Abstract
Glucocorticosteroids are a group of structurally related molecules that includes natural hormones and synthetic drugs with a wide range of anti-inflammatory potencies. For synthetic corticosteroid analogues it is commonly assumed that the therapeutic index cannot be improved by increasing their glucocorticoid receptor binding affinity. The validity of this assumption, particularly for inhaled corticosteroids, has not been fully explored. Inhaled corticosteroids exert their anti-inflammatory activity locally in the airways, and hence this can be dissociated from their potential to cause systemic adverse effects. The molecular structural features that increase glucocorticoid receptor binding affinity and selectivity drive topical anti-inflammatory activity. However, in addition, these structural modifications also result in physicochemical and pharmacokinetic changes that can enhance targeting to the airways and reduce systemic exposure. As a consequence, potency and therapeutic index can be correlated. However, this consideration is not reflected in asthma treatment guidelines that classify inhaled corticosteroid formulations as low-, mid- and high dose, and imbed a simple dose equivalence approach where potency is not considered to affect the therapeutic index. This article describes the relationship between potency and therapeutic index, and concludes that higher potency can potentially improve the therapeutic index. Therefore, both efficacy and safety should be considered when classifying inhaled corticosteroid regimens in terms of dose equivalence. The historical approach to dose equivalence in asthma treatment guidelines is not appropriate for the wider range of molecules, potencies and device/formulations now available. A more robust method is needed that incorporates pharmacological principles.
Keywords: Corticosteroid, dose equivalence, inhaled, potency, therapeutic index
Introduction
Glucocorticosteroids are natural and synthetic analogues of the hormones secreted by the hypothalamic–anterior pituitary–adrenocortical (HPA) axis which have anti-inflammatory activity. It is a widely held assumption that the therapeutic index of synthetic glucocorticoids, generally termed corticosteroids, cannot be improved by increasing their potency via enhanced glucocorticoid receptor binding affinity. This is probably valid for systemically administered corticosteroids, unless selectivity for glucocorticoid receptors vs. nontarget receptors is greatly increased, as the efficacy and safety are both attributable to circulating drug concentrations and common receptor interactions [1]. However, a similar rationale is commonly adopted for inhaled corticosteroids, where potency is not considered to affect the topical efficacy to systemic activity ratio [2], with efficacy and potency differences being overcome by giving larger doses of the less potent drug [3].
There are several reasons why this rationale may not be valid for inhaled corticosteroids. First, they exert their anti-inflammatory activity at the site of action in the airways, which is not in equilibrium with the downstream systemic drug concentrations responsible for the unwanted systemic effects [4]. Secondly, it assumes that increasing inhaled corticosteroid potency is not associated with changes in other features of the molecule [5]. However, in reality, the molecular structural features that increase glucocorticoid receptor binding affinity and selectivity also result in physicochemical and pharmacokinetic changes that together may potentially enhance targeting to the airways and reduce systemic exposure.
Currently, there are eight inhaled corticosteroid molecules approved for clinical use that span a wide range of potency and other attributes. This article explores the relationship between inhaled corticosteroid potency and therapeutic index
Potency and molecular structure
Beclomethasone dipropionate (BDP) was introduced in 1972 as the first synthetic corticosteroid asthma controller medication administered via the inhaled route [6]. At the time, it was heralded as a major breakthrough that freed asthma sufferers from the fear of the adverse effects associated with chronic systemic corticosteroid use. Since then, a further seven inhaled corticosteroids have been approved for clinical use, with a range of glucocorticoid receptor selectivity, potency, physicochemical properties, pharmacokinetic parameters and inhaler/formulation options (Table 1).
Table 1.
Corticosteroid physicochemical, pharmacokinetic and pharmacological characteristics
| Corticosteroid/dose form | Relative glucocorticoid receptor binding affinity | Lipophilicity (log P) | Aqueous solubility (µg ml–1) | PPB (%) | Vss l | CL l h–1 | F (%) | Sources |
|---|---|---|---|---|---|---|---|---|
| Fluticasone furoate DPI | 2989 | 4.17 | 0.03 | 99.7 | 608 | 65 | 15DPI 1oral | [7, 21, 23, 33, 35] |
| Mometasone furoate DPI | 2100 | 4.73 | <0.1 | 99.5 | 332 | 54 | 11DPI 1oral | [7, 21, 23, 31, 32, 39–42] |
| Fluticasone propionate DPI | 1775 | 3.89 | 0.14 | 99.3 | 318 | 69 | 16DPI 1oral | [7, 21, 23, 31, 32, 43–45, 40, 46, 47, 42] |
| Beclomethasone dipropionate (BMP) MDI | 53 (1345) | 4.59 (3.27) | 0.13 (15.5) | 95.9 | 424 | 120 | 62CFC 82HFA 41oral | [7, 21, 22, 43, 39] |
| Ciclesonide (des-CIC) MDI | 12 (1200) | 3.2 (3.0) | <0.1 (7) | 98.7 | 396 | 228 | 63HFA 1oral | [7, 21, 23, 39, 44, 42] |
| Budesonide DPI | 935 | 2.32 | 16 | 91.4 | 180 | 84 | 39DPI 11oral | [7, 21, 23, 43, 39, 45, 46, 48, 49, 42] |
| Triamcinolone acetonide MDI | 233 | 1.85 | 21 | 73.2 | 103 | 37 | 25CFC 23oral | [7, 21, 22, 43, 39, 45, 50, 51, 42] |
| Flunisolide MDI | 190 | 1.36 | 140 | 61.2 | 96 | 58 | 33CFC 70HFA 20oral | [7, 21, 23, 43, 39, 45] |
| Prednisolone oral | 12 | 1.65 | 223 | 57.6 | 93 | 37 | 82oral | [7, 21, 23, 45, 52, 42] |
For beclomethasone dipropionate (BDP) and ciclesonide (CIC), values in parenthesis are for the active metabolites – beclomethasone 17-monopropionate (BMP) and desisobutyryl ciclesonide (des-CIC). Glucocorticoid receptor binding affinity is relative to dexamethasone where dexamethasone affinity = 100. Log P values are defined as the log10 of the octanol/water partition coefficient. CFC, chlorofluorocarbon propellant MDI; CL, plasma clearance; DPI, dry-powder inhaler; F, absolute bioavailability determined in healthy subjects; HFA, hydrofluoroalkane propellant MDI; MDI; metered-dose inhaler; PPB, plasma protein binding; Vss, volume of distribution at steady state.
