity was still normal [49]. Comparing ROC curves suggests that HMGB1 may be the most accurate biomarker at first presentation, but this needs to be tested in larger studies.
Glutamate dehydrogenase
Key properties:
Conserved between rodent models and humans.
Predicts ALT rise
Glutamate dehydrogenase (GLDH) is a mitochondrial enzyme that catalyzes the reversible deamination of glutamate to α-ketoglutarate plus free ammonia by using NAD or NADP as a co-factor [73]. Circulating GLDH has been suggested to be a specific mechanistic marker for mitochondrial damage. It was reported that mice treated with furosemide, a loop diuretic deemed to cause centrilobular liver necrosis without affecting mitochondrial function [74], produced a significant increase in serum ALT activity with only a non-significant increase in serum GLDH activity [75]. By contrast, paracetamol treated mice had a substantial increase in GLDH along with ALT suggesting that the increase of circulating GLDH is indicative of paracetamol-induced mitochondrial damage and not simply caused by leakage of the enzyme from necrotic cells [75].
In patients with established ALI circulating GLDH is elevated. At first presentation to hospital GLDH is less accurate than miR-122, K18 and HMGB1 with regard to identifying patients with subsequent ALI despite NAC with an AUC value (sensitivity at 90% specificity) of 0.80 (0.19, 95% CI 0.68, 0.93, P = 0.0003) [49].
Mitochondrial DNA fragments
Circulating mitochondrial DNA (mtDNA) has been reported to act as a DAMP molecule via TLR mediated activation of inflammatory cells [76,77]. Increased concentrations of circulating mtDNA have been associated with the systemic inflammatory response syndrome, multiple organ dysfunction syndrome and mortality in patients admitted to intensive care [78,79].
In patients with established ALI circulating mtDNA was increased as measured by absolute quantification of mtDNA encoding NADH dehydrogenase and cytochrome c oxidase. The plasma concentration of mtDNA in patients with abnormal liver function increased over time and peak levels correlated with peak ALT [75]. As is the case with GLDH, circulating mtDNA may be a mechanistic marker for paracetamol-induced mitochondrial injury [75].
Kidney injury molecule-1
Key properties:
Prognostic
Translational between humans and rodents
Point of care tested developed with rapid turn around
Formally qualified by regulatory authorities for the investigation of drug-induced renal injury in preclinical drug development
Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein that confers phagocytic activity on the proximal tubule cells of the kidney. During AKI, KIM-1 is rapidly up-regulated and its ecto-domain is shed into urine and blood where it is a sensitive and specific biomarker of acute kidney injury. Furthermore, KIM-1 has been formally qualified by regulatory authorities for its use to monitor acute kidney injury in the preclinical setting [80]. In patients with paracetamol overdose, secondary injury to the kidney and specifically the proximal tubule epithelia is a major determinate of mortality. Indeed, biomarkers such as serum creatinine are often incorporated into prognostic algorithms. However, serum creatinine is delayed in its onset and data from animal models and in humans have repeatedly demonstrated the ability of KIM-1 to increase earlier following acute kidney injury [81,82]. In patients with established ALI circulating KIM-1 has been recently reported to be elevated, particularly in those patients who subsequently died or required a liver transplant compared with spontaneous survivors [83]. The fold change in KIM-1 in this poor prognostic group was higher than creatinine and KIM-1 outperformed creatinine in a ROC analysis. Furthermore, circulating KIM-1 was an independent predictor of outcome in a logistic regression model [83].
Current biomarker performances and future challenges
Table 2 gives an overview of the characteristics of each marker. Each of our characteristics has at least one biomarker that meets the acceptable or desired specifications. However, all these biomarkers have only been measured in relatively small numbers of patients and large multicentre trials are required to qualify current findings and explore and confirm further the attributes of these biomarkers. These studies should confirm at which time after paracetamol overdose the biomarker is able to predict liver injury and the sensitivity for ruling out injury. These studies should also determine the signal-to-noise ratio of each marker, identify which markers (if any) can distinguish between benign and clinically relevant increases in ALT, and establish if there is a quantitative relationship between biomarker level and outcome.
Table 2.