Drug discovery and development in this area has identified molecules with greater selectivity, potency and improved targeting to the lung via low oral bioavailability and high systemic clearance. However, in the minds of many prescribers and patients, it is unclear whether having a wider choice of inhaled corticosteroid molecules and inhaler options available offers any advantages.
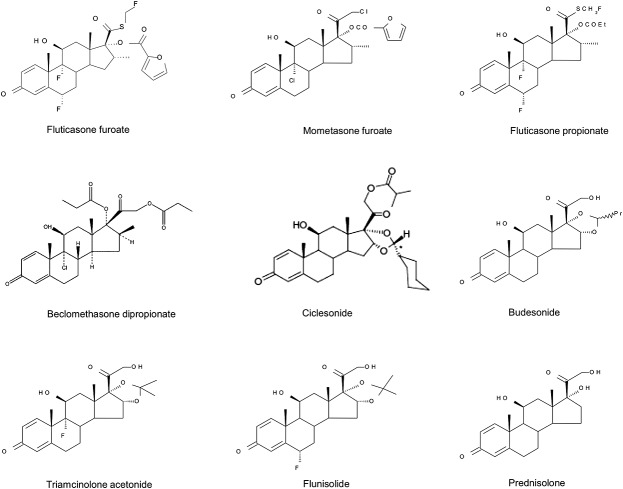
The main structural feature shared by the synthetic analogues and the endogenous glucocorticoid, cortisol, is the 17-carbon androstane nucleus derived from cholesterol (Figure1). In the synthetic glucocorticoids, the addition of the 1,2 double bond and halogen atoms in the alpha position on carbon atoms 6 and 9 (Figure1) confer greater metabolic stability. Esters and cyclic esters on the 17 and 16 positions, and hydrophobic groups on the 20 and 21 positions (Figure1) lead to greater affinity for the glucocorticoid receptor. These structural modifications also result in greater specificity for the glucocorticoid receptor, a longer duration of receptor occupancy and less association with nontarget steroid receptors. They also lead to increased lipophilicity and reduced aqueous solubility [7]. The available inhaled corticosteroid molecules are listed in Table 1 in order of potency, with flunisolide (FLU) the least and fluticasone furoate (FF) the most potent. Lipophilicity, aqueous solubility, plasma protein binding and tissue distribution all follow the same trend. In comparison, the oral corticosteroid, prednisolone, ranks the lowest in these attributes (Table 1).
Figure 1.
Chemical structures of synthetic glucocorticoids
High first-pass metabolism and consequently negligible oral bioavailability are found for FF, fluticasone propionate (FP), mometasone furoate (MF) and ciclesonide (CIC), whereas significant oral bioavailability is found for FLU, triamcinolone acetonide (TAA), budesonide (BUD) and beclomethasone dipropionate (BDP) (Table 1).
Metabolic stability is important for efficacy but is only an advantage if the rate of systemic clearance is also high. This is the case for most glucocorticoids (Table 1), with more lipophilic molecules being good substrates for hepatic cytochrome P450 3A polypeptide 4 (CYP3A4) metabolism [7]. For BDP and CIC, clearance includes extra-hepatic metabolism as they are also pro-drugs and converted to their active metabolites by esterases found in lung and others tissues. For BDP, 97% is converted in the lung to the more potent beclomethasone monopropionate (BMP); for CIC, the conversion rate to its active principle in the lung appears to be less complete [7]. By contrast, FP and FF are not pro-drug esters of fluticasone, and their efficacy is dependent on the intact molecules. The two molecules are distinct, with different properties – the furoate ester in FF being responsible for the greater lipophilicity, lower solubility and enhanced glucocorticoid receptor binding affinity compared with FP and other inhaled corticosteroid molecules [8]. Furthermore, fluticasone is not a metabolite and is devoid of activity.
The duration of action of glucocorticoids in the lung has also been related to their residence time there [8–10]. A prolonged pulmonary residence time is apparent when the elimination half-life following inhaled administration is significantly longer than found following intravenous administration. This tendency has been noted for the more lipophilic inhaled corticosteroids, with the following order of lung retention times: FF >> MF ≥ FP > TAA >> BUD ≥ desisobutyryl-CIC (des-CIC) > FLU ≥ BMP [6]. In addition, FF has greater glucocorticoid receptor affinity in vitro and a longer duration of action in experimental models of lung inflammation than FP [11,12]. It has also been reported that BUD, BMP and des-CIC may undergo a reversible intracellular reaction to form esters with fatty acids such as oleate and palmitate. This is a feature of glucocorticoids with 21-hydroxyl groups and has been proposed as an alternative mechanism of prolonged tissue retention in the lung, although it is unclear whether this has any benefit in prolonging the duration of action [7,13].
Potency and therapeutic dose equivalence
The potential advantage of higher inhaled corticosteroid potency is that a lower inhaled dose is required to occupy the same numbers of glucocorticoid receptors in the airways, resulting in a lower daily dose for equivalent efficacy. This relationship is depicted in Figure2, where mid-range nominal therapeutic daily doses of inhaled corticosteroid used in adult asthma are plotted against the corresponding potency, expressed as the relative glucocorticoid receptor binding affinity. Theoretically, the major factors expected to drive the relative efficacy of an inhaled corticosteroid are potency, device efficiency (delivered lung dose) and pulmonary residency time. However, it is notable that Figure2 shows a clear exponential decline in therapeutic daily dose with increasing potency, without the need to take account of factors other than potency. Although the other factors are likely to contribute to differences in efficacy, it is clear that topical potency in the airways is the most important.
Figure 2.
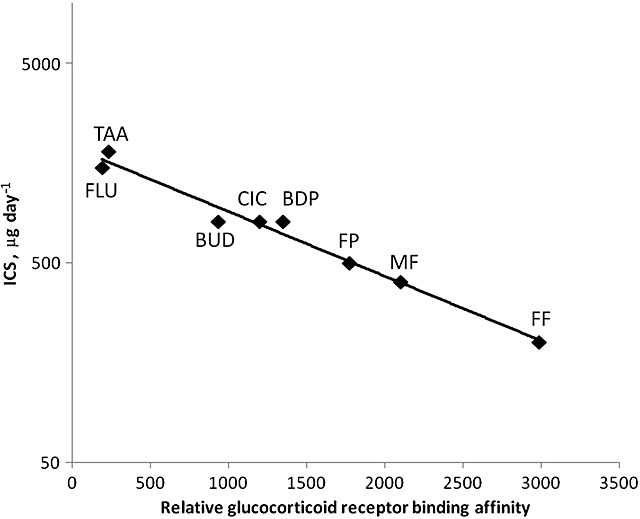
Relationship between glucocorticoid receptor binding affinity (Table 1) and mid-range nominal therapeutic daily doses [53] of inhaled corticosteroids (ICS) (r2 = 0.980)
Despite this observation, the pulmonary residence time (described above) does appear to influence some aspects of efficacy. The main consequence of this appears to be a longer duration of action rather than greater efficacy per se, with the corticosteroid with the longest lung retention time (FF) being suitable for once-daily dosing, and those with shorter lung retention times requiring twice- (FP), three- or four-times (TAA) daily dosing regimens [7]. Once-daily dosing of MF, FP, BUD and CIC is efficacious but twice-daily dosing is generally better [14]. The exception to this is FF, which has the longest lung retention and highest potency, where administering the same total daily dose as either a once-daily or twice-daily regimen has equivalent efficacy [15].
The inhaler device efficiency is expected to influence inhaled corticosteroid therapeutic dose equivalence. Device efficiency (lung deposited dose/nominal dose) varies for dry-powder (DPI) and metered-dose (MDI) inhalers. The largest differences are seen between DPIs and chlorofluorocarbon (CFC) MDIs of low- to mid-range efficiency that emit particles mostly in the 3–5 µm range when compared with MDIs that contain drug dissolved in hydrofluoroalkane (HFA) propellant and generate an ultrafine aerosol plume with smaller particles (≈1 µm) and a greater proportion of the particles in the respirable range (<5 µm) [16]. The impact of device efficiency on therapeutic dose is explored in Figure3, which has on the y-axis the total daily dose estimated to be deposited in the lungs, obtained by correcting the nominal dose for the device efficiency. Figure3 also includes data for DPIs and MDIs of both the CFC and higher-efficiency ultrafine aerosol HFA MDIs (FLU, BDP, CIC) (Table 1). Also included are low-, mid- and high-dose regimens of all currently available inhaled corticosteroids, illustrating for each dose level a distinct exponential decline in therapeutic daily dose with increasing potency. Therefore, one might expect that all dose regimens in the low-, mid- or high-dose categories, as defined by each regression line, should have equivalent efficacy. This may be the case, but is difficult to verify as the extent to which each product's recommended doses are based on comprehensive dose ranging in all severities of asthma is variable.
Figure 3.

Relationship between glucocorticoid receptor binding affinity (Table 1) and estimated daily lung dose [53, 54] (Table 1) for therapeutic doses in low- (♦) (r2 = 0.825), mid- (▲) (r2 = 0.934) and high- (▪) dose ranges (r2 = 0.947) of inhaled corticosteroids (ICS). This analysis includes ICS dose regimens that are not approved for clinical use
Clinical experience with inhaled corticosteroids in asthma indicates that most of the benefit in terms of improving lung function is achieved with low–mid doses, with fewer patients benefiting from higher doses [17,18]. Consequently, for inhaled corticosteroids it is difficult to demonstrate a clear dose response for clinical endpoints within the efficacious dose range. Figure4 shows the relationship between the glucocorticoid receptor binding affinity and the glucocorticoid receptor dissociation constant (Kd, nmol l–1), the concentration of the inhaled corticosteroid needed to occupy 50% of the receptors. If one calculates the lung concentrations of the various inhaled corticosteroids that would result from daily doses in the recommended range being evenly distributed throughout the entire ≈900 g of human lung tissue [19], these inhaled corticosteroid concentrations far exceed the Kd values. Although this calculation is a worst-case scenario for drug availability at the site of action, it nevertheless suggests the potential for a high degree of glucocorticoid receptor occupancy, even for low doses of the least potent inhaled corticosteroid molecules. Considering these factors, all commonly prescribed inhaled corticosteroid doses would be at the top of the dose response curve, unless only a small fraction of the lung dose reaches the site of action and is pharmacologically active. If this premise is correct, it underlines the importance of potency in driving receptor occupancy and clinical efficacy.
Figure 4.