Comparative biomarker profiles
| Attribute | Desired | Acceptable |
|---|---|---|
| Specific for paracetamol overdose | APAP-CYS | miR-122, GLDH |
| Sensitivity for ruling out injury | – | miR-122, Keratin-18, HMGB1, |
| Rapidly assayed | – | GLDH, KIM-1 |
| Feasibility of assay | GLDH? | GLDH |
| Invasiveness/sample preparation time | – | miR-122, Keratin-18, HMGB1, GLDH, mtDNA |
| Conserved (translational) across in vitro models, in vivo models and humans | miR-122 | Keratin-18, HMGB1, GLDH, mtDNA, KIM-1 |
| Time after overdose at which it is able to predict the onset of liver injury | – | miR-122, Keratin-18, HMGB1, GLDH |
| Signal to noise | miR-122, Keratin-18, APAP-CYS, HMGB1, GLDH, KIM-1 | – |
| Quantitative relationship with disease severity | – | miR-122, HMGB1 |
| Distinguish benign and clinical relevant increase in ALT | Keratin-18, HMGB1 | miR-122, GLDH |
| Mediator of liver injury | HMGB1 |
There is considerable scope for improvement in the ‘rapidly assayed’ and ‘feasibility of assay’ characteristics in our TBP. At the time of writing, only the calorimetric assay for GLDH has been validated with these performance characteristics in mind. It can be automated in modern clinical chemistry laboratories with a turnaround time of less than 1 h. All other biomarkers are typically measured manually in research laboratories with time consuming and expensive kits. There is an urgent need for standardized and validated commercial assays that can be used at point-of-care to stratify paracetamol overdose patients for entry into trials of new therapeutic approaches.
In order to have the driver for introducing one or more of these biomarkers into clinical practice their measurement must add value to patient care [84]. In the setting of paracetamol overdose, a normal test result might add value by giving the treating clinician more confidence in discharging a patient. Given the large number of patients, this could reduce the pressure on acute hospital beds and save the health provider money. Conversely, an abnormal test result might trigger entry into a stratified clinical trial, indicate need for different treatment with a new therapy or referral for specialist care.
In conclusion, our target biomarker profile could be useful for biomarker research and development in the settings of drug overdose. Studies to date demonstrate the utility of a number of mechanistic biomarkers that should be evaluated against our TBP in prospective studies. These attributes can be used to define the strengths and weaknesses of a new marker in development and can help in identifying potential clinical benefit.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
ADBV was supported by an NC3Rs PhD Studentship (NC/K001485/1). JWD acknowledges the support of an NHS Research Scotland (NRS) Career Research Fellowship through NHS Lothian and the UK Regenerative Medicine Platform Niche Hub.
References
- 1.Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, Dow M, Coyle J, Cranfield KR, Hook C, Sandilands EA, Veiraiah A, Webb D, Gray A, Dargan PI, Wood DM, Thomas SH, Dear JW, Eddleston M. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol. 2014;78:610–8. doi: 10.1111/bcp.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–94. [PubMed] [Google Scholar]
- 4.Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39:11–21. doi: 10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- 5.Ferner RE, Dear JW, Bateman DN. Management of paracetamol poisoning. BMJ. 2011;342:d2218. doi: 10.1136/bmj.d2218. [DOI] [PubMed] [Google Scholar]
- 6.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–62. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 7.Waring WS. Criteria for acetylcysteine treatment and clinical outcomes after paracetamol poisoning. Exp Rev Clin Pharmacol. 2012;5:311–8. doi: 10.1586/ecp.12.15. [DOI] [PubMed] [Google Scholar]
- 8.Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther. 2012;92:332–9. doi: 10.1038/clpt.2012.108. [DOI] [PubMed] [Google Scholar]
- 9.Prescott K, Stratton R, Freyer A, Hall I, Le Jeune I. Detailed analyses of self-poisoning episodes presenting to a large regional teaching hospital in the UK. Br J Clin Pharmacol. 2009;68:260–8. doi: 10.1111/j.1365-2125.2009.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hourani K, Mansi R, Pettie J, Dow M, Bateman DN, Dear JW. The predictive value of hospital admission serum alanine transaminase activity in patients treated for paracetamol overdose. QJM. 2013;106:541–6. doi: 10.1093/qjmed/hct062. [DOI] [PubMed] [Google Scholar]
- 11.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Rumack BH, Peterson RC, Koch GG, Amara IA. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380–5. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- 13.Prescott LF, Roscoe P, Wright N, Brown SS. Plasma paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet. 1971;1:519–22. doi: 10.1016/s0140-6736(71)91125-1. [DOI] [PubMed] [Google Scholar]
- 14.Beringer RM, Thompson JP, Parry S, Stoddart PA. Intravenous paracetamol overdose: two case reports and a change to national treatment guidelines. Arch Dis Child. 2011;96:307–8. doi: 10.1136/adc.2010.192005. [DOI] [PubMed] [Google Scholar]
- 15.FDA. 2007. Draft guidance for industry and review staff, target product profile—a strategic development process tool. Available at http://www.fda.gov/cder/guidance/index.htm: (last accessed 8 July 2015) FDA.