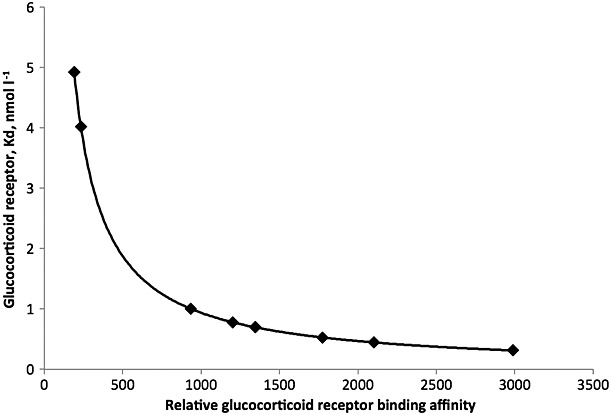
Relationship between glucocorticoid receptor binding affinity (Table 1) and the corresponding inhaled corticosteroid glucocorticoid receptor dissociation constant (Kd, nmol l–1), which is the concentration needed to occupy 50% of glucocorticoid receptors
Potency and systemic effects
The undesirable effects of inhaled corticosteroids comprise a broad range of class-related adverse events that include hoarseness/dysphonia, candidiasis of the mouth and throat, adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataract, glaucoma, hyperglycaemia, contusions and pneumonia (in patients with chronic obstructive pulmonary disease). Some of the commonly reported and minor adverse effects, such as hoarseness/dysphonia, are related to local topical activity, whereas adverse effects related to systemic exposure, such as adrenal suppression, although more serious, are very rarely reported [20].
The factors that contribute to a low potential for systemic effects are those which minimize circulating drug concentrations. These are a low dose, which leads to low absorption from the lung; low bioavailability of the swallowed faction of the dose; and high clearance of the absorbed dose. The increased lipophilicity, which accompanies high potency, is also associated with a higher plasma protein binding and larger volumes of distribution (Table 1). These lead to lower total and unbound systemic drug concentrations. Plasma protein binding is probably a less important factor for the more potent inhaled corticosteroid molecules as evidence suggests that this is a relatively low-affinity interaction and therefore may have less impact on systemic bioactivity [21]. The volume of distribution is a major determinant, together with the clearance, of the elimination half-life and time taken to reach steady state for systemic concentrations, but the all-important steady-state drug concentration that the patient is continually exposed to with chronic long-term use is a consequence of the clearance rate and input rate (dose rate and bioavailability). The systemic activity and associated adverse effects are related to this concentration, together with the glucocorticoid receptor binding potency. A higher potency alone would lead to greater systemic effects but the structural changes that lead to higher potency and a lower dose also result in a lower rate and extent of bioavailability and high clearance.
The measurement of inhaled corticosteroid-mediated adrenal suppression, such as inhibition of cortisol secretion, is the most sensitive and easily monitored biomarker of adverse systemic inhaled corticosteroid effects. This is a risk factor in inhaled corticosteroid therapy as the body does not distinguish between endogenous and synthetic exogenous glucocorticoids. The administration of exogenous glucocorticoids elevates the circulating concentrations of total glucocorticoids (endogenous + exogenous), resulting in reduced adrenocorticotrophic hormone and corticotropin-releasing hormone release and a corresponding reduction in cortisol production [22]. Low-dose therapy with inhaled glucocorticoids may make only a small contribution to the glucocorticoid pool. Therefore, homeostasis is maintained and the daily glucocorticoid requirements remain within physiological limits. However, when high doses of glucocorticoids are administered, it is possible that the extra glucocorticoid added to the endogenous pool may become the majority of the daily requirements. Under these circumstances, the normal daily requirements can be exceeded, even if endogenous glucocorticoid production is suppressed to very low levels, and if this is maintained for a prolonged period, there is a risk of adrenal insufficiency [22].
Using physiologically based pharmacokinetic/pharmacodynamic modelling [23], it is possible to estimate the extent to which common glucocorticoid dose regimens have an impact on the HPA axis. This approach relates the normal endogenous glucocorticoid production rate to the exogenous contributions from inhaled corticosteroids by converting them into cortisol-equivalent exposures. The calculation takes account of the bioavailability, relative potency, plasma protein binding and systemic clearance of the exogenous glucocorticoids to express the systemic exposure for each exogenous corticosteroid as a cortisol-equivalent area under the plasma concentration–time curve [23]. For example, when high-dose inhaled regimens of FP, BUD, TAA and BDP were investigated, they were estimated to correspond to an additional daily input of 2–4 mg of cortisol into the body and to result in a cortisol suppression at steady state of 22–34%, which was in agreement with published values [23].
The same method was used to estimate the daily dose that would result in 20% cortisol suppression for each inhaled corticosteroid and formulation shown in Table 1: FF DPI 580 µg day–1, MF DPI 660 µg day–1, FP DPI 900 µg day–1, BDP HFA MDI 390 µg day–1, BDP CFC MDI 500 µg day–1, CIC HFA MDI 1200 µg day–1, BUD DPI 600 µg day–1, TAA CFC MDI 700 µg day–1, FLU HFA MDI 700 µg day–1 and FLU CFC MDI 1500 µg day–1.[22–32] These values were then used to calculate the corresponding therapeutic indices for each inhaled corticosteroid shown in Figure5 and discussed below. This approach was used as published data documenting the doses associated with 20% cortisol suppression for each inhaled corticosteroid formulation, using the same methodology, were not available. However, where data of this type were available, the estimated values were in close agreement [24–34]. The cortisol suppression estimates were a worst-case scenario as they assumed that lung delivery and systemic exposure was as seen in healthy subjects or mild asthmatics. However, it has been shown that inhaled corticosteroid lung deposition and systemic exposure to inhaled corticosteroid are lower in more severe asthma, when lung function is lower [35]. Although a value of 20% reduction in serum cortisol appears small and intrinsically not associated with adverse effects, it is close to the lower boundary of detectable systemic exposure for an exogenous corticosteroid and was therefore used as the cut-off above which a wider range of unwanted effects become more likely.
Figure 5.
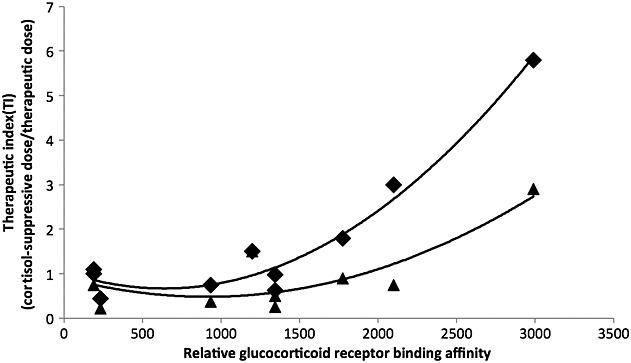
Relationship between glucocorticoid receptor binding affinity (Table 1) and the therapeutic index for various inhaled corticosteroid dose regimens. Therapeutic index is defined as the daily dose that produces 20% cortisol suppression divided by either the low–mid (♦) or mid–high (▲) therapeutic daily dose [23,53,54]
Potency and therapeutic index
The glucocorticoid receptor binding potency of an inhaled corticosteroid can influence both its efficacy and systemic effects, but for potency to influence the therapeutic index there needs to be a differential effect on efficacy or systemic exposure. Figure5 shows the relationship between potency and therapeutic index for various inhaled corticosteroid dose regimens. This relationship is approximately exponential or linear on a log-dose scale. The higher the therapeutic index, the greater the separation between systemic adverse effects and the desired local effects in the airways. In this example, the therapeutic index is defined as the dose that produces 20% cortisol suppression (as described above) divided by the therapeutic dose. The therapeutic doses are shown in Figure3 but, for consistency and as each inhaled corticosteroid varies in the number of recommended doses, two dose levels are shown in Figure5 – a low–mid dose and a mid–high dose. Figure5 shows that, with increasing potency, the therapeutic index increases and, as expected, the low–mid doses have a better therapeutic index than the mid/high doses. Furthermore, it is the inhaled corticosteroid molecules with highest potency, longest lung retention, lowest oral bioavailability and highest systemic clearance (FF, MF, FP, CIC) that have the highest therapeutic index. The therapeutic index values are <1 for mid/high doses of BDP, BUD and TAA, but for FLU the therapeutic index was approximately 1, due to its lower potency, higher dose and greater systemic exposure. Therapeutic index values >1 were seen for CIC, FP, MF and FF, with the highest value of 5.8 for the 100 µg day–1 dose of FF. To put these values into context, 5 mg day–1 and 20 mg day–1 dose regimens of oral prednisolone had corresponding therapeutic index values of 0.32 and 0.08, respectively.
Current asthma treatment guidelines [36,37] make assumptions about dose equivalence that position low doses as effective doses without significant risk of adverse effects, and high doses as those achievable with an acceptable systemic adverse-effect profile. It is also recognized that most of the therapeutic benefit is achieved at low–mid doses and that not all patients benefit from high doses [17,18]. Asthma treatment guidelines [36,37] also classify the various inhaled corticosteroid formulations into low-, mid- and high doses. Although it is not claimed that within these classification doses are therapeutically equivalent, this is unavoidably implied and leads to the perception that efficacy and safety cannot be separated and that they are interchangeable products. There is little evidence to support the current dose-equivalence approach that includes benchmarking to the now obsolete BDP CFC MDI. Indeed, most inhaled corticosteroid molecules have been evaluated in isolation using different dose ranges in each severity of asthma. Few studies have compared more than two inhaled corticosteroids and none has explored multiple products across a range of doses comparing both efficacy and safety endpoints [2]. It is acknowledged that the major determinants of inhaled corticosteroid therapeutic equivalence are potency and the efficiency of the device used for lung delivery, but there is incomplete consideration of the systemic exposure and relative risk of adverse effects so as to arrive at a relative therapeutic index for each dose of each inhaled corticosteroid. Historically, when this approach was applied to a narrow range of similar inhaled corticosteroid molecules, the consequences probably had less of an impact. However, it is questionable whether we should simply extrapolate this simple approach to the full range of inhaled corticosteroid molecules, potencies and device/formulations currently available.
Another area of difficult interpretation is that of inhaler performance and its impact on dose equivalence. Two questions often arise: is it possible to improve the therapeutic index of an inhaled corticosteroid by (i) increasing the efficiency of the inhalation device and/or (ii) by reducing the particle size in the emitted aerosol? The answer is not simple to arrive at as improving the device efficiency is often accompanied by a reduction in the average particle size emitted, which may also lead to a shift in the pattern of lung deposition. Although it has been proposed that small particles may be better able to treat small airways disease, this hypothesis has not been proven [38]. Smaller particles may also have a higher rate of dissolution and reduced mucociliary clearance, resulting in increased absorption and systemic exposure. This may confound the interpretation of changes in device efficiency as consequences occur for both efficacy and systemic exposure.
For an inhaled corticosteroid that has minimal oral absorption of the swallowed dose (FF, MF, FP, CIC), it is not likely that increasing lung deposition would have much impact on the therapeutic index as most of the inhaled dose that reaches the lungs and site of action is also available for systemic absorption. By contrast, for a drug with significant oral absorption (FLU, TA, BUD, BDP), increasing lung deposition may have some impact on the therapeutic index. A more efficient device would allow a lower dose to be administered to achieve an effective dose at the site of action but the swallowed dose would also be lower, and hence the systemic absorption.