- 16.Watkins PB, Merz M, Avigan MI, Kaplowitz N, Regev A, Senior JR. The clinical liver safety assessment best practices workshop: rationale, goals, accomplishments and the future. Drug Saf. 2014;37(Suppl 1):S1–7. doi: 10.1007/s40264-014-0181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 18.Muller-Bardorff M, Rauscher T, Kampmann M, Schoolmann S, Laufenberg F, Mangold D, Zerback R, Remppis A, Katus HA. Quantitative bedside assay for cardiac troponin T: a complementary method to centralized laboratory testing. Clin Chem. 1999;45:1002–8. [PubMed] [Google Scholar]
- 19.Hawkins RC. Laboratory turnaround time. Clin Biochem Rev. 2007;28:179–94. [PMC free article] [PubMed] [Google Scholar]
- 20.Pollock NR, Rolland JP, Kumar S, Beattie PD, Jain S, Noubary F, Wong VL, Pohlmann RA, Ryan US, Whitesides GM. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med. 2012;4:152ra29. doi: 10.1126/scitranslmed.3003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2:1097–100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, Lindahl B, Giannitsis E, Hasin Y, Galvani M, Tubaro M, Alpert JS, Biasucci LM, Koenig W, Mueller C, Huber K, Hamm C, Jaffe AS. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197–204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 23.Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem Biol Interact. 1984;48:349–66. doi: 10.1016/0009-2797(84)90145-5. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–71. [PMC free article] [PubMed] [Google Scholar]
- 25.Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. Immunochemical quantitation of 3-(cystein-S-yl)acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 1989;248:190–6. [PubMed] [Google Scholar]
- 26.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–51. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 27.Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–94. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 28.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, Squires RH. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–81. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 29.Alonso EM, James LP, Zhang S, Squires RH. Acetaminophen adducts detected in serum of pediatric patients with acute liver failure. J Pediatr Gastroenterol Nutr. 2015;61:102–7. doi: 10.1097/MPG.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James LP, Capparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, Blumer JL, Sullivan JE. Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84:684–90. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–8S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James L, Roberts D, Hinson JA. 2009. Inventors. Acetaminophen-protein adduct assay device and method. In eds The Board of Trustees of The University of Arkansas. Arkansas Children's Hospital Research Institute I.