There is a lack of evidence that an inhaled corticosteroid with smaller aerosol particles (≈1 µm) offers a therapeutic advantage over the established particle size range (3–5 µm). On the positive side, small particles may deposit less in the oropharynx and more easily reach the peripheral airways. However, on the negative side, smaller particles are more likely to be exhaled and if they do deposit in the airways they are more likely to dissolve and be absorbed rapidly. Therefore, apart from improving device efficiency, it may be preferable to have a range of particle sizes in the respirable range (<5 µm), rather than predominantly small particles, as this will be likely to favour deposition both centrally and peripherally, and minimize systemic absorption.
There are examples where device efficacy has been improved for inhaled corticosteroid molecules, e.g. when replacing BDP and FLU CFC MDIs with HFA MDIs that emit smaller particles. The increase in device efficiency for both HFA MDIs appears to result in a small improvement in the therapeutic index (Figure5) but not by enough to reclassify them from low- to high-therapeutic-index inhaled corticosteroid products. For BDP, a greater therapeutic index improvement might be expected but the efficacy of BDP relies on its conversion to the active metabolite BMP in the airways, whereas the more rapid dissolution and absorption of the ultrafine BDP particles from the HFA MDI result in less conversion in the airways and more unchanged BDP reaching the systemic circulation [16].
Conclusions
The exponential relationship between in vitro glucocorticoid receptor binding affinity and therapeutic dose for inhaled corticosteroids is evidence that more potent molecules can be administered at much lower doses to achieve similar clinical efficacy. Furthermore, the structural features of inhaled corticosteroids that give rise to more potent molecules also drive lower systemic exposure, and together these factors can improve the therapeutic index. In this way, enhanced inhaled corticosteroid potency leads to greater lipophilicity, slower dissolution and pulmonary absorption of inhaled drug particles with longer retention times in the airways. This also results in a longer duration of action and permits less frequent dosing. Once absorbed, more potent inhaled corticosteroids have higher plasma protein binding, lower unbound fractions in the plasma and larger volumes of distribution. These molecules are also good substrates for drug-metabolizing enzymes and have high systemic clearance, high first-pass metabolism and low oral bioavailability of the swallowed dose. All these factors, together with the lower dose that greater potency affords, favour low systemic drug concentrations, effectively improving the targeting of drug to the site of action.
As a higher potency can improve the therapeutic index, both efficacy and safety should be considered when classifying inhaled corticosteroid regimens in terms of dose equivalence. Current asthma treatment guidelines rely on a simple historical approach to dose equivalence but this is not appropriate for the wider range of molecules, potencies and devices/formulations now available. A more fit-for-purpose method is needed that incorporates pharmacological principles.
Competing Interests
The author has completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the author) and declared PDY is an employee and shareholder of GlaxoSmithKline. This work was funded by GSK. There are no financial relationships with any other organizations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–27. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 2.Kelly HW. Establishing a therapeutic index for the inhaled corticosteroids: part I. Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. J Allergy Clin Immunol. 1998;102:S36–51. doi: 10.1016/s0091-6749(98)70004-1. [DOI] [PubMed] [Google Scholar]
- 3.Kamada AK, Szefler SJ, Martin RJ, Boushey HA, Chinchilli VM, Drazen JM, Fish JE, Israel E, Lazarus SC, Lemanske RF. Issues in the use of inhaled glucocorticoids. The Asthma Clinical Research Network. Am J Respir Crit Care Med. 1996;153(6):1739–48. doi: 10.1164/ajrccm.153.6.8665030. [DOI] [PubMed] [Google Scholar]
- 4.Daley-Yates PT. The clinical utility of pharmacokinetics in demonstrating bioequivalence of locally acting orally inhaled drugs. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory Drug Delivery. Davis. River Grove: Healthcare International Publishing; 2010. pp. 273–83. 1. [Google Scholar]
- 5.Hochhaus G, Mollmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic and pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37:881–92. doi: 10.1002/j.1552-4604.1997.tb04262.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark TJH. Effect of beclomethasone dipropionate delivered by aerosol in patients with asthma. Lancet. 1972;1:1361–4. doi: 10.1016/s0140-6736(72)91094-x. [DOI] [PubMed] [Google Scholar]
- 7.Daley-Yates PT. Pharmacological aspects of glucocorticoid therapy. In: Wolthers OD, editor. Exogenous Glucocorticoids in Paediatric Asthma. Kerala: Transworld Research Network; 2007. pp. 1–18. [Google Scholar]
- 8.Valotis A, Högger P. Human receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoate. Respir Res. 2007;8:54. doi: 10.1186/1465-9921-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brindley C, Mackie AE, Falcoz C, Bye A. Absorption kinetics after inhalation of fluticasone propionate via the Diskhaler, Diskus and metered-dose inhaler in healthy subjects. Clin Pharmacokinet. 2000;39(Suppl. 1):8. doi: 10.2165/00003088-200039001-00001. [DOI] [PubMed] [Google Scholar]
- 10.Derendorf H. Pharmacokinetic and pharmacodynamic properties of inhaled corticosteroids in relation to efficacy and safety. Respir Med. 1997;91(Suppl. A):22–8. doi: 10.1016/s0954-6111(97)90102-5. [DOI] [PubMed] [Google Scholar]
- 11.Salter M, Biggadike K, Matthews JL, West MR, Haase MV, Farrow SN, Uings IJ, Gray DW. Pharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:660–7. doi: 10.1152/ajplung.00108.2007. [DOI] [PubMed] [Google Scholar]
- 12.Rossios C, To Y, To M, Ito M, Barnes PJ, Adcock IM, Johnson M, Ito K. Long-acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. Eur J Pharmacol. 2011;670:244–51. doi: 10.1016/j.ejphar.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Jerre A, Sjölin P, Silberstein D, Wieslander E. Cellular uptake and fatty acid esterification of budesonide and ciclesonide in protein-rich medium. Proc Am Thoracic Society. 2006;3:A73. [Google Scholar]
- 14.Boulet LP, Cowie RL, Negro RD, Brett W, Gold M, Marques A, Thorburn WS, Stepner NM, Robson R. Comparison of once- with twice-daily dosing of fluticasone propionate in mild and moderate asthma. Can Respir J. 2000;7:239–47. doi: 10.1155/2000/464639. [DOI] [PubMed] [Google Scholar]
- 15.Woodcock A, Bleecker ER, Busse WW, Lötvall J, Snowise NG, Frith L, Jacques L, Haumann B, Bateman ED. Fluticasone furoate: once-daily evening treatment versus twice-daily treatment in moderate asthma. Respir Res. 2011;12:160. doi: 10.1186/1465-9921-12-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daley-Yates PT. Relationship between pharmacokinetic and aerodynamic particle size distribution properties for inhalers containing corticosteroids. Respiratory Drug Delivery. 2014;1:163–72. [Google Scholar]
- 17.Masoli M, Holt S, Weatherall M, Beasley R. Dose–response relationship of inhaled budesonide in adult asthma: a meta-analysis. Eur Respir J. 2004;23:552–8. doi: 10.1183/09031936.04.00076604. [DOI] [PubMed] [Google Scholar]
- 18.Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose–response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ. 2001;323:253–6. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina DK, DiMaio VJ. Normal organ weights in men: part II – the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2012;33:368–72. doi: 10.1097/PAF.0b013e31823d29ad. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer KJ, Tata LJ, Smith CJP, West J, Harrison TW, Tattersfield AE, Hubbard RB. Oral and inhaled corticosteroids and adrenal insufficiency: a case-control study. Thorax. 2006;61:405–8. doi: 10.1136/thx.2005.052456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daley-Yates PT, Harker AJ, Taylor S, Daniel MJ. Plasma protein binding of inhaled corticosteroids: reappraisal of its significance in systemic pharmacological activity. J Allergy Clin Immunol. 2005;115:S4. [Google Scholar]
- 22.Wolthers OD, Daley-Yates PT. Exogenous Glucocorticoids in Paediatric Asthma, eds Wolthers OD. Kerala: Transworld Research Network; 2007. Inhaled glucocorticoids and hypothalamic–pituitary–adrenal axis function; pp. 19––34. [Google Scholar]
- 23.Daley-Yates PT, Pierre LN. A physiologically based pharmacokinetic/pharmacodynamic model predicting cortisol suppression for inhaled corticosteroids. Am J Respir Crit Care Med. 2001;163:A518. [Google Scholar]
- 24.Casale TB, Nelson HS, Stricker WE, Kenneth HR, Newman B. Suppression of hypothalamic–pituitary–adrenal axis activity with inhaled flunisolide and fluticasone propionate in adult asthma patients. Ann Allergy Asthma Immunol. 2001;87:379–85. doi: 10.1016/S1081-1206(10)62918-3. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AM, McFarlane LC, Lipworth BJ. Dose–response effect for adrenal suppression with repeated twice daily inhaled fluticasone propionate and triamcinolone acetonide in adult asthmatics. Am J Respir Crit Care Med. 1997;156:1274–7. doi: 10.1164/ajrccm.156.4.97-03029. [DOI] [PubMed] [Google Scholar]
- 26.Sorkness CA, LuForce C, Storms W, Lincourt WR, Edwards L, Rogenes PR. Effects of the inhaled corticosteroids fluticasone propionate, triamcinolone acetonide, and flunisolide and oral prednisone on the hypothalamic–pituitary–adrenal axis in adult patients with asthma. Clin Ther. 1999;2(1):353–67. doi: 10.1016/S0149-2918(00)88292-2. [DOI] [PubMed] [Google Scholar]
- 27.Möllmann H, Wagner M, Krishnaswami S, Dimova H, Tang Y, Falcoz C, Daley-Yates PT, Krieg M, Stöckmann R, Möllmann AC, Derendorf H, Hochhaus G. Single dose and steady state pharmacokinetic and pharmacodynamic evaluation of equipotent doses of inhaled fluticasone propionate and budesonide in healthy subjects. J Clin Pharmacol. 2001;41:1329–38. doi: 10.1177/00912700122012913. [DOI] [PubMed] [Google Scholar]
- 28.Daley-Yates PT, Bagen S, Tournant J, Pereira A. Beclomethasone dipropionate chlorofluorocarbon and hydrofluoroalkane metered dose inhalers: relationship between systemic exposure, dose, fine particle mass and particle size in healthy volunteers. Eur Resp J. 1999;14(30):P1358. [Google Scholar]
- 29.Derom E, Van De Velde V, Marissens S, Engelstatter R, Vincken W, Pauwels R. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and airway responsiveness to adenosine monophosphate in asthmatic patients. Pulm Pharmacol Ther. 2005;18:328–36. doi: 10.1016/j.pupt.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Chrousos GP, Ghaly L, Shedden A, Iezzoni DG, Harris AG. Effects of mometasone furoate dry powder inhaler and beclomethasone dipropionate hydrofluoroalkane and chlorofluorocarbon on the hypothalamic–pituitary–adrenal axis in asthmatic subjects. Chest. 2005;128:70–7. doi: 10.1378/chest.128.1.70. [DOI] [PubMed] [Google Scholar]
- 31.Crim C, Pierre LN, Daley-Yates PT. A review of the pharmacology and pharmacokinetics of fluticasone propionate and mometosone furoate. Clin Ther. 2001;23:1339–54. doi: 10.1016/s0149-2918(01)80113-2. [DOI] [PubMed] [Google Scholar]