- 34.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 35.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, Xiao T, Schafer J, Lee ML, Schmittgen TD, Nana-Sinkam SP, Jarjoura D, Marsh CB. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003694. e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–24. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 40.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 42.Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–97. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–7. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su YW, Chen X, Jiang ZZ, Wang T, Wang C, Zhang Y, Wen J, Xue M, Zhu D, Zhang Y, Su YJ, Xing TY, Zhang CY, Zhang LY. A panel of serum microRNAs as specific biomarkers for diagnosis of compound- and herb-induced liver injury in rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037395. e37395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrill AH, Eaddy JS, Rose K, Cullen JM, Ramanathan L, Wanaski S, Collins S, Ho Y, Watkins PB, Lecluyse EL. Liver biomarker and in vitro assessment confirm the hepatic origin of aminotransferase elevations lacking histopathological correlate in beagle dogs treated with GABAA receptor antagonist NP260. Toxicol Appl Pharmacol. 2014;277:131–7. doi: 10.1016/j.taap.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Vliegenthart AD, Starkey Lewis P, Tucker CS, Del Pozo J, Rider S, Antoine DJ, Dubost V, Westphal M, Moulin P, Bailey MA, Moggs JG, Goldring CE, Park BK, Dear JW. Retro-orbital blood acquisition facilitates circulating microRNA measurement in zebra fish with paracetamol hepatotoxicity. Zebrafish. 2014;11:219–26. doi: 10.1089/zeb.2013.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson P, Gidlof O, Braun OO, Gotberg M, van der Pals J, Olde B, Erlinge D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock. 2012;37:234–8. doi: 10.1097/SHK.0b013e31823f1811. [DOI] [PubMed] [Google Scholar]
- 48.Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM, Neoptolemos JP, Moggs J, Goldring CE, Park BK. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–76. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 49.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. ‘Heads and tails’ of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–94. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Vijayaraj P, Sohl G, Magin TM. Keratin transgenic and knockout mice: functional analysis and validation of disease-causing mutations. Methods Mol Biol. 2007;360:203–51. doi: 10.1385/1-59745-165-7:203. [DOI] [PubMed] [Google Scholar]
- 52.Ku NO, Zhou X, Toivola DM, Omary MB. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol. 1999;277:G1108–37. doi: 10.1152/ajpgi.1999.277.6.G1108. [DOI] [PubMed] [Google Scholar]
- 53.Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, Nap M, Bjorklund V, Bjorklund P, Bjorklund B, Lane EB, Omary MB, Jornvall H, Ramaekers FC. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Cummings J, Hodgkinson C, Odedra R, Sini P, Heaton SP, Mundt KE, Ward TH, Wilkinson RW, Growcott J, Hughes A, Dive C. Preclinical evaluation of M30 and M65 ELISAs as biomarkers of drug induced tumor cell death and antitumor activity. Mol Cancer Ther. 2008;7:455–63. doi: 10.1158/1535-7163.MCT-07-2136. [DOI] [PubMed] [Google Scholar]
- 55.Kramer G, Schwarz S, Hagg M, Havelka AM, Linder S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer. 2006;94:1592–8. doi: 10.1038/sj.bjc.6603129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 2011;252:289–97. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Possamai LA, McPhail MJ, Quaglia A, Zingarelli V, Abeles RD, Tidswell R, Puthucheary Z, Rawal J, Karvellas CJ, Leslie EM, Hughes RD, Ma Y, Jassem W, Shawcross DL, Bernal W, Dharwan A, Heaton ND, Thursz M, Wendon JA, Mitry RR, Antoniades CG. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure. Crit Care Med. 2013;41:2543–50. doi: 10.1097/CCM.0b013e31829791a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 60.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 62.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, Morser J, Stern D, Schmidt AM. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 63.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 64.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93:865–73. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–9. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 68.Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359–63. doi: 10.1097/01.mpa.0000236741.15477.8b. [DOI] [PubMed] [Google Scholar]
- 69.Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–81. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 70.Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112:521–31. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- 71.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Jenkins RE, Antoine DJ, Schwabe RF. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–50. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–90. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Fang J, Hsu BY, MacMullen CM, Poncz M, Smith TJ, Stanley CA. Expression, purification and characterization of human glutamate dehydrogenase (GDH) allosteric regulatory mutations. Biochem J. 2002;363:81–7. doi: 10.1042/0264-6021:3630081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong SG, Card JW, Racz WJ. The role of mitochondrial injury in bromobenzene and furosemide induced hepatotoxicity. Toxicol Lett. 2000;116:171–81. doi: 10.1016/s0378-4274(00)00218-6. [DOI] [PubMed] [Google Scholar]
- 75.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258:591–8. doi: 10.1097/SLA.0b013e3182a4ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001577. e1001577; discussion e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, Park BK, Smyth RL, Pirmohamed M. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043809. e43809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–86. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Antoine DJ, Sabbisetti VS, Francis B, Jorgensen AL, Craig DG, Simpson KJ, Bonventre JV, Park BK, Dear JW. Circulating kidney injury molecule-1 predicts prognosis and poor outcome in patients with acetaminophen-induced liver injury. Hepatology. 2015 doi: 10.1002/hep.27857. doi: 10.1002/hep.27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McShane LM. Statistical challenges in the development and evaluation of marzker-based clinical tests. BMC Med. 2012;10:52. doi: 10.1186/1741-7015-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]