- 32.Daley-Yates PT, Derks MGM, Weeks AT, Mehta RS, Beerahee M, Sousa A. Pharmacokinetics and pharmacodynamics of fluticasone propionate and mometasone furoate dry powder inhalers in healthy and asthmatic subjects. Am J Respir Crit Care Med. 2005;2:A354. [Google Scholar]
- 33.Allen A. The relationship between fluticasone furoate systemic exposure and cortisol suppression. Clin Pharmacokinet. 2013;52:885–96. doi: 10.1007/s40262-013-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FFA103096. A randomised, double blind, placebo controlled, incomplete block, five-way cross-over study to investigate the effect of one week repeat dosing of fluticasone furoate (GW685698X) and fluticasone propionate (FP) on twenty-four hour serum cortisol in healthy subjects. Study Period: October 2004 – March 2005. Available at http://www.gsk-clinicalstudyregister.com (la st accessed 8 March 2015)
- 35.Brutsche MH, Brutsche IC, Munavvar M, Langley S, Masterson C, Daley-Yates PT, Brown R, Woodcock AA. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in subjects with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356:556–61. doi: 10.1016/S0140-6736(00)02581-2. [DOI] [PubMed] [Google Scholar]
- 36.British Thoracic Society and Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2003;58(Suppl. 1):i1–94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Global Initiative for Asthma. 2014. Global strategy for asthma management and prevention. Available at http://www.ginasthma.org (last accessed 8 March 2015)
- 38.Suarez E, Fang S, Abraham J, DiSantostefano RL, Stempel DA, Frith L, Barnes NC. Effect of inhaled corticosteroid particle size on asthma efficacy and safety outcomes: a systematic literature review. Thorax. 2014;69(Suppl. 2):A182. [Google Scholar]
- 39.Hogger P, Rohdewald P. Glucocorticoid receptors and fluticasone propionate. Rev Contemp Pharmacother. 1998;9:501––22. [Google Scholar]
- 40.Daley-Yates PT, Kunka RL, Shen YY, Andrews SM, Callejas S, Ng C. Bioavailability of fluticasone propionate and mometasone furoate aqueous nasal sprays. Eur J Clin Pharmacol. 2004;60:265–8. doi: 10.1007/s00228-004-0763-y. [DOI] [PubMed] [Google Scholar]
- 41.Falcoz C, Oliver R, McDowall J, Ventresca GP, Bye A, Daley-Yates PT. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39(Suppl. 1):9–15. doi: 10.2165/00003088-200039001-00002. [DOI] [PubMed] [Google Scholar]
- 42.Taylor S, Harker A. Modification of the ultrafiltration technique to overcome solubility and non-specific binding challenges associated with the measurement of plasma protein binding of corticosteroids. J Pharm Biomed Anal. 2006;41:299–303. doi: 10.1016/j.jpba.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Derendorf H, Hochhaus G, Meibohm B, Mollmann H, Bart J. Pharmacokinetics and pharmacodynamics of inhaled corticosteroids. J Allergy Clin Immunol. 1998;101:S440–6. doi: 10.1016/s0091-6749(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 44.Kaliner MA. Pharmacologic characteristics and adrenal suppression with newer inhaled corticosteroids: a comparison of ciclesonide and fluticasone propionate. Clin Ther. 2006;28:319–31. doi: 10.1016/j.clinthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Mager DE, Jusko WJ. Quantitative structure–pharmacokinetic/pharmacodynamic relationships of corticosteroids in man. J Pharm Sci. 2001;91:2442–51. doi: 10.1002/jps.10231. [DOI] [PubMed] [Google Scholar]
- 46.Agertoft L, Pedersen S. Lung deposition and systemic bioavailability of fluticasone Diskus and budesonide Turbuhaler. Am J Respir Crit Care Med. 2003;168:779–82. doi: 10.1164/rccm.200302-200OC. [DOI] [PubMed] [Google Scholar]
- 47.Mackie AE, McDowall JE, Falcoz C, Ventresca GP, Bye A. Daley-Yates PT. Pharmacokinetics of fluticasone propionate inhaled via the Diskhaler and Diskus powder devices in healthy subjects. Clin Pharmacokinet. 2000;39(1):23–30. doi: 10.2165/00003088-200039001-00004. [DOI] [PubMed] [Google Scholar]
- 48.Thorsson L, Edsbacker S, Conradson TB. Lung deposition from Turbuhaler is twice that from a pressurised metered dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44. doi: 10.1183/09031936.94.07101839. [DOI] [PubMed] [Google Scholar]
- 49.Agertoft L, Andersen A, Weibull E, Pedersen S. Systemic availability and pharmacokinetics of nebulised budesonide in pre-school children with asthma. Arch Dis Child. 1999;80:241–7. doi: 10.1136/adc.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeal W, Faulds D. Triamcinolone acetonide. A review of its pharmacological properties and therapeutic efficacy in the management of allergic rhinitis. Drugs. 1997;53:257–80. doi: 10.2165/00003495-199753020-00006. [DOI] [PubMed] [Google Scholar]
- 51.Derendorf H, Hochhaus G, Rohatagi S, Möllmann H, Barth J, Sourgens H, Erdmann M. Pharmacokinetics of triamcinolone acetonide after intravenous, oral, and inhaled administration. J Clin Pharmacol. 1995;35:302–5. doi: 10.1002/j.1552-4604.1995.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 52.Barth J, Damoiseaux M, Möllmann H, Brandis KH, Hochhaus G, Derendorf H. Pharmacokinetics and pharmacodynamics of prednisolone after intravenous and oral administration. Int J Clin Pharmacol Ther Toxicol. 1992;30:317–24. [PubMed] [Google Scholar]
- 53. Electronic Medicines Compendium. Datapharm Communications Limited,1999. Available at https://www.medicines.org.uk/emc (last accessed 8 March 2015)
- 54. FDA approved drug products. FDA center for drug evaluation and research, office of communications, division of online communications, 2014. Available at http://www.accessdata.fda.gov/scripts/cder/drugsatfda (last accessed 8 March 2015)